Abstract
The myxoma virus (MYXV) carries three tandem C7L-like host range genes (M062R, M063R, and M064R). However, despite the fact that the sequences of these three genes are similar, they possess very distinctive functions in vivo. The role of M064 in MYXV pathogenesis was investigated and compared to the roles of M062 and M063. We report that M064 is a virulence factor that contributes to MYXV pathogenesis but lacks the host range properties associated with M062 and M063.
TEXT
The poxvirus family is comprised of a group of large DNA viruses encoding a wealth of viral factors to combat host defense mechanisms (5, 6). The poxvirus C7L family members, recognized as host range factors, are broadly distributed among the majority of sequenced mammalian poxviruses except molluscum contagiosum virus and parapoxviruses. In vaccinia virus (VACV), the C7 host range factor has been shown to regulate host cellular tropism and antagonize effectors of type 1 interferon (IFN) (10). Interestingly, within the myxoma virus (MYXV) genome, three C7L-like genes (M062R, M0623R, and M064R) are located in a tandem cluster in the conserved central region of the genome (4). Of these three genes, M062 has been shown to be the sole functional homolog of VACV C7 in the context of a recombinant VACV construct (9), while M063 is a host range factor critical for viral replication in rabbits. In addition, M063 binds to M062 to form a complex that antagonizes the cellular antiviral factor SAMD9 (8). It has been previously proposed that M064 may also be a host range factor due to its high similarity to C7 (4). Therefore, in this study, we created a targeted M064 knockout virus to investigate the roles of M064 in MYXV infection, host range function, and pathogenesis.
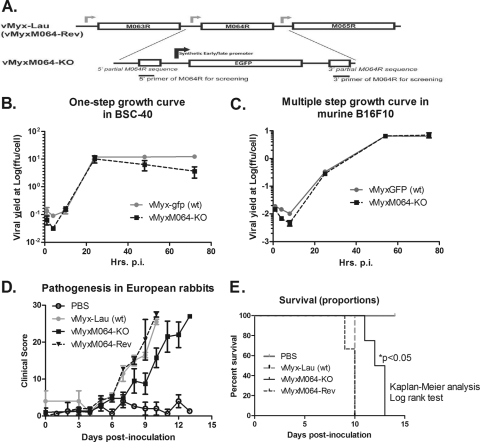
To study the role of M064 during viral infection, a recombinant MYXV with the M064R gene knocked out (vMyxM064-KO) was constructed for in vitro and in vivo analyses (Fig. 1A). Except for the 5′ 83 bp and the 3′ 86 bp of M064R, the central region of the gene (about 82%) was deleted and replaced by an enhanced green fluorescent protein (EGFP) expression cassette driven by a poxvirus early/late synthetic promoter (8). The recombination and purification procedures were conducted as previously described (7, 8). First, the growth of the knockout virus was evaluated in vitro by one-step or multiple-step growth curves, as previously reported (7, 8), in various different indicator cell lines and compared with that of the wild-type (wt) MYXV (vMyxGFP). In many of these cell lines, both the M063 and M062 knockout viruses manifested dramatic and severe replication defects (2, 8). Surprisingly, vMyxM064-KO replication was similar to that of wt MYXV not only in rabbit cells, such as RK-13 (ATCC CCL-37) (Fig. 1B), but also in cells from other species including humans (not shown), mice (e.g., B16F10 ATCC CRL-6475) (Fig. 1C), and nonhuman primates (not shown). These results suggested that M064 does not contribute to MYXV host range functions.
Fig 1.
Knocking out M064R from the MYXV genome (vMyxM064R-KO) does not alter the host range tropism of the virus, but it does delay the pathogenic phenotype in vivo. (A) Construct of M064R knockout MYXV. The central region of M064R was replaced by a GFP expression cassette driven by a poxvirus synthetic early/late promoter. A revertant virus was constructed by putting back the intact sequence of M064R. (B) M064R knockout virus replicates very similarly to the wild-type virus in rabbit cells. RK-13 cells were infected at an MOI of 0.1, and cell lysates were harvested at given time points (15, 41, and 67 hours p.i.). Titration was conducted to estimate viral yields. (C) M064R knockout virus replicates similarly to the wild-type virus in murine B16F10 cells. Infection was conducted at an MOI of 0.5, and at given time points (1, 4, 8, 25, 54, and 75 h p.i.) the cell lysates were harvested for titration to estimate the viral yield. (D) M064R knockout virus showed a delayed development of myxomatosis in European rabbits. Four groups of animals were intradermally inoculated with vehicle (PBS only), 1,000 FFU of vMyx-Lau (wt), vMyxM064-KO, or vMyxM064-Rev (revertant virus). Pathogenesis was evaluated daily for each animal using a clinical score system, and the average score of each group is shown in the graph. The error bars represent the standard error of the mean (SEM). (E) M064R knockout virus infection led to a significantly delayed pathogenesis. From the same set of experiments as those shown in panel D, the survival of animals from each group was evaluated. Statistical analysis was conducted using Kaplan-Meier analysis followed by a log rank test, and a P value of <0.05 is defined as statistically significant.
Next, the role of M064 in the pathogenesis of MYXV infection in vivo was evaluated. The animal study was approved by the Institutional Animal Care and Usage Committee (IACUC) at the University of Florida. Four groups of European rabbits that had been treated with vehicle (phosphate-buffered saline [PBS] only) (n = 2), 1,000 focus-forming units (FFU) of wt MYXV Lausanne strain (vMyx-Lau) (n = 2), vMyxM064-KO (n = 4), and the revertant virus (vMyxM064-Rev) (n = 3) were inoculated intradermally with the corresponding reagent in a 100-μl volume of vehicle. A clinical scoring system that has been described in detail previously (7, 8) was used for daily evaluation of the progression of myxomatosis in these animals. The average daily score from each group was plotted and used as a measure of disease progression. As shown in Fig. 1C, a delayed development of myxomatosis was observed in the vMyxM064-KO group, while wt and revertant virus groups were indistinguishable. None of the animals infected with vMyxM064-KO survived the infection. However, a statistical significance in the delay of disease progression and endpoint was observed, suggesting the importance of M064 in the kinetics of infection in vivo. Histological analysis of organs (spleen, Peyer's patches, and primary and secondary lesions, etc.) at the endpoint showed no differences between wt virus or vMyxM064-KO-infected rabbits. This is the first report of a delay in disease progression following infection with a poxvirus knockout lacking a C7-like gene that is not accompanied by a detectable host range function.
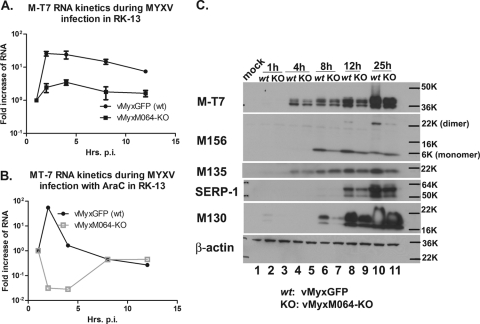
We further investigated the role of M064 in viral gene expression and hypothesized that M064 might regulate an early event in viral infection. First, viral RNA transcription was evaluated during wt or vMyxM064-KO infection in rabbit cells (e.g., RK-13). Briefly, a multiplicity of infection (MOI) of 5 was used to infect cells, and the cell lysates were harvested with TRIzol (Invitrogen) for RNA extraction (8) at a series of time points postinfection (p.i.), such as 1, 2, 4, 8, and 12 h. After DNase treatment (Ambion), total RNA was reverse transcribed followed by Sybr green real-time PCR amplification as described previously (8). The rabbit 18S rRNA (8) was used as an internal control, and the comparative cycle threshold (CT) method was used for comparison of viral gene expression levels. We observed a significantly lower viral early/late RNA expression, such as M-T7 (Fig. 2A) and M062R (not shown), in vMyxM064-KO-infected cells than in wt MyxGFP-infected cells. When cytosine arabinoside (AraC) was introduced before and during infection in the same setting as described above, a significant defect in RNA transcription at early time points was observed during the M064 knockout virus infection (Fig. 2B). This reduction in early gene expression observed in M064 knockout virus-infected cells is also consistent with lower protein levels of MYXV early/late gene products, e.g., M-T7, M135, and M156 compared to wt virus-infected cells (Fig. 2C). In addition, the expression of MYXV late genes (e.g., SERP-1 and M130) was also reduced significantly (Fig. 2C) by the absence of M064. Therefore, we conclude that M064 facilitates the efficient expression of viral genes during infection in cultured cells.
