Abstract
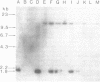
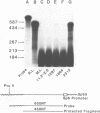
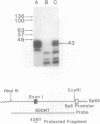
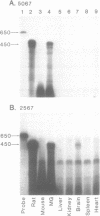
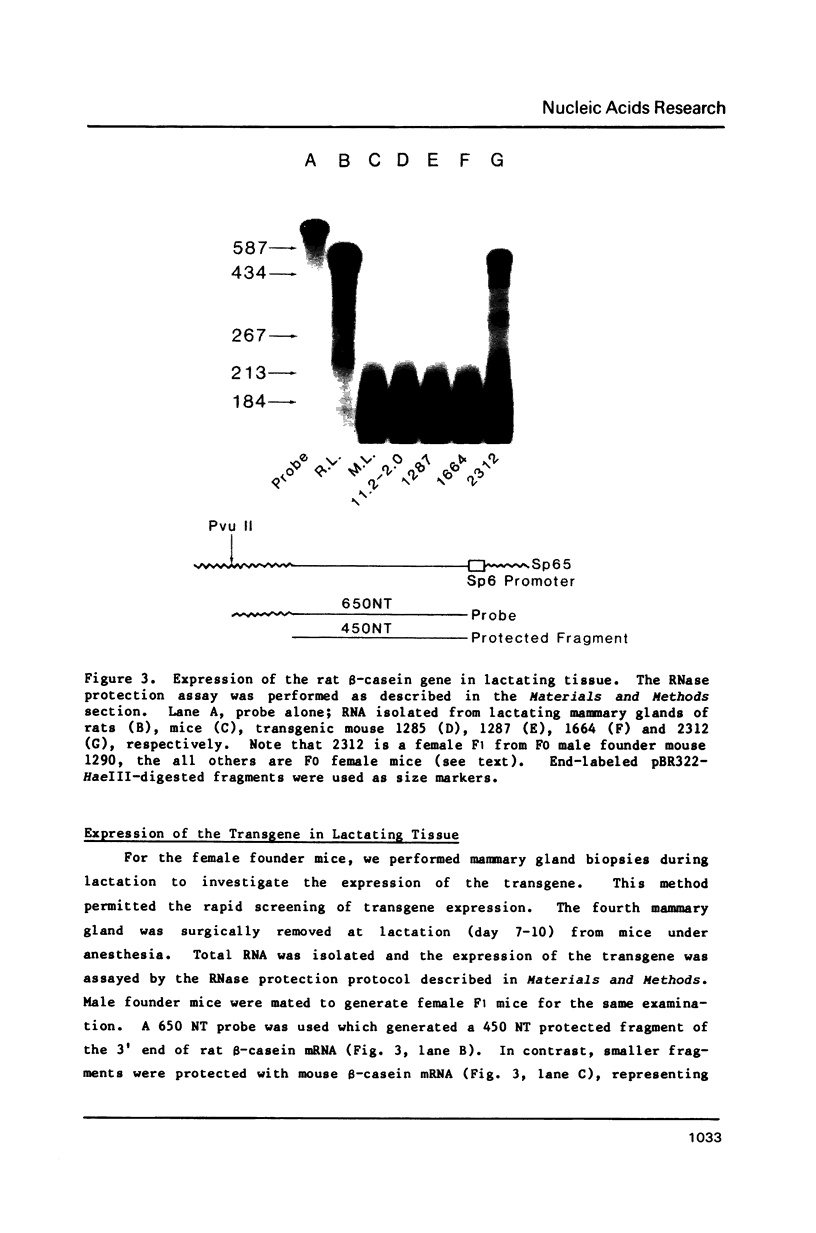
The rat beta-casein gene is a member of a small gene family, encoding the principal milk proteins. In order to understand the mechanisms by which its stage- and tissue-specific expression are regulated, initially, a 14 kb genomic clone containing the entire 7.5 kb rat beta-casein gene with 3.5 kb of 5' and 3.0 kb of 3' flanking DNA was microinjected into the germline of mice. Eight F0 transgenic mice were generated with copy numbers ranging from 1-10; five transmitted the transgene to their offspring in a Mendelian pattern. A specific RNase protection assay was developed to quantitate the level of expression of the rat beta-casein transgene as compared to the endogenous mouse beta-casein gene. Using this assay expression was demonstrated predominantly in the lactating mammary gland of transgenic mice at a level of 0.01-1% of the endogenous mouse beta-casein gene. The transgene employed the authentic transcription initiation site observed previously in the analogous rat beta-casein gene. In one line, a reduced level of expression of the transgene was also observed in the brain. The site of integration, therefore, plays an important role in influencing the level of expression of the transgene, but not its general pattern of tissue-specificity. The transgene appears to be developmentally-regulated in accordance with the endogenous mouse beta-casein gene. These lines of mice generated carrying the rat beta-casein transgene should provide useful models for studying the developmental and hormonