Abstract
The adenovirus early region 1A (E1A) protein promotes cell immortalization and transformation by mediating the activities of key cellular regulators. The repressor element 1-silencing transcription factor (REST), which is a major neuronal and tumor suppressor, was previously found mainly in the cytoplasm rather than in the nuclei of adenovirus-transformed rodent cells (22). We now demonstrate that the loss of REST in the nucleus is due to its rapid degradation by the ubiquitin-proteasome system. Only nuclear REST, but not its cytoplasmic counterpart, was ubiquitinated and degraded. REST degradation was blocked by the ubiquitination inhibitor PYR-41 and the proteasome inhibitor MG-132 but not by the nuclear export inhibitor leptomycin B. REST degradation required both of its two C-terminal degrons that are recognized by the ubiquitin ligase SCFβ-TrCP, since deletion or mutation of either degron eliminated degradation. Importantly, E1A was shown to mediate REST ubiquitination and degradation by upregulating β-TrCP. Knockdown of E1A in virus-transformed cells reduced both β-TrCP and ubiquitination of nuclear REST. In contrast, when expressed in HeLa cells, E1A enhanced the degradation of nuclear REST. Reconstitution of REST in virus-transformed cells negatively affected E1A-mediated cell proliferation and anchorage-independent growth. These data strongly indicate that E1A stimulates ubiquitination and proteolysis of REST in the nucleus, thereby abolishing the tumor suppressor functions of REST.
INTRODUCTION
The immediate early gene product E1A of human adenovirus is an oncoprotein capable of driving normally quiescent cells into the cell cycle. E1A itself is not a DNA-binding protein but a promiscuous host factor-binding protein. Therefore, E1A exerts its oncogenic function by interaction with cellular factors. It is well documented that E1A interacts with multiple key cellular regulators, including the tumor suppressor pRB, the transcriptional coactivator CBP/p300, and the transcriptional corepressor CtBP (38). Such interactions can lead to chromatin remodeling and cellular gene reprogramming (10). As a manifest consequence, cells become immortalized or transformed.
All 51 types of human adenovirus are capable of transforming rodent cells in culture, though only a subgroup, including adenovirus type 12 (Ad12), can further cause tumorigenesis in immunocompetent adult mice and rats (40–42, 54). We previously reported that the repressor element 1-silencing transcription factor (REST), which has recently been identified as a tumor suppressor in epithelial cells (23, 52, 53), was functionally defective in both tumorigenic Ad12-transformed and nontumorigenic Ad5-transformed rodent cells (22). REST was originally discovered as a master transcriptional repressor of neuronal genes and thus is also called the neuron-restrictive silencer factor. REST is rarely or not expressed in neuronal cells but is widely expressed in nonneuronal cells. REST recognizes and binds to cis-acting DNA sequences known as repressor element 1 (RE1), which is found in more than 1,000 cellular genes (9, 35). REST consists of two N- and C-terminal repressor domains, nine Cys2His2 zinc finger motifs (eight of which form the DNA-binding domain), and lysine- and proline-rich domains. The two repressor domains serve as scaffolds for the formation of distinct, large repressor complexes via recruitment of multiple corepressors such as Sin3, histone deacetylase, and CoREST (9, 35).
REST regulates many aspects of cellular functions. For example, it has been reported that REST plays an important role in the maintenance of embryonic stem cell self-renewal and pluripotency (47). In addition, REST deregulation and/or dysfunction has been associated with multiple human diseases, including Down's syndrome and Huntington's disease (9, 18, 35). Knockdown of REST in epithelial cells has been shown to cause cell transformation (53). In accordance with this, loss of REST function has been implicated in several human cancers, including breast, prostate, ovarian, colorectal, pancreatic, and small-cell lung cancers (1, 6, 9, 15, 19, 35, 51, 56). In breast cancer cells, REST has been found to repress the oncogenic TAC1 gene, as well as cell proliferation and migration (39). These findings indicate that REST plays a pivotal role in suppressing cancer formation and may represent a potential therapeutic target of some human cancers.
Our previous study revealed that REST was barely present in the nucleus but located mainly in the cytoplasm of nontumorigenic Ad5-transformed and tumorigenic Ad12-transformed rodent cells (22). This suggests that the loss of REST in the nuclei of these cells is likely related to viral transformation rather than tumorigenicity. In this study, we reveal for the first time that the nuclear absence of REST in adenovirus-transformed cells is due to its rapid degradation by the ubiquitin-proteasome system. E1A is responsible for promoting REST proteasomal degradation in the nucleus, which plays an essential role in E1A-mediated transformation.
MATERIALS AND METHODS
Cell lines.
Hooded Lister rat kidney cell lines transformed by Ad5 (DP5-2) and Ad12 (12-1) were previously described (26, 27). These and HeLa cells were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. NIH 3T3 cells were cultured in Eagle's minimal essential medium (BioWhittaker) supplemented with 10% FBS, 2 mM l-glutamine, and 50 μg/ml gentamicin sulfate.
Plasmids.
pCMV-Ad12E1A 13S was previously described (21). pEGFP-Ad12E1A 13S was constructed by subcloning E1A-12 13S cDNA into the HindIII/BamHI sites of the pEGFP-N1 vector (Clontech). This plasmid encodes the E1A-12 protein fused to enhanced green fluorescence protein (EGFP) at the C terminus. To construct pCMV-Myc-REST, REST cDNA was first generated by reverse transcription of mRNA from 12-1 rat cells, followed by PCR using primers 5′AGTGCGGCCGCTACAGTTATGGCCACCCAGGTGATG3′ and 5′AGTTCTAGATACTCTACTCCTGCTCCTCAGCTG3′ (REST sequence underlined). The PCR product was then cloned into the NotI and XbaI sites of the pCMV-Myc tag vector, which was generated by inserting a Myc tag sequence (5′ATGGAGCAGAAACTCATCTCTGAAGAGGATCTG3′) between the HindIII and NotI sites of the pRc/CMV vector (Invitrogen). The REST cDNA clone, which encodes 1,069 amino acids with an N-terminal Myc tag, was confirmed by sequencing. To construct a REST mutant with a deletion of the C-terminal 69 residues, the REST cDNA sequence encoding amino acids 1 to 1,000 was amplified and subcloned into the pCMV-Myc tag vector. pCMV-Myc-REST(S1002A) was constructed by replacing serine with alanine at position 1002 of REST using the QuikChange site-directed mutagenesis kit (Stratagene). pCMV-Myc-REST(E981A/S985A) was similarly constructed by replacing both the glutamic acid and serine residues at positions 981 and 985, respectively, with alanine. To create pSilencer-E1A12 plasmids for E1A knockdown, double-stranded cDNA sequences that encode E1A-12 small interfering RNAs (siRNAs) 5′GGGUACCUCGUUAAAAAAGUU (complementary to positions 73 to 93) and 5′CCGCCUGUUCAUUAUUAUCUU (complementary to positions 161 to 181) were cloned into the HindIII and BamHI sites of the pSilencer 4.1-CMV neo vector (Ambion).