Fig 2.
Knocking out M064R from the MYXV genome leads to a delayed expression of viral gene products during infection of rabbit cells. (A) M064R knockout virus (vMyxM064-KO) infection in rabbit cells leads to a reduced RNA transcription for viral early/late genes. RK-13 cells were infected with either wild-type MYXV (vMyxGFP) or vMyxM064-KO at an MOI of 5. At given time points (1, 2, 4, 8, and 12 h p.i.), cell lysates were harvested for RNA extraction and DNase treatment, followed by reverse transcription and then Sybr green real-time PCR to detect the transcription of an early/late gene, M-T7. The comparative CT method was used to calculate and compare the relative level of RNA. The results of the two independent experiments combined are shown. (B) The delay of vMyxM064-KO infection is at the early stage of viral gene expression. RK-13 cells were treated with AraC before and during the infection with either vMyxGFP or vMyxM064-KO. At given time points (1, 2, 4, 8, and 12 h p.i.), cell lysates were harvested and processed as described above for panel A. The results shown are representative of the results of two independent experiments. (C) The delay of viral gene expression by infection of vMyxM064-KO can be detected at the protein level. RK-13 cells were infected by either vMyxGFP (wt) or vMyxM064-KO at an MOI of 10. At given time points (1, 4, 8, 12, and 25 h p.i.), cell lysates were harvested for Western blotting. Early/late gene expression (M-T7, M156, and M135) and late gene expression (SERP-1 and M130) were compared between wt and vMyxM064-KO. The results shown are representative of the results of two independent experiments. The positions of molecular size markers in daltons are shown to the right of the gels (e.g., 50K, 50,000).
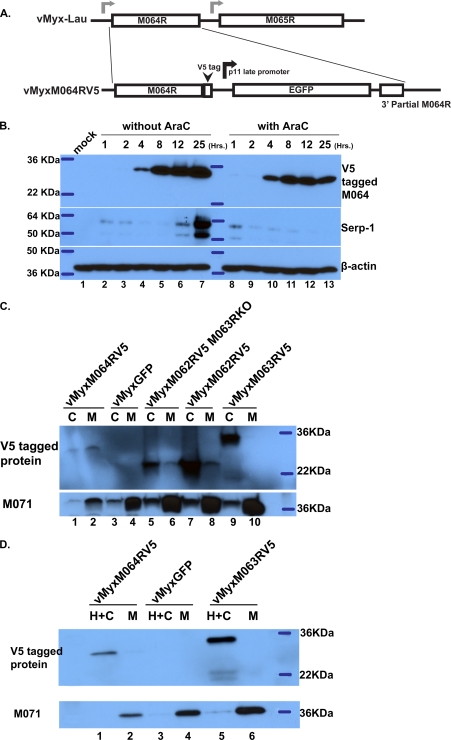
To confirm the expression kinetics of M064 in coordination with its role during infection, a recombinant MYXV with V5-tagged M064 (vMyxM064RV5) was constructed (Fig. 3A). The sequence of the V5 and downstream EGFP expression cassette has been reported elsewhere (8). A time course study was conducted by infecting RK-13 cells at an MOI of 8 with vMyxM064RV5 in the absence or presence of AraC. Cell lysates were harvested at different time points p.i. (1, 4, 8, 12, and 25 h) and analyzed by Western blotting. The anti-V5 antibody (Invitrogen) was used to probe for V5-tagged M064 expression. An anti-SERP-1 antibody was used to show that AraC treatment abolished late gene expression (Fig. 3B). M064 was expressed as an early/late gene product; interestingly, its expression can be detected starting at 4 h p.i. (Fig. 3B), which is later than other known MYXV early/late gene products and which also coincides with the late detection of the peak level of M064 RNA (not shown). We therefore tested whether M064 resides in the virion, and thus could be primed to function at the earliest time points of infection prior to the de novo expression of new M064 protein. Velocity sedimentation on sucrose gradients was conducted (1) to purify MYXV virions, and the measurement of viral particles was performed as described previously (13). We detected V5-tagged M064 in the MYXV virions by Western blotting (not shown), using V5-tagged M013 protein (12) as a control for a V5-tagged viral protein not present in the MYXV virion (M. M. Rahman, personal communication). The separation of membrane and core fractions of MYXV virions (13) was conducted to identify the fraction in which V5-tagged M064 resides. As shown in Fig. 3C, lanes 1 and 2, in the presence of both detergent (0.5% NP-40 in 50 mM Tris buffer) and dithiothreitol (DTT) (50 mM), most M064 is associated with the membrane fraction. In the absence of DTT, however, M064 remains insoluble with core structure (Fig. 3D, lanes 1 and 2). M062, the functional homolog of VACV C7, and M063, the rabbit-specific host range factor, have been shown to form a heteromeric complex during viral infection