Abstract
The rapid transmission of the pandemic 2009 H1N1 influenza virus (pH1N1) among humans has raised the concern of a potential emergence of reassortment between pH1N1 and highly pathogenic influenza strains, especially the avian H5N1 influenza virus. Here, we report that the cold-adapted pH1N1 live attenuated vaccine (CApH1N1) elicits cross-reactive immunity to seasonal and H5 influenza A viruses in the mouse model. Immunization with CApH1N1 induced both systemic and mucosal antibodies with broad reactivity to seasonal and H5 strains, including HAPI H5N1 and the avian H5N2 virus, providing complete protection against heterologous and heterosubtypic lethal challenges. Our results not only accentuate the merit of using live attenuated influenza virus vaccines in view of cross-reactivity but also represent the potential of CApH1N1 live vaccine for mitigating the clinical severity of infections that arise from reassortments between pH1N1 and highly pathogenic H5 subtype viruses.
TEXT
The unexpected emergence of the novel H1N1 influenza virus (pH1N1) in 2009 was recorded as the first influenza pandemic of the 21st century. Fortunately, this pandemic caused fewer deaths than previous pandemics before moving into its postpandemic period. However, the rapid human-to-human transmission of pH1N1 and the sporadic outbreak of highly pathogenic avian H5N1 virus (HPAI) infections have raised a serious concern that a reassortment between these viruses would lead to the generation of a highly pathogenic influenza virus with an increased ability to infect humans (8, 20, 26, 27). The unpredictability of the emergence of a pandemic strain such as pH1N1, together with the possibility of a reassortment event that would create a new virus strain, evidently calls for the development of a universal vaccine that would induce a broad range of protection against antigenically different influenza virus strains. That an HPAI H5N1 strain with enhanced transmissibility among humans can be generated in the laboratory is also being debated as a potential new threat, in addition to those represented by natural outbreaks (29). Efforts to develop such universal vaccines encompass a wide range of aspects, including the identification of previously unknown conserved regions or T cell epitopes and new antibodies with broad reactivity that, in turn, could expedite the development of new and more effective strategies (11, 13, 16, 24, 28, 35, 38, 41). In addition, extensive studies of the humoral and cell-mediated immunity responsible for inducing cross-protection are being carried out (3, 33). The potential importance of cross-protection also triggered many recent studies addressing the effect of either prior exposure to seasonal influenza viruses or vaccination against them on the immune responses to a pandemic virus, particularly the 2009 pandemic A H1N1 influenza virus (1, 6, 7, 14, 36, 40).
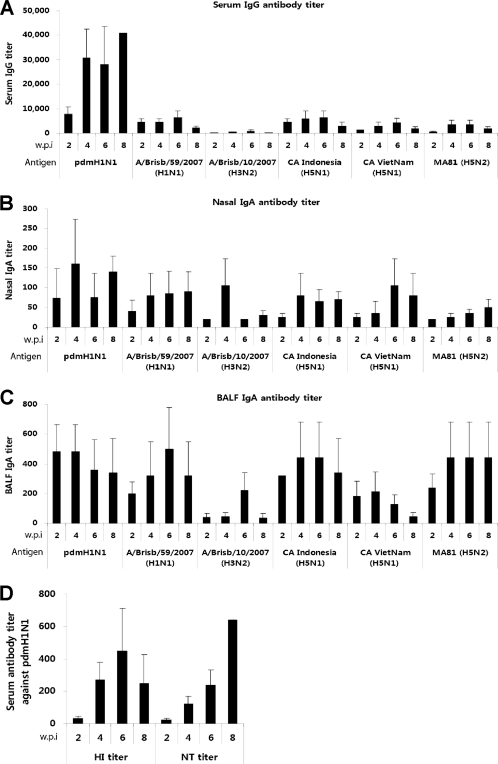
Thus far, the cold-adapted (ca) live attenuated pH1N1 monovalent vaccine has shown promising immunogenicity and safety for human use, and its protective efficacy was intensively evaluated in animal models (10, 15, 44). Many studies have shown that live attenuated influenza vaccine (LAIV), mimicking the natural process of viral infection, induces both humoral and cellular immune responses, providing broad and long-lasting immunity (25, 26). Along with its effectiveness as a pandemic vaccine, the pH1N1 live vaccine needs to be extensively evaluated for the breadth of its cross-reactivity in nature against cocirculating influenza virus strains such as the seasonal influenza viruses and the HPAI H5N1 influenza virus. Both pH1N1 and the seasonal influenza viruses can easily infect and spread among humans, which substantially increases the possibility of exchange of their genetic materials. Of further concern, it was reported that pH1N1 can infect a wide range of species, including pigs and poultry (4), both of which are highly susceptible hosts to the avian H5N1 influenza virus. We previously developed and characterized the X-31 cold-adapted donor strain (X-31ca) and showed it to have an excellent safety, immunogenicity, and protection