Cell transfection and establishment of stable cell lines.
Cell transfection with plasmids was performed using TurboFect transfection reagent (Fermentas) based on the manufacturer's protocol. For β-TrCP knockdown, β-TrCP siRNA (sc-37179) and control siRNA (sc-37007), which were purchased from Santa Cruz Biotechnology, were transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. To establish stable NIH 3T3 cell lines expressing E1A-5 or E1A-12, NIH 3T3 cells were transfected with the pCMV-Ad5E1A or pCMV-Ad12E1A 13S plasmid and single-cell colonies resistant to Geneticin (Invitrogen) were selected. Expression of E1A-5 and E1A-12 was confirmed by Western blot analysis. As a control, stable NIH 3T3 cell lines transfected with empty plasmid pRc/CMV were also generated.
Cell proliferation and soft-agar growth assays.
12-1 cells were transfected with the pCMV-Myc-REST plasmid and then treated with 2.0 mg/ml Geneticin (Invitrogen) for 3 days. Drug-resistant cells were used for both cell proliferation and soft-agar growth assays. For the cell proliferation assay, 2 × 105 cells were seeded into six-well plates in triplicate. After culture for 48, 72, or 96 h, cells were trypsinized, resuspended in phosphate buffer, and counted. The soft-agar assay was carried out with 24-well plates. Briefly, 0.5 ml of 0.5% agar in DMEM supplemented with 10% FBS was plated as base agar. Two thousand Geneticin-resistant cells, which were resuspended in 0.35% agar dissolved in DMEM (10% FBS), were then added on the top of solidified base agar. Cells were cultured in 0.3 ml of DMEM (10% FBS) per well for 2 to 4 weeks, and the medium was replaced every 3 or 4 days. Anchorage-independent colonies were stained with 0.01% crystal violet and counted. Each assay was performed in quadruplicate.
Confocal immunofluorescence microscopy.
Confocal immunofluorescence microscopy was conducted as previously described (22). Briefly, for endogenous REST immunostaining, cells grown on glass coverslips were incubated first with mouse (ab52850; Abcam) or rabbit (H-290, sc-25398; Santa Cruz Biotechnology) anti-REST antibody and then with Alexa Fluor 488- or 594-conjugated anti-mouse or rabbit antibody (Invitrogen). For Myc-tagged REST staining, rabbit anti-Myc tag antibody 2272 (Cell Signaling Technology) was used. The cells were then mounted in ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) and visualized with a Nikon confocal microscope (Eclipse TE300; 60× objective lens).
IP and Western blot analyses.
Immunoprecipitation (IP) and Western blot analyses were carried out as described previously (20, 30). Rabbit anti-E1A-12 antibody was raised against purified E1A-12 13S protein. Rabbit anti-REST antibody (07-579) and mouse anti-ubiquitin monoclonal antibody (MAB1510) were purchased from Millipore. Rabbit anti-β-TrCP/HOS antibody (H-300, sc-15354) was obtained from Santa Cruz Biotechnology. Mouse β-actin (AC-15) monoclonal antibody was acquired from Sigma.
RESULTS
REST is rapidly degraded in the nucleus via ubiquitin-mediated proteolysis.
As a transcriptional repressor, REST is normally located in the nucleus. However, we previously reported that REST was essentially absent from the nuclei but substantially present in the cytoplasm of both nontumorigenic Ad5-transformed and tumorigenic Ad12-transformed rodent cells (22). To determine if REST could be made to accumulate in the nucleus by blocking nuclear export, rat kidney cells transformed by Ad5 (DP5-2) or Ad12 (12-1) were treated with the nuclear export inhibitor leptomycin B (LMB). As shown in Fig. 1A, treatment with LMB had little or no effect on nuclear REST accumulation, whereas significant increases in other proteins, including NF-κB, in the nucleus were observed (see Fig. S1 in the supplemental material). This inability of REST to accumulate in the nuclei when the nuclear export pathway is blocked suggested that REST could either be blocked from nuclear entry or rapidly degraded within the nucleus. To test this hypothesis, we first treated DP5-2 and 12-1 cells with the proteasome inhibitor MG-132. This treatment led to a dramatic increase in REST in the nuclei of both cell lines (Fig. 1A). It was noted that MG-132-treated cells became smaller and that cytoplasmic REST accumulated mainly in certain area instead of being evenly distributed in the cytoplasm (Fig. 1A). Treatment with PYR-41, an inhibitor of ubiquitin-activating enzyme E1, also resulted in great elevation of nuclear REST levels (Fig. 1A). These results indicate that REST is able to efficiently translocate into the nucleus and accumulate there when proteasomal degradation is inhibited. The nuclear translocation capability of REST was further demonstrated by transient transfection using its cDNA generated from 12-1 cells. This REST cDNA was fused to a Myc tag at the N terminus and reintroduced into the 12-1 cells (Fig. 1B) and DP5-2 cells (see Fig. S2 in the supplemental material). These data strongly suggest that the absence of nuclear REST in both Ad5- and Ad12-transformed cells is attributable to proteasomal degradation in the nucleus rather than a block in nuclear entry or enhanced nuclear export. Since both the 12-1 and DP5-2 cell lines exhibited very similar patterns of cellular REST localization, mainly 12-1 cells were used for the remainder of this study.