that inhibits the cellular antiviral factor SAMD9 (8). Recombinant MYXVs with V5-tagged M062, V5-tagged M062 but with M063R knocked out, or V5-tagged M063, have been reported previously (8). M063 does not affect the localization of M062 in the virion (Fig. 3C, lanes 5 to 8), and only a small fraction of V5-tagged M062 appears to be soluble along with the membrane fraction in the presence of DTT. In contrast, V5-tagged M063 is associated with the core structure regardless of the presence of DTT (Fig. 3C, lanes 9 and 10, and D, lanes 5 and 6). M071, a homolog of VACV H3L (a membrane protein), was probed as an indicator of the membrane fraction. Therefore, we conclude that M064 does not localize directly in the membrane structure of the MYXV virion, which is consistent with the observation that vMyxM064-KO showed no defect in binding to the cell surface (not shown). In addition, the in vitro transcription assay (3) was conducted using gradient-purified virions to determine whether vMyxM064-KO has defective transcriptional machinery, but no difference was detected in the in vitro core transcription between vMyxM064-KO and vMyxGFP (wt) (not shown). Finally, when viral DNA replication of both viruses during infection of rabbit cells was measured by Sybr green real-time PCR, except for a delay in the M064 knockout virus at the early time point (before 8 h p.i.), the rise in viral DNA levels is comparable to that of wt MYXV (not shown). However, we cannot rule out the possibility that M064 might play a role in the events of virion entry, uncoating, and/or the stability of RNA, based on our data thus far. Therefore, we conclude that M064 is a virion component that can facilitate the early events of MYXV infection after binding but before late gene expression during infection of cultured cells and that M064 controls the kinetics of disease progression in vivo.
Fig 3.
M064 is an early/late viral factor that is packaged into the progeny virions. (A) Construction of the MYXV with V5-tagged M064. A V5 tag was inserted before the stop codon of M064R, and an EGFP expression cassette driven by a vaccinia virus p11 late promoter was inserted after the V5-tagged M064R. The purity of the recombinant virus was confirmed by PCR, and this recombinant virus remains the wild-type phenotype of MYXV in vitro. (B) M064 is expressed early during viral infection, and the expressed protein stably accumulates throughout the course of infection. RK-13 cells were pretreated with AraC or not pretreated, followed by mock infection or infection with vMyxM064RV5 at an MOI of 5 in the presence or absence of AraC. At given time points (1, 2, 4, 8, 12, and 25 h p.i.), cell lysates were harvested for Western blotting. V5-tagged M064 was detected by probing with the anti-V5 antibody. Serp-1 expression was probed to show the effective AraC treatment that abolishes late gene expression. (C) M064 appears to be packaged into MYXV virions. Gradient-purified MYXV virions (0.15 optical density [OD] units for each virus) were used to coarsely separate the membrane and core components in the presence of detergent and DTT. The resulting fractions were separated on 12% SDS-polyacrylamide gels for Western blotting. V5-tagged protein was probed by anti-V5 antibody, and a known membrane component of MYXV virion, M071, was also probed as the control for a successful separation of virion core and membrane. Abbreviations: C, core component; M, membrane fraction. (D) M064 does not appear to be located in the membrane component of the purified MYXV virion. Gradient-purified MYXV virions (0.15 OD units for each virus) were used to separate only membrane and core component along with the intervened network structure which is insoluble in the absence of DTT, designated the H fraction. H+C, core fraction and H fraction that are insoluble in the absence of DTT; M, outer membrane component that is soluble in the presence of detergent while without DTT.