profile in the mouse model (25). Using reverse genetics, we generated a candidate live vaccine against pH1N1 carrying hemagglutinin (HA) and neuraminidase (NA) from A/Korea/01/2009 (H1N1) in the genetic background of X-31ca and investigated whether this live vaccine (CApH1N1) induced cross-reactive immunity to seasonal or avian H5 viruses. To analyze the cross-reactive antibody responses, mice were intranasally inoculated with a single dose of 105 PFU of CApH1N1 or phosphate-buffered saline (PBS) as a control, and sera and nasal washes were collected from the immunized mice at 2-week intervals. Using an enzyme-linked immunosorbent assay (ELISA), each of samples was analyzed for titers of antibody that reacted to the seasonal and H5 influenza virus strains. The 2008 to 2009 seasonal influenza viruses for testing included A/Brisbane/59/2007 (H1N1) and A/Brisbane/10/2007 (H3N2). For H5 subtypes, we generated two 6:2 reassortant viruses containing surface antigens, HA and NA, from A/Indonesia/05/2005 (H5N1) and A/Vietnam/HN31242/2007 (H5N1) as surrogates for highly virulent avian H5 strains. In addition, A/Aquatic bird/Korea/W81/2005 (MA81) (H5N2) (32), a mouse-adapted and highly virulent avian influenza virus, was included. Our results showed that the CApH1N1 vaccine elicited substantial levels of cross-reactive serum IgG antibodies against seasonal H1N1 and H5 strains but showed low reactivity to strain H3N2 (Fig. 1A). It should be noted that the titers of heterosubtypic cross-reactive serum IgG against three H5 strains were commensurate with those observed against A/Brisbane/59/2007 (H1N1), which belongs to the same subtype of vaccine strain, although their cross-reactivity was approximately 1/10 of the level seen against the homologous swine influenza virus strain (CApH1N1). Intriguingly, the cross-reactivity of mucosal antibody responses was much more robust than that of systemic responses. The secretory IgA titers of antibody in nasal washes (Fig. 1B) and bronchoalveolar lavage fluid (BALF) (Fig. 1C) against the same set of viruses were similar to those against CApH1N1. This observations agree with previous studies showing that the mucosal immunity mediated by secretory IgA antibodies, through their polymeric nature and hence higher avidity, is more cross-protective against heterologous viral infection than the systemic immunity mediated by IgG antibodies (22, 37).
Fig 1.
Cross-reactive systemic and mucosal antibody responses to seasonal and H5 influenza viruses. To collect serum samples, eight 6-week-old female BALB/c mice were divided into two groups; the mice in one of the groups were immunized with one dose of 105 PFU of CApH1N1, and the mice in the other group received PBS as a control. Every 2 weeks, serum samples were collected from each of the eight mice. For bronchoalveolar fluid (BALF) and nasal wash sampling, 32 mice were divided into two groups, one for immunization with one dose of 105 PFU of CApH1N1 and the other for treatment with PBS as a control. Every 2 weeks, eight mice from the two groups were sacrificed by cervical dislocation, and the BALF and nasal washes were harvested in PBS. The virus-specific IgG or IgA antibody titers were analyzed by ELISA. (A to C) Titers of serum IgG (A), nasal wash IgA (B), and BALF IgA (C) against six viruses are shown. Antibody titers are expressed as the reciprocal of the dilution that yielded an optical density above the mean plus two times the standard deviation (SD) of PBS control sample results. (D) With the same serum samples, the hemagglutinin inhibition (HI) antibody titers and viral neutralization (NT) antibody titers against homologous vaccine strain were also estimated. None of the PBS groups produced a detectable level of antibody titer. Data represent the means of the results determined for each cohort (n = 4), and error bars indicate ±SD. Detection limits were 160 for serum IgG titer, 20 for IgA titer, 8 for HI antibody titer, and 20 for NT antibody titer. w.p.i, weeks postimmunization. pdmH1N1, 2009 pandemic H1N1 ca vaccine (CApH1N1). All experimental procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Yonsei Laboratory Animal Research Center (YLARC).