Fig 1.
REST is degraded by proteasomes in nuclei of adenovirus-transformed cells. (A) Enhancement of nuclear REST accumulation by proteasome inhibitor. DP5-2 and 12-1 cells were either left untreated (control) or treated for 12 h with the nuclear exporter inhibitor LMB (10 ng/ml), the proteasome inhibitor MG-132 (1 μM for DP5-2 and 5 μM for 12-1), or the ubiquitin-activating enzyme E1 inhibitor PYR-41 (5 μM). Confocal immunofluorescence microscopy was then carried out using an antibody against REST (green). Nuclei were stained with DAPI (blue). (B) Nuclear translocation of REST. N-terminally Myc-tagged REST was transiently transfected into 12-1 cells. At 24 h posttransfection, the cells were immunostained with an antibody against the Myc tag (red).
To further examine how proteosome inhibition affects REST distribution in cells, nuclear and cytoplasmic extracts of 12-1 cells were examined by Western blot analysis following treatment with MG-132. Since REST was barely present in the nucleus, as revealed by immunostaining (Fig. 1A), we used 2.5-fold more nuclear extract than cytoplasmic extract for Western blotting. As shown in Fig. 2A, a REST band of about 180 kDa was detected in the nuclei of both untreated and dimethyl sulfoxide-treated 12-1 cells (lanes 5 and 6). It is important to note that REST migrates more slowly (∼180 kDa) than its predicted molecular mass (∼120 kDa) due to posttranslational modifications that include glycosylation (8, 28, 32). In addition to the 180-kDa band, a very faint band at about 110 kDa, which corresponds to the molecular mass of unmodified REST, was marginally detected in the nuclear extracts (Fig. 2A, lanes 5 and 6). Inhibition of proteasomes by MG-132 resulted in increased levels and formation of multiple bands of nuclear REST (110 to 180 kDa) in a dose-dependent manner (Fig. 2A, lanes 7 and 8). Considering that proteasomal degradation requires the target protein to be ubiquitinated first (11, 45), these 110- to 180-kDa bands generated by MG-132 are likely polyubiquitinated forms of native REST. In contrast, cytoplasmic REST, which is more abundant and much lower in mass (about 90 kDa), was not affected by MG-132 (Fig. 2A, lanes 1 to 4). This cytoplasmic 90-kDa band was not found in the nucleus (Fig. 2A, lanes 5 to 8), which might represent an alternative splice isoform (17, 37) or partially degraded REST. These data, together with the immunostaining results shown in Fig. 1, suggest that only a small amount of posttranslationally modified REST (∼180 kDa) survives proteasomal degradation in the nucleus.
Fig 2.
REST is ubiquitinated in nuclei of adenovirus-transformed cells. (A) Enhancement of nuclear REST accumulation by inhibition of proteasomes. 12-1 cells were treated with the proteasome inhibitor MG-132 at the indicated concentrations for 12 h. Cytoplasmic extract (CE) and nuclear extract (NE) (8.0 μg and 20 μg, respectively) were analyzed by Western blotting with an antibody against REST. The same blot was reprobed with a β-actin antibody. (B) Ubiquitination of REST in the nucleus. Equal amounts of cytoplasmic and nuclear extracts from 12-1 cells were immunoprecipitated with control IgG or an anti-REST antibody and then Western blotted with an antibody against ubiquitin. n.s., nonspecific. (C) Inhibition of REST ubiquitination by PYR-41. 12-1 cells were treated with PYR-41, an inhibitor of the ubiquitin-activating E1 enzyme, at the indicated concentrations for 24 h. Nuclear extracts were then analyzed by IP-Western blotting as described for panel B. DMSO, dimethyl sulfoxide.
To examine if REST is polyubiquitinated in adenovirus-transformed cells, REST was immunoprecipitated from cytoplasmic and nuclear extracts of 12-1 cells, followed by Western blotting with a ubiquitin antibody. As shown in Fig. 2B, no ubiquitinated REST was immunoprecipitated from the cytoplasm (lanes 1 and 2). In strong contrast, a ladder of ubiquitin antibody-recognized bands ranging in size from 110 to 180 kDa was immunoprecipitated by REST antibody from the nucleus (lane 4) but not by control IgG (lane 3). This confirms that REST is polyubiquitinated in the nucleus. Interestingly, the 180-kDa REST band recognized by ubiquitin antibody is weaker than other lower-molecular-mass (110- to 180-kDa) bands (Fig. 2B, lane 4). This indicates that the higher-molecular-mass 180-kDa residual REST detected by regular Western blotting as described above is due mainly to glycosylation (8, 28, 32), as well as other possible posttranslational modifications.
We next examined if REST polyubiquitination in 12-1 cells can be inhibited by PYR-41, which is an inhibitor of ubiquitin-activating enzyme E1. Following PYR-41 treatment, nuclear extracts were subjected to IP-Western analysis as described above. As shown in Fig. 2C, PYR-41 treatment gave rise to a dose-dependent decrease in ubiquitinated REST. These data further demonstrate that REST is polyubiquitinated in the nucleus.
Proteasomal degradation of REST requires its C-terminal degrons.
It has recently been found that human REST contains two adjacent but distinct degrons near the C terminus. Both degrons, upon being phosphorylated by an unknown kinase(s), are recognized by the E3 ubiquitin ligase SCFβ-TrCP and can elicit REST degradation by proteasomes (23, 52). Amino acid sequence analysis revealed that REST derived from 12-1 rat cells also contains the two degron sequences (designated D1 and D2, Fig. 3A). To examine if REST proteasomal degradation in 12-1 cells requires the two degron sequences, a deletion mutant (ΔD2) form containing only the D1 degron sequence was constructed by deleting the C-terminal 69 amino acids that encode D2 (Fig. 3A). The ΔD2 mutant and wild-type (WT) REST proteins, both Myc tagged at their N termini, were expressed in 12-1 cells by transient transfection, followed by protein stability analysis in a cycloheximide (CHX) time course. As shown in Fig. 3B, WT REST was rapidly degraded in the nucleus, with a half-life of less than 2 h. In strong contrast, ΔD2 REST became very stable in the nucleus, with no apparent degradation even after 8 h following CHX treatment (Fig. 3B). It was noted that no Myc-tagged REST, either the WT or the D2 deletion mutant form, was found in the cytoplasm (data not shown), which is consistent with the data obtained by immunofluorescence microscopy (Fig. 1B). These data demonstrate that REST is degraded exclusively in the nucleus and its degradation requires at least the very C-terminal degron D2.