Although the M064R gene knockout in MYXV (Lausanne strain) did not affect end-stage disease in laboratory European rabbits nearly as dramatically as the M062 and M063 knockouts did, M064R is nevertheless highly conserved between virulent parental MYXV and attenuated field strains recovered in Australia (P. Kerr, personal communication). In addition, comparison of the genome of the attenuated nonpathogenic field strain of MYXV with the genome of its ancestor Lausanne strain released in Europe showed that the M064R gene is also strictly conserved (11). Finally, M064 is conserved in both MYXV and with gp064 of Shope (rabbit) fibroma virus (SFV) (4), another closely related member of the Leporipoxvirus genus (14). Because the phenotypes of vMyxM064-KO in vitro and in vivo show delayed progression of infection, the evolutionary selection pressure in keeping M064R intact may well be at the vector-host transmission step. Interestingly, our in vitro study of the M064R knockout MYXV did not reveal any obvious host tropism defects compared to the wild-type parental virus, and is thus very different from the knockout viruses constructed for M062 or M063, suggesting that M064R does not function as a host range factor, at least in the cultured cells tested. This study shows a fundamentally different role of M064 from those of its closely related C7L family members, including M062R and M063R of MYXV.
ACKNOWLEDGMENT
This work was supported by NIH grant RO1 AI080607.
Footnotes
Published ahead of print 29 February 2012
REFERENCES
- 1. Ausubel FM, et al. 1994. Current protocols in molecular biology. John Wiley and Sons, Inc., New York, NY [Google Scholar]
- 2. Barrett JW, et al. 2007. Myxoma virus M063R is a host range gene essential for virus replication in rabbit cells. Virology 361:123–132 [DOI] [PubMed] [Google Scholar]
- 3. Boyd O, Strahl AL, Rodeffer C, Condit RC, Moussatche N. 2010. Temperature-sensitive mutant in the vaccinia virus E6 protein produce virions that are transcriptionally inactive. Virology 399:221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cameron C, et al. 1999. The complete DNA sequence of myxoma virus. Virology 264:298–318 [DOI] [PubMed] [Google Scholar]
- 5. Johnston JB, McFadden G. 2003. Poxvirus immunomodulatory strategies: current perspectives. J. Virol. 77:6093–6100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu J, Wennier S, McFadden G. 2010. The immunoregulatory properties of oncolytic myxoma virus and their implications in therapeutics. Microbes Infect. 12:1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, et al. 2009. Myxoma virus expressing interleukin-15 fails to cause lethal myxomatosis in European rabbits. J. Virol. 83:5933–5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Wennier S, Zhang L, McFadden G. 2011. M062 is a host range factor essential for myxoma virus pathogenesis and functions as an antagonist of host SAMD9 in human cells. J. Virol. 85:3270–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meng X, Chao J, Xiang Y. 2008. Identification from diverse mammalian poxviruses of host-range regulatory genes functioning equivalently to vaccinia virus C7L. Virology 372:372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meng X, et al. 2009. Vaccinia virus K1L and C7L inhibit antiviral activities induced by type I interferons. J. Virol. 83:10627–10636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morales M, et al. 2009. Genome comparison of a nonpathogenic myxoma virus field strain with its ancestor, the virulent Lausanne strain. J. Virol. 83:2397–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rahman MM, McFadden G. 2011. Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-kappaB innate response pathways. J. Virol. 85:12505–12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turner PC, et al. 2007. Vaccinia virus temperature-sensitive mutants in the A28 gene produce non-infectious virions that bind to cells but are defective in entry. Virology 366:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Willer DO, McFadden G, Evans DH. 1999. The complete genome sequence of Shope (rabbit) fibroma virus. Virology 264:319–343 [DOI] [PubMed] [Google Scholar]