However, we failed to detect significant levels of serum virus-neutralizing titers (NT) or hemagglutinin inhibition (HI) antibody titers against those viruses even after booster immunization (data not shown), while a single immunization induced a robust immune response with high titers of NT and HI antibodies against a homologous vaccine strain (Fig. 1D). In vitro neutralizing and HI activities are mediated by the antibodies that directly interact with the receptor binding region of HA molecules, which is highly variable among influenza virus subtypes. It is most likely, therefore, that the antibodies induced by CApH1N1 do not bind avidly to this variable region of antigenically distant strains. However, as our ELISA results suggest, it is possible that antibodies that can interact with relatively more conserved regions of HA proteins were generated, yielding clearly detectable titers in ELISA. This interpretation is in agreement with recent findings that identified antibodies that interact with highly conserved domains of surface proteins, providing protective immune responses (16, 17, 28).
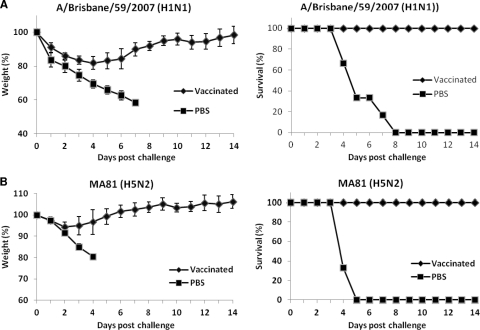
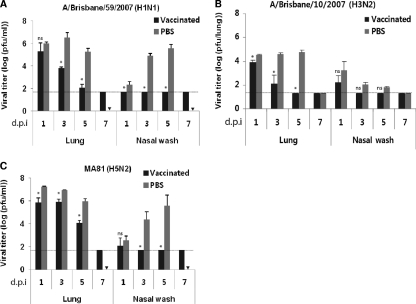
The cross-reactivity by CApH1N1 was further analyzed by challenging preimmunized mice with a lethal dose of wild-type virus. Mice were intranasally inoculated with two doses of 105 PFU of CApH1N1 or PBS administered 2 weeks apart. Two weeks after the second immunization, the mice were challenged with 5 times the mouse 50% lethal dose (5 MLD50) of A/Brisbane/59/2007 (H1N1) or A/Aquatic bird/Korea/W81/2005 (MA81) (H5N2); A/Brisbane/10/2007 (H3N2) was not lethal in mouse at the highest infection dose in a preliminary study. The challenges with A/Brisbane/59/2007 (H1N1) or A/Aquatic bird/Korea/W81/2005 (MA81) (H5N2) were lethal to the unvaccinated mice, causing rapid weight loss and finally death, whereas vaccinated mice developed only temporary weight loss of about 10% to 20% and all survived (Fig. 2). To assess the rapidity of viral clearance in the lungs and nasal washes, mice were intranasally inoculated with 105 PFU of CApH1N1 vaccine or PBS two times and were challenged 2 weeks later with 5 MLD50 of A/Brisbane/59/2007 (H1N1) or A/Aquatic bird/Korea/W81/2005 (MA81) (H5N2) or with 106 PFU of A/Brisbane/10/2007 (H3N2). Every 2 days after the challenge, mice were sacrificed by cervical dislocation, and samples from lungs and nasal washes were collected for residual viral titration by plaque assay. In the PBS groups, the titers of lung virus from all the challenged mice remained high up to day 5 after the challenge, while the nasal wash titers of A/Brisbane/59/2007 (H1N1) and A/Aquatic bird/Korea/W81/2005 (MA81) (H5N2) steadily increased by day 5, finally leaving no mouse alive at day 7. A/Brisbane/10/2007 (H3N2) also showed efficient replication in the lungs, albeit with relatively lower titers than the other two viruses and without notable clinical symptoms. On the other hand, in the vaccinated mouse groups, the titers of virus from lungs and nasal washes decreased rapidly, and complete viral clearance in the lungs of vaccinated mice was accomplished at day 5 or 7 after the challenge. Although, with respect to HA, pH1N1 is antigenically more closely related to MA81 (H5N2) (80% homology in amino acid sequence) than it is to A/Brisbane/10/2007 (H3N2) (57% homology), the clearance of MA81 in the vaccinated mice was less efficient than that of A/Brisbane/10/2007 (Fig. 3B and C). We assume that MA81, a mouse-adapted and highly virulent strain with an MLD50 of 103 PFU, is able to replicate more robustly upon infection than the much less virulent A/Brisbane/10/2007 strain, resulting in delayed viral clearance in the vaccinated mice. In the nasal washes, viruses were completely cleared by day 3 for all three challenge viruses, in contrast to the high-titer growth observed in those without vaccination. Taken together, these results suggest that the CApH1N1 live vaccine can induce heterosubtypic protection against not only the seasonal influenza virus but also antigenically distant H5N2 viruses.
Fig 2.
Cross-protection ability conferred by immunization with CApH1N1. Two doses of 105 PFU of CApH1N1 or PBS were inoculated intranasally into mice (n = 6 per group) 2 weeks apart. Two weeks after the second inoculation, mice were challenged with five times the mouse 50% lethal dose (5 MLD50) of A/Brisbane/59/2007 (H1N1) and A/Aquatic bird/Korea/W81/2005 (MA81) (H5N2). Daily weight changes (left) and survival rates (right) of mice challenged with A/Brisbane/59/2007 (H1N1) (A) or A/Aquatic bird/Korea/W81/2005 (MA81) (H5N2) (B) were monitored for 2 weeks after the challenge. Data represent the means of the results determined for each cohort (n = 6), and error bars indicate ±SD.
Fig 3.
Enhanced clearance of challenge virus in the mice preimmunized with CApH1N1. Two doses of 105 PFU of CApH1N1 or PBS were inoculated intranasally into mice (n = 3 per group) 2 weeks apart. Two weeks after the second inoculation, the mice were challenged with 5 MLD50 of A/Brisbane/59/2007 (H1N1) or A/Aquatic bird/Korea/W81/2005 (MA81) (H5N2) or with 106 PFU of A/Brisbane/10/2007 (H3N2). Every 2 days postchallenge, the mice from each group were sacrificed, and lung and nasal washes were collected for viral titration. Each viral titer was determined by a plaque assay using MDCK cells. Data represent the means of determined for each cohort (n = 4), and error bars indicate ±SD. The dashed horizontal lines indicate the detection limit, 1.6990. ▾, no mouse left alive at day 7 after the challenge in the PBS group. *, P < 0.05 is considered significant. ns, nonsignificant. d.p.i, days postinfection.