Fig 3.
The C-terminal degrons are required for REST proteasomal degradation in the nucleus. (A) Mutant forms of REST. Rat WT REST (1,069 amino acids, denoted by the gray bar at the top) contains two distinct C-terminal degron-like sequences (D1 and D2, indicated by asterisks), as originally identified in its human counterpart (23, 52). Represented at the bottom are the degron mutants that were generated: ΔD2, in which the C-terminal 69 residues harboring D2 are deleted; D1, a double point mutant (E981A/S985A) protein; and D2, a single point mutant (S1002A) protein. Amino acids conserved in the two degrons are in boldface. (B) Elimination of REST degradation in the nucleus by deletion of C-terminal D2. 12-1 cells were transfected with the control vector, N-terminally Myc-tagged WT REST, or D2 deletion mutant ΔD2 REST. At 40 h posttransfection, cells were treated with 20 μg/ml CHX to block protein synthesis. Cells were collected at different time points, and nuclear extracts were then prepared and used for Western blotting with antibodies against the Myc tag (for REST detection) and E1A-12. Nonspecific bands (n. s.) generated by the Myc tag antibody as a quantitative control are also shown. Quantification of relative REST levels is shown at the bottom. The vertical dotted line indicates the half-life of WT REST (∼1.8 h). (C) Requirement of both D1 and D2 for REST proteasomal degradation in the nucleus. 12-1 cells were transfected with Myc-tagged double point mutant (E981A/S985A) D1 or single point mutant (S1002A) D2 REST, treated with CHX, and subjected to Western blot analysis as described for panel B. The same blot was reprobed with a β-actin antibody.
To further investigate if disruption of either of the two degrons could abolish REST degradation in 12-1 cells, substitutions of conserved amino acid residues were introduced into D1 (E981A/S985A, Fig. 3A) or D2 (S1002A). The stability of these point mutants was then analyzed in a CHX time course as described above. As shown in Fig. 3C, neither of the point mutants was degraded in 12-1 cells. This conclusively demonstrated that both degrons are indispensable for REST proteasomal degradation.
E1A induces REST ubiquitination and proteasomal degradation in the nucleus by upregulating the ubiquitin ligase β-TrCP.
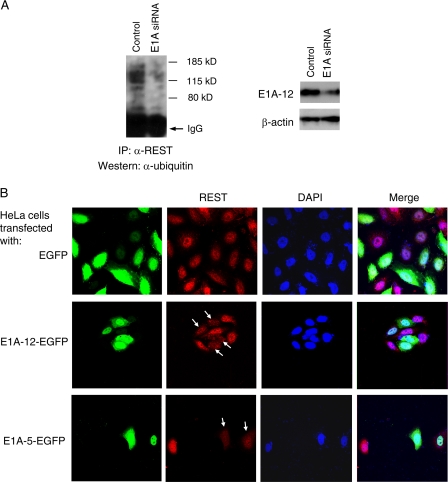
Given the opposing functions of E1A and REST in tumorigenesis, we hypothesized that E1A might be involved in promoting REST ubiquitination. To investigate the role of E1A in REST ubiquitination, we first knocked down E1A expression with siRNA in 12-1 cells. REST was immunoprecipitated from nuclear extracts and analyzed by Western blotting using an anti-ubiquitin antibody. As shown in Fig. 4A, knockdown of E1A-12 led to a reduction in ubiquitinated REST in the nucleus (left panel) compared with the level seen after mock treatment. This demonstrates that E1A-12 is really involved in inducing REST ubiquitination in the nucleus. It was expected that E1A-mediated ubiquitination of REST would result in degradation of the repressor. To confirm this notion, E1A-12 was expressed as an EGFP fusion protein in HeLa cells. As revealed by immunofluorescence microscopy, expression of the E1A-12–EGFP fusion resulted in a significant reduction in REST protein in the nucleus (Fig. 4B, middle panel), whereas the expression of EGFP alone had little or no effect on REST nuclear accumulation (upper panel). A similar result was obtained when E1A-5–EGFP was expressed in HeLa cells (Fig. 4B, lower panel). These findings clearly substantiate that E1A mediates the proteasomal degradation of REST, thus relieving its neuronal and tumor suppressor function.
Fig 4.
E1A promotes REST ubiquitination and proteasomal degradation in the nucleus. (A) Reduction of REST ubiquitination by E1A knockdown. 12-1 cells were transfected with empty vector DNA or a plasmid encoding E1A-12 siRNA. At 60 h posttransfection, nuclear extracts were used for IP with an anti-REST antibody. Immunoprecipitated pellets were then analyzed by Western blotting with an antibody against ubiquitin. Knockdown of E1A was confirmed by Western blotting using antibodies against E1A-12 and β-actin separately. (B) Reduction of REST nuclear accumulation by E1A. HeLa cells were transiently transfected with either the EGFP vector or a plasmid expressing an E1A-12–EGFP or an E1A-5–EGFP fusion protein. At 40 h posttransfection, cells were analyzed by confocal immunofluorescence microscopy to detect REST (red). Nuclei were stained with DAPI (blue). Arrows indicate E1A-transfected cells that exhibit decreased REST in their nuclei.