Here, we showed that the cold-adapted X-31 live attenuated pH1N1 vaccine (CApH1N1) induced cross-reactive immunity to seasonal and H5 influenza virus A strains. Previous studies have extensively examined the impact of prior infection with or vaccination against seasonal influenza viruses on protection against pH1N1 infection (1, 6, 7, 14, 36, 40). Our present study instead explored cross-reactive immunity to the cold-adapted pH1N1 LAIV and other influenza virus strains with a high likelihood of reassortment in nature, with a view to preventing or mitigating the potential risks posed by an emergence of reassortant virus among them rather than by pH1N1 itself. In this regard, interesting observations have recently been reported indicating that a heterosubtypic cross-reactive neutralizing antibody pool could be preferentially recruited by exploiting preexisting influenza-related immunological memory in humans by the use of inactivated pH1N1 vaccine (31) or prior infection with pH1N1 virus (43). However, the contribution of the neutralizing antibody elicited by the inactivated pH1N1 vaccine to protective efficacy has not been reported. It is now known that cross-reactive neutralizing antibodies from prior infection with pH1N1 recognize the stalk region rather than the globular domain of HA and that the passive transfer of the antibodies into naïve mice provides only partial protection against homologous laboratory H1N1 strains (43). Parallel studies with pH1N1 DNA vaccine failed to show cross-reactive neutralizing activity, even after four administrations of vaccination to immunologically naïve mice (31). Collectively, these findings provide new insights into the nature of preestablished cross-reactive immunity to influenza viruses, and yet their contribution to protection from heterologous infection needs to be further addressed. Similarly, the breadth of the cross-reactive immune responses afforded by cold-adapted pH1N1 LAIV has not yet been elucidated. Our present results, demonstrating very efficient heterologous protection from H5N2 even in the absence of cross-neutralizing antibodies, suggest that the nature of the protective immune response largely depends on the immunization route and the nature of vaccine formulation. It should be remembered that the cold-adapted LAIV is in the form of a reassortant virus carrying two surface genes derived from a wild-type virus strain in the genetic background of a preestablished cold-adapted donor strain. The potential contribution of internal genes (or proteins) to cross-protective immune responses and the differences from natural infection remain to be further explored. The results of a recently reported study have indicated that A/Ann Arbor/6/60 ca pH1N1 vaccine induced a significant level of cellular immune responses to seasonal H1N1 and H5N1 influenza viruses in mice (31), suggesting another possible mechanism for cross-reactivity of X-31 ca pH1N1 vaccine, in addition to the cross-reactive systemic and mucosal antibody responses observed in the present work.
Although neither neutralizing nor HI antibody was detectable under our experimental conditions, the cross-protective potential was sufficiently proven in an animal model. Our results agree with previous reports that the absence of a neutralizing antibody response was not necessarily associated with a lack of protective efficacy of live attenuated vaccines (2, 12, 23, 34). It should also be noted that prior infection or vaccination with the H1N1 strain has been shown to induce cross-protective immunity to avian H5N1 viruses (39, 43). Those previous reports support our present results with respect to the induction of cross-reactivity by cold-adapted LAIV. The most likely explanation for the protection seen in the absence of neutralizing activity is the induction of cross-reactive CD8+ T cells upon vaccination, which is one of the common features of the live attenuated vaccine (18, 19). In addition, there has been convincing evidence presented indicating that nonneutralizing antibodies are intimately associated with protection against heterosubtypic influenza viruses (5, 30) potentially related to the elimination of the influenza virus via FcR-mediated phagocytosis (21). In particular, the pH1N1 LAIV was previously reported to induce expression of the NA antibody that is cross-reactive against H5N1 influenza virus in a ferret model (9), implying that cross-reactive immunity is also feasible in models that employ higher animals. The cross-reactive nature of the HA (29, 42) and NA (9) of the pH1N1 virus in ferret or humans renders the pH1N1 LAIV an attractive candidate for development of cross-protective vaccine.
Remarkably, the strength of cross-reactivity mediated by mucosal IgA antibody, which was more pronounced than that induced by systemic IgG antibody, was comparable to that seen with the homologous pH1N1 virus. In addition, the cross-reactivity of the IgG antibody to H5 strains was almost equal to that seen with the seasonal H1N1, the same antigenic subtype of the vaccine strain. It is worth testing whether such robust cross-reactive systemic and mucosal antibody responses and the protection ability afforded by CApH1N1 in animal model could be further translated into definite benefits at the clinical level such as reducing the susceptibility and morbidity associated with potential reassortments between pH1N1 and other influenza virus strains, especially H5N1 viruses.
The heterosubtypic cross-protective immune response of the cold-adapted pH1N1 live influenza virus vaccine represents a practical and rational resource to meet the challenge of a future pandemic involving pH1N1 influenza viruses and merits further investigation in higher animals, including ferrets, to more closely model the vaccination to humans.