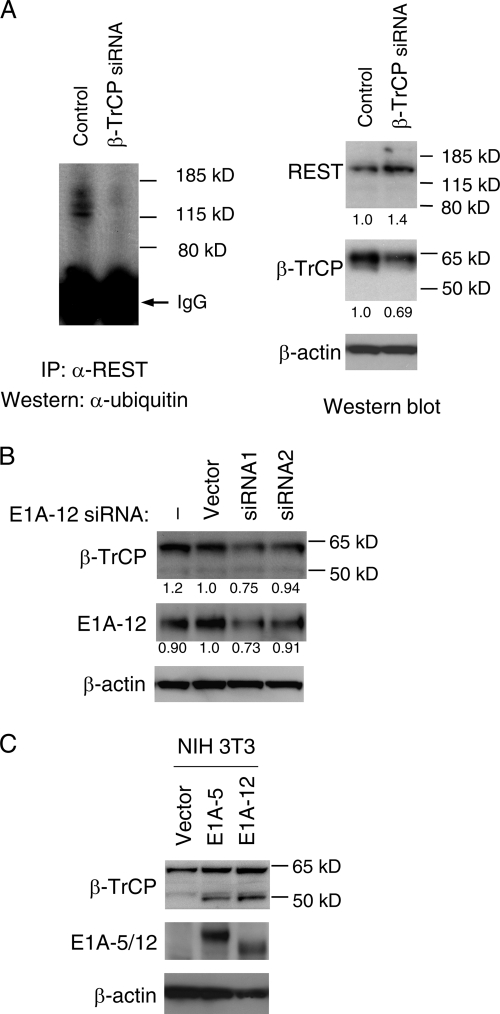
It has been demonstrated that the ubiquitin ligase β-TrCP is responsible for REST ubiquitination (23, 52). There are two forms of β-TrCP, i.e., β-TrCP1 (∼60 kDa) and β-TrCP2 (∼50 kDa), which differ mainly in their N-terminal regions and usually play a redundant role in regulating the proteasomal degradation of cellular proteins, including REST (14, 23, 52). To investigate if β-TrCP is required for REST ubiquitination and degradation in adenovirus-transformed cells, we conducted β-TrCP knockdown by siRNA in 12-1 cells. As shown in Fig. 5A, the 60-kDa form of β-TrCP1 protein was knocked down by β-TrCP siRNA (center panel on right). The knockdown of β-TrCP1 led to a severe reduction in ubiquitinated REST, as determined by IP-Western analysis (Fig. 5A, left panel). As a consequence, more REST was accumulated in the nuclei of β-TrCP knockdown cells (right upper panel). This confirms that β-TrCP is responsible for REST degradation in these cells.
Fig 5.
E1A upregulates β-TrCP, the ubiquitin ligase responsible for REST degradation. (A) Reduction of REST ubiquitination by β-TrCP knockdown. 12-1 cells were transfected with either control or β-TrCP siRNA. At 48 h posttransfection, REST was immunoprecipitated from nuclear extracts and analyzed by Western blotting using an antibody against ubiquitin. The same nuclear extracts were also analyzed by Western blotting using antibodies against REST, β-TrCP, and β-actin separately. Quantification of relative REST and β-TrCP levels (with levels obtained from control siRNA transfections arbitrarily set to 1.0) after normalization to β-actin is shown under each blot. (B) Decreased expression of β-TrCP incurred by E1A knockdown. 12-1 cells were either left untreated or transfected with empty vector DNA or plasmids encoding two distinct E1A-12 siRNAs. At 48 h posttransfection, cellular extracts were analyzed by Western blotting using antibodies against β-TrCP1/2, E1A-12, and β-actin separately. Quantification of relative β-TrCP and E1A-12 levels is shown under each blot. (C) Upregulation of β-TrCP by E1A. NIH 3T3 cells were stably transfected with control vector, E1A-5, or E1A-12 13S cDNA. Western blotting was conducted using a β-TrCP antibody that recognizes both β-TrCP1 (∼60 kDa) and β-TrCP2 (∼50 kDa). The same blot was reprobed with antibodies against E1A5, E1A-12, and β-actin.
We next examined if β-TrCP is upregulated by E1A. E1A knockdown in 12-1 cells led to a reduction in β-TrCP1 (Fig. 5B). A similar result was also obtained with an Ad12-transformed mouse cell line when E1A was knocked down (see Fig. S3 in the supplemental material). The inability to completely knock down E1A (Fig. 5B, middle) most likely accounts for the moderate reduction in β-TrCP1 (Fig. 5B, top). To further confirm that β-TrCP upregulation is linked to E1A expression, we examined β-TrCP levels in stably E1A-transfected NIH 3T3 cell lines. As shown in Fig. 5C, stable transfection of NIH 3T3 cells with E1A-5 or E1A-12 enhanced the expression of both β-TrCP1 (60 kDa) and, to a greater extent, β-TrCP2 (50 kDa). These data indicate that E1A promotes REST ubiquitination and degradation by upregulating the ubiquitin ligase β-TrCP.
E1A-mediated REST degradation contributes to viral transformation.
The above data suggest that the relief of REST tumor suppression is critical for E1A to stimulate cell proliferation and transformation. To confirm this notion, we examined the proliferation rate and anchorage-independent growth of 12-1 cells following transfection with Myc-tagged REST cDNA plasmids. As shown in Fig. 6A, expression of Myc-REST in 12-1 cells dramatically reduced the number of proliferated cells over a period of 96 h compared with transfection with an empty vector (Fig. 6A). It is important to note that the number of transfected cells in the first 24 h of the proliferation assay decreased (Fig. 6A), suggesting that in addition to negatively affecting cell proliferation, Myc-REST likely also caused the death of some of the transfected cells. The expression of Myc-REST in 12-1 cells prior to the proliferation assay was confirmed by Western blotting (Fig. 6C). At 96 h, the transfected cells expressed smaller amounts of REST, similar to the levels found in untransfected or empty-vector-transfected cells (data not shown), suggesting that only those cells lacking REST survived and proliferated. Furthermore, soft-agar colony assay also revealed that REST expression greatly inhibited the formation of anchorage-independent colonies by 12-1 cells (Fig. 6B), indicating that REST has a negative effect on E1A-driven, anchorage-independent growth (transformation). These data demonstrate for the first time that E1A mediates REST ubiquitination of degradation in the nucleus, which likely contributes to viral transformation.
Fig 6.

REST inhibits E1A-mediated cell proliferation and anchorage-independent growth. (A) Inhibition of cell proliferation by REST. 12-1 cells were transfected with the empty vector or a plasmid expressing N-terminally Myc-tagged REST (Myc-REST). Following transfection, 2 × 105 cells were seeded in triplicate and counted at the indicated times of culture. (B) Inhibition of anchorage-independent colony formation by REST. Two thousand cells from the experiment described in panel A were subjected to a soft-agar assay in quadruplicate. Anchorage-independent cell colonies were counted after culture for 4 weeks. Representative pictures of the cell colonies are shown at the bottom. (C) Western blot analysis of REST. Total lysates of transfected 12-1 cells as described in panel A were analyzed by Western blotting using antibodies against REST and β-actin separately.
DISCUSSION
E1A is an oncoprotein capable of immortalizing and transforming mammalian cells by mediating or interacting with key cellular factors. We previously discovered that the neuronal and tumor suppressor REST is dysfunctional in adenovirus-transformed rodent cells and that relief of REST repression activity is required for neuronal and tumor-related gene induction by E1A-12 (22). In the present study, we show for the first time that E1A-mediated cell transformation appears to require relief of REST repression function. We found that E1A is able to promote ubiquitination and proteasomal degradation of REST in the nuclei of both Ad5- and Ad12-transformed cells by upregulating the ubiquitin ligase SCFβ-TrCP. Degradation of REST requires the two adjacent C-terminal degrons that are recognized by β-TrCP.
REST is well documented as a master regulator of neuronal development due to its overwhelming role in repressing a large number of neuronal differentiation genes. An oncogenic role for REST in neuronal cells has been suggested since its persistent expression, which dedifferentiates neuronal cells, often correlates with neuronal oncogenesis (9, 18, 34). In strong contrast, REST has recently been identified as a tumor suppressor in nonneuronal cells (52, 53). Our previous finding also revealed that loss of REST repression activity is required for the induction of neuronal and tumor-related genes in tumorigenic Ad12-transformed cells (22). Consistent with its tumor suppressor function, this study substantiates that by introducing REST into cells, exogenous REST is capable of inhibiting E1A-mediated oncogenic transformation. Knockdown of REST in epithelial cells has been shown to cause cell transformation, probably via release of its repression on phosphoinositide 3-kinase-dependent signaling (53). Loss of REST in nonneuronal cells could also cause dedifferentiation by perturbing cell specificity/identity through derepression of neuronal genes.
REST is a frequent target for mutation in nonneuronal cancers, and loss of its function with concomitant neuronal gene expression has been implicated in several human tumors, such as breast, prostate, ovarian, colorectal, and pancreatic cancers (9, 18, 35). It is important to note that degradation of REST by the ubiquitin-proteasome system is required for neuronal differentiation, as well as for oncogenic transformation of epithelial cells (52). In both cases, REST degradation is controlled by the F-box protein β-TrCP, which recognizes two distinct degrons near the C terminus of REST following their phosphorylation by an unknown kinase(s) (23, 52). While these two degrons could function independently, our data indicate that neither one is sufficient to trigger proteasomal degradation of REST in 12-1 cells. This suggests that these two degrons function together to more efficiently elicit REST degradation. In this regard, E1A could act as a mediator to stimulate β-TrCP binding to the two degrons, thus effectively promoting REST ubiquitination and degradation.
The ubiquitin-proteasome system plays a critical role in controlling many cellular activities, and deregulation of this system has been implicated in multiple diseases, including cancer (13). In addition to REST, many other key transcription factors, such as p53, Smad2, MyoD, Stat5A, and Myc, are regulated by the ubiquitin-proteasome system (7, 12, 33, 46, 49). Proteasomal degradation of these factors can occur in the cytoplasm and/or the nucleus, where the ubiquitin-proteasome system exists (2, 44). For example, p53 is ubiquitinated in the nucleus but is shuttled to the cytoplasm by its ubiquitin ligase HDM2 for degradation (4, 43). In contrast, Smad2, MyoD, and Stat5A, as found with REST in this study, are ubiquitinated and degraded in the nucleus (7, 12, 33). Other proteins, such as the serine/threonine kinase AKT and the mitogen-activated protein kinase scaffold protein Ste5, which are involved in cell signaling, were also found to be degraded in the nucleus (16, 48). Since these cellular regulators, especially the transcription factors, normally function in the nucleus, degradation by the ubiquitin-proteasome system without the need for nuclear export could render quick control of their activities and cellular processes. In view of the fact that REST is a potent tumor suppressor that can directly regulate more than 1,000 cellular genes, its rapid degradation induced by E1A in the nucleus could enable the viral oncoprotein to easily reprogram the transcription of REST target genes.
E1A-mediated degradation of REST in the nucleus by the ubiquitin-proteasome system reveals for the first time another major pathway by which this viral oncoprotein hijacks cellular mechanisms for tumorigenesis. It is well known that viral oncoproteins manipulate the ubiquitin-proteasome pathway to promote the degradation of tumor suppressors and/or the stability of cellular oncoproteins. For example, the adenovirus E1B and human papillomavirus E6 oncoproteins are engaged in inducing p53 degradation by assembling the E3 ubiquitin ligases, whereas E1A and simian virus 40 large T antigen are able to increase Myc oncoprotein stability by inhibiting the F-box protein and tumor suppressor SCFFbw7 (3, 25). It has been reported that E1A is capable of interacting with enzymes involved in ubiquitination and the 26S proteasome (24, 25, 31, 36, 50). It is intriguing to speculate that such an interaction between E1A and β-TrCP and/or the 26S proteasome could enhance REST degradation. In view of the fact that E1A upregulates β-TrCP, as demonstrated in this study, E1A could possibly mediate the upregulation of nuclear proteasomes and/or the kinase activity responsible for phosphorylating the REST degrons. It has been found that REST can form a complex with telomere repeat factor 2 (TRF2) in promyelocytic leukemia (PML) nuclear bodies, and dissociation of the complex causes proteasomal degradation of REST (55). E1A is capable of disrupting PML nuclear bodies (5), which may, in turn, lead to dissociation of the REST-TRF2 complex and enhancement of REST degradation. Further study is needed to resolve these issues.
In contrast to negatively regulating transcription by binding to canonical and noncanonical RE1 sites on its target genes in the nucleus, REST, when located in the cytoplasm, can positively regulate the translation of its target genes. Cytoplasmic REST has been found to upregulate the cap-dependent translation of RE1-containg mRNA by enhancing the phosphorylation of the translation initiation factor eIF4G (29). Our data revealed that compared with the readily degradable REST in the nuclei of adenovirus-transformed cells, there exists a smaller form of REST that is prevalent and stable in the cytoplasm. It is possible that E1A uses two different strategies to mediate the expression of REST target genes: first by derepressing the transcription of these genes and second by enhancing their translation. This could maximize the effect of E1A in upregulating REST target genes for cell transformation.
In summary, E1A promotes ubiquitination and proteasomal degradation of the neuronal and tumor suppressor REST in the nucleus by upregulating the ubiquitin ligase β-TrCP, which likely plays an important role in E1A-mediated cell transformation. Degradation of REST by the ubiquitin-proteasome system requires both of the C-terminal degrons that are recognized by β-TrCP. E1A-mediated proteasomal degradation of REST provides an effective means by which the viral oncoprotein counteracts this tumor suppressor, eliminating another barrier to the progression of cancer.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by grant CA29797 from the National Cancer Institute to R.P.R.
Footnotes
Published ahead of print 14 March 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Albert ML, Darnell RB. 2004. Paraneoplastic neurological degenerations: keys to tumour immunity. Nat. Rev. Cancer 4:36–44 [DOI] [PubMed] [Google Scholar]
- 2. Bader N, Jung T, Grune T. 2007. The proteasome and its role in nuclear protein maintenance. Exp. Gerontol. 42:864–870 [DOI] [PubMed] [Google Scholar]
- 3. Blanchette P, Branton PE. 2009. Manipulation of the ubiquitin-proteasome pathway by small DNA tumor viruses. Virology 384:317–323 [DOI] [PubMed] [Google Scholar]
- 4. Boyd SD, Tsai KY, Jacks T. 2000. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat. Cell Biol. 2:563–568 [DOI] [PubMed] [Google Scholar]
- 5. Carvalho T, et al. 1995. Targeting of adenovirus E1A and E4-ORF3 proteins to nuclear matrix-associated PML bodies. J. Cell Biol. 131:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen W, Böcker W, Brosius J, Tiedge H. 1997. Expression of neural BC200 RNA in human tumours. J. Pathol. 183:345–351 [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Dai X, Haas AL, Wen R, Wang D. 2006. Proteasome-dependent down-regulation of activated Stat5A in the nucleus. Blood 108:566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chong JA, et al. 1995. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 80:949–957 [DOI] [PubMed] [Google Scholar]
- 9. Coulson JM. 2005. Transcriptional regulation: cancer, neurons and the REST. Curr. Biol. 15:R665–R668 [DOI] [PubMed] [Google Scholar]
- 10. Ferrari R, et al. 2008. Epigenetic reprogramming by adenovirus e1a. Science 321:1086–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finley D. 2009. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78:477–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Floyd ZE, Trausch-Azar JS, Reinstein E, Ciechanover A, Schwartz AL. 2001. The nuclear ubiquitin-proteasome system degrades MyoD. J. Biol. Chem. 276:22468–22475 [DOI] [PubMed] [Google Scholar]
- 13. Frescas D, Pagano M. 2008. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat. Rev. Cancer 8:438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuchs SY, Spiegelman VS, Kumar KG. 2004. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene 23:2028–2036 [DOI] [PubMed] [Google Scholar]
- 15. Garber ME, et al. 2001. Diversity of gene expression in adenocarcinoma of the lung. Proc. Natl. Acad. Sci. U. S. A. 98:13784–13789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garrenton LS, et al. 2009. Nucleus-specific and cell cycle-regulated degradation of mitogen-activated protein kinase scaffold protein Ste5 contributes to the control of signaling competence. Mol. Cell. Biol. 29:582–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gillies SG, et al. 2011. Distinct gene expression profiles directed by the isoforms of the transcription factor neuron-restrictive silencer factor in human SK-N-AS neuroblastoma cells. J. Mol. Neurosci. 44:77–90 [DOI] [PubMed] [Google Scholar]
- 18. Gopalakrishnan V. 2009. REST and the RESTless: in stem cells and beyond. Future Neurol. 4:317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gröne J, et al. 2006. Robo1/Robo4: differential expression of angiogenic markers in colorectal cancer. Oncol. Rep. 15:1437–1443 [PubMed] [Google Scholar]
- 20. Guan H, Hou S, Ricciardi RP. 2005. DNA binding of repressor nuclear factor-kappaB p50/p50 depends on phosphorylation of Ser337 by the protein kinase A catalytic subunit. J. Biol. Chem. 280:9957–9962 [DOI] [PubMed] [Google Scholar]
- 21. Guan H, Jiao J, Ricciardi RP. 2008. Tumorigenic adenovirus type 12 E1A inhibits phosphorylation of NF-kappaB by PKAc, causing loss of DNA binding and transactivation. J. Virol. 82:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guan H, Williams JF, Ricciardi RP. 2009. Induction of neuronal and tumor-related genes by adenovirus type 12 E1A. J. Virol. 83:651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guardavaccaro D, et al. 2008. Control of chromosome stability by the beta-TrCP-REST-Mad2 axis. Nature 452:365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hateboer G, et al. 1996. mUBC9, a novel adenovirus E1A-interacting protein that complements a yeast cell cycle defect. J. Biol. Chem. 271:25906–25911 [DOI] [PubMed] [Google Scholar]