ACKNOWLEDGMENTS
This work was supported by the R&D Program of MKE/KEIT (10031969) and MEST (2010-0001932). This study was also supported in part by a grant from the Republic of Korea CDC (2009-E0066800) and a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A085105).
We thank Yong Ki Choi (Chungbuk University, South Korea) for providing us with the H5N2 A/Aquatic bird/Korea/W81/05 (MA81) virus.
Footnotes
Published ahead of print 21 March 2012
REFERENCES
- 1. Alam S, Sant AJ. 2011. Infection with seasonal influenza virus elicits CD4 T cells specific for genetically conserved epitopes that can be rapidly mobilized for protective immunity to pandemic H1N1 influenza virus. J. Virol. 85:13310–13321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belshe RB, et al. 2000. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J. Infect. Dis. 181:1133–1137 [DOI] [PubMed] [Google Scholar]
- 3. Benton KA, et al. 2001. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or γδ T cells. J. Immunol. 166:7437–7445 [DOI] [PubMed] [Google Scholar]
- 4. Berhane Y, et al. 2010. Molecular characterization of pandemic H1N1 influenza viruses isolated from turkeys and pathogenicity of a human pH1N1 isolate in turkeys. Avian Dis. 54:1275–1285 [DOI] [PubMed] [Google Scholar]
- 5. Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. 2008. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J. Immunol. 181:4168–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen GL, Lau Y-F, Lamirande EW, McCall AW, Subbarao K. 2011. Seasonal influenza infection and live vaccine prime for a response to the 2009 pandemic H1N1 vaccine. Proc. Natl. Acad. Sci. U. S. A. 108:1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen GL, et al. 2011. Comparison of a live attenuated 2009 H1N1 vaccine with seasonal influenza vaccines against 2009 pandemic H1N1 virus infection in mice and ferrets. J. Infect. Dis. 203:930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L-M, Davis CT, Zhou H, Cox NJ, Donis RO. 2008. Genetic compatibility and virulence of reassortants derived from contemporary avian H5N1 and human H3N2 influenza A viruses. PLoS Pathog. 4:e1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Z, Kim L, Subbarao K, Jin H. 2012. The 2009 pandemic H1N1 virus induces anti-neuraminidase (NA) antibodies that cross-react with the NA of H5N1 viruses in ferrets. Vaccine 30:2516–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Z, et al. 2010. Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J. Virol. 84:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corti D, et al. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856 [DOI] [PubMed] [Google Scholar]
- 12. Edwards KM, et al. 1994. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J. Infect. Dis. 169:68–76 [DOI] [PubMed] [Google Scholar]
- 13. Ekiert DC, et al. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang Y, et al. 2012. Seasonal H1N1 influenza virus infection induces cross-protective pandemic H1N1 virus immunity through a CD8-independent, B cell-dependent mechanism. J. Virol. 86:2229–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Girard MP, Katz JM, Pervikov Y, Hombach J, Tam JS. 2011. Report of the 7th meeting on evaluation of pandemic influenza vaccines in clinical trials, World Health Organization, Geneva, 17–18 February 2011. Vaccine 29:7579–7586 [DOI] [PubMed] [Google Scholar]
- 16. Gocník M, et al. 2008. Antibodies induced by the HA2 glycopolypeptide of influenza virus haemagglutinin improve recovery from influenza A virus infection. J. Gen. Virol. 89:958–967 [DOI] [PubMed] [Google Scholar]
- 17. Gocník M, et al. 2007. Antibodies specific to the HA2 glycopolypeptide of influenza A virus haemagglutinin with fusion-inhibition activity contribute to the protection of mice against lethal infection. J. Gen. Virol. 88:951–955 [DOI] [PubMed] [Google Scholar]
- 18. Gorse GJ, Belshe RB. 1991. Enhanced lymphoproliferation to influenza A virus following vaccination of older, chronically ill adults with live-attenuated viruses. Scand. J. Infect. Dis. 23:7–17 [DOI] [PubMed] [Google Scholar]
- 19. Gorse GJ, et al. 1995. Increased anti-influenza A virus cytotoxic T cell activity following vaccination of the chronically ill elderly with live attenuated or inactivated influenza virus vaccine. J. Infect. Dis. 172:1–10 [DOI] [PubMed] [Google Scholar]
- 20. Herfst S, et al. 2010. Introduction of virulence markers in PB2 of pandemic swine-origin influenza virus does not result in enhanced virulence or transmission. J. Virol. 84:3752–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. 2001. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 166:7381–7388 [DOI] [PubMed] [Google Scholar]
- 22. Ichinohe T, Iwasaki A, Hasegawa H. 2008. Innate sensors of influenza virus: clues to developing better intranasal vaccines. Expert Rev. Vaccines 7:1435–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joseph T, et al. 2008. A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 378:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krause JC, et al. 2011. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J. Virol. 85:10905–10908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee K-H, et al. 2006. Characterization of live influenza vaccine donor strain derived from cold-adaptation of X-31 virus. Vaccine 24:1966–1974 [DOI] [PubMed] [Google Scholar]
- 26. Octaviani CP, Li C, Noda T, Kawaoka Y. 2011. Reassortment between seasonal and swine-origin H1N1 influenza viruses generates viruses with enhanced growth capability in cell culture. Virus Res. 156:147–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Octaviani CP, Ozawa M, Yamada S, Goto H, Kawaoka Y. 2010. High level of genetic compatibility between swine-origin H1N1 and highly pathogenic avian H5N1 influenza viruses. J. Virol. 84:10918–10922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prabhu N, et al. 2009. Monoclonal antibodies against the fusion peptide of hemagglutinin protect mice from lethal influenza A virus H5N1 infection. J. Virol. 83:2553–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiu C, et al. 2012. Boosting heterosubtypic neutralization antibodies in recipients of 2009 pandemic H1N1 influenza vaccine. Clin. Infect. Dis. 54:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rangel-Moreno J, et al. 2008. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J. Immunol. 180:454–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi J, et al. 2012. Protective efficacy of an H1N1 cold-adapted live vaccine against the 2009 pandemic H1N1, seasonal H1N1, and H5N1 influenza viruses in mice. Antiviral Res. 93:346–353 [DOI] [PubMed] [Google Scholar]
- 32. Song M-S, et al. 2009. The polymerase acidic protein gene of influenza A virus contributes to pathogenicity in a mouse model. J. Virol. 83:12325–12335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Staneková Z, Vareckova E. 2010. Conserved epitopes of influenza A virus inducing protective immunity and their prospects for universal vaccine development. Virol. J. 7:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suguitan AL, Jr, et al. 2006. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 3:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sui J, et al. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun K, Ye J, Perez DR, Metzger DW. 2011. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J. Immunol. 186:987–993 [DOI] [PubMed] [Google Scholar]
- 37. Tamura SI, Tanimoto T, Kurata T. 2005. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn. J. Infect. Dis. 58:195–207 [PubMed] [Google Scholar]
- 38. Tan PT, Khan AM, August JT. 2011. Highly conserved influenza A sequences as T cell epitopes-based vaccine targets to address the viral variability. Hum. Vaccin. 7:402–409 [DOI] [PubMed] [Google Scholar]
- 39. Van Reeth K, et al. 2009. Prior infection with an H1N1 swine influenza virus partially protects pigs against a low pathogenic H5N1 avian influenza virus. Vaccine 27:6330–6339 [DOI] [PubMed] [Google Scholar]
- 40. Weinfurter JT, et al. 2011. Cross-reactive T cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLoS Pathog. 7:e1002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whittle JRR, et al. 2011. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc. Natl. Acad. Sci. U. S. A. 108:14216–14221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wrammert J, et al. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie H, et al. 2009. A live attenuated H1N1 M1 mutant provides broad cross-protection against influenza A viruses, including highly pathogenic A/Vietnam/1203/2004, in mice. J. Infect. Dis. 200:1874–1883 [DOI] [PubMed] [Google Scholar]
- 44. Yang P, et al. 2011. Immunogenicity and protective efficacy of a live attenuated vaccine against the 2009 pandemic A H1N1 in mice and ferrets. Vaccine 29:698–705 [DOI] [PubMed] [Google Scholar]