- 25. Isobe T, et al. 2009. Adenovirus E1A inhibits SCF(Fbw7) ubiquitin ligase. J. Biol. Chem. 284:27766–27779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jelinek T, Graham FL. 1992. Recombinant human adenoviruses containing hybrid adenovirus type 5 (Ad5)/Ad12 E1A genes: characterization of hybrid E1A proteins and analysis of transforming activity and host range. J. Virol. 66:4117–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jelinek T, Pereira DS, Graham FL. 1994. Tumorigenicity of adenovirus-transformed rodent cells is influenced by at least two regions of adenovirus type 12 early region 1A. J. Virol. 68:888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kallunki P, Edelman GM, Jones FS. 1997. Tissue-specific expression of the L1 cell adhesion molecule is modulated by the neural restrictive silencer element. J. Cell Biol. 138:1343–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim CS, et al. 2008. Novel function of neuron-restrictive silencer factor (NRSF) for posttranscriptional regulation. Biochim. Biophys. Acta 1783:1835–1846 [DOI] [PubMed] [Google Scholar]
- 30. Kushner DB, Ricciardi RP. 1999. Reduced phosphorylation of p50 is responsible for diminished NF-kappaB binding to the major histocompatibility complex class I enhancer in adenovirus type 12-transformed cells. Mol. Cell. Biol. 19:2169–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lafarga M, et al. 2002. Clastosome: a subtype of nuclear body enriched in 19S and 20S proteasomes, ubiquitin, and protein substrates of proteasome. Mol. Biol. Cell 13:2771–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee JH, Shimojo M, Chai YG, Hersh LB. 2000. Studies on the interaction of REST4 with the cholinergic repressor element-1/neuron restrictive silencer element. Brain Res. Mol. Brain Res. 80:88–98 [DOI] [PubMed] [Google Scholar]
- 33. Lo RS, Massague J. 1999. Ubiquitin-dependent degradation of TGF-beta-activated smad2. Nat. Cell Biol. 1:472–478 [DOI] [PubMed] [Google Scholar]
- 34. Lunyak VV, Rosenfeld MG. 2005. No rest for REST: REST/NRSF regulation of neurogenesis. Cell 121:499–501 [DOI] [PubMed] [Google Scholar]
- 35. Majumder S. 2006. REST in good times and bad: roles in tumor suppressor and oncogenic activities. Cell Cycle 5:1929–1935 [DOI] [PubMed] [Google Scholar]
- 36. Nakajima T, et al. 1997. Induction of ubiquitin conjugating enzyme activity for degradation of topoisomerase II alpha during adenovirus E1A-induced apoptosis. Biochem. Biophys. Res. Commun. 239:823–829 [DOI] [PubMed] [Google Scholar]
- 37. Palm K, Belluardo N, Metsis M, Timmusk T. 1998. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J. Neurosci. 18:1280–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pelka P, Ablack JN, Fonseca GJ, Yousef AF, Mymryk JS. 2008. Intrinsic structural disorder in adenovirus E1A: a viral molecular hub linking multiple diverse processes. J. Virol. 82:7252–7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reddy BY, Greco SJ, Patel PS, Trzaska KA, Rameshwar P. 2009. RE-1-silencing transcription factor shows tumor-suppressor functions and negatively regulates the oncogenic TAC1 in breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 106:4408–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ricciardi R. 1995. Transformation and tumorigenesis mediated by the adenovirus E1A and E1B oncogenes, p 195–225 In Barbanti-Brodano G, Bendinelli M, Friedman H. (ed), DNA tumor viruses: oncogenic mechanisms. Plenum Press, New York, NY [Google Scholar]
- 41. Ricciardi RP. 1999. Adenovirus transformation and tumorigenicity, p 217–227 In Seth P. (ed), Adenoviruses: from basic research to gene therapy applications. Landes Biosciences/Springer, New York, NY [Google Scholar]
- 42. Ricciardi RP, Zhao B, Guan H. 2006. Mechanism of tumorigenesis mediated by adenovirus-12 E1A, p 107–124 In Tognon M. (ed), Viral oncogenesis. Research Signpost, Kerala, India [Google Scholar]
- 43. Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ. 1998. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 17:554–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scharf A, Rockel TD, von Mikecz A. 2007. Localization of proteasomes and proteasomal proteolysis in the mammalian interphase cell nucleus by systematic application of immunocytochemistry. Histochem. Cell Biol. 127:591–601 [DOI] [PubMed] [Google Scholar]
- 45. Schrader EK, Harstad KG, Matouschek A. 2009. Targeting proteins for degradation. Nat. Chem. Biol. 5:815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sears RC. 2004. The life cycle of C-myc: from synthesis to degradation. Cell Cycle 3:1133–1137 [PubMed] [Google Scholar]
- 47. Singh SK, Kagalwala MN, Parker-Thornburg J, Adams H, Majumder S. 2008. REST maintains self-renewal and pluripotency of embryonic stem cells. Nature 453:223–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suizu F, et al. 2009. The E3 ligase TTC3 facilitates ubiquitination and degradation of phosphorylated Akt. Dev. Cell 17:800–810 [DOI] [PubMed] [Google Scholar]
- 49. Thompson SJ, Loftus LT, Ashley MD, Meller R. 2008. Ubiquitin-proteasome system as a modulator of cell fate. Curr. Opin. Pharmacol. 8:90–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turnell AS, et al. 2000. Regulation of the 26S proteasome by adenovirus E1A. EMBO J. 19:4759–4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wagoner MP, et al. 2010. The transcription factor REST is lost in aggressive breast cancer. PLoS Genet. 6:e1000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Westbrook TF, et al. 2008. SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature 452:370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Westbrook TF, et al. 2005. A genetic screen for candidate tumor suppressors identifies REST. Cell 121:837–848 [DOI] [PubMed] [Google Scholar]
- 54. Williams JF, et al. 2004. E1A-based determinants of oncogenicity in human adenovirus groups A and C. Curr. Top. Microbiol. Immunol. 273:245–288 [DOI] [PubMed] [Google Scholar]
- 55. Zhang P, et al. 2008. Nontelomeric TRF2-REST interaction modulates neuronal gene silencing and fate of tumor and stem cells. Curr. Biol. 18:1489–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Y, Dang C, Ma Q, Shimahara Y. 2005. Expression of nerve growth factor receptors and their prognostic value in human pancreatic cancer. Oncol. Rep. 14:161–171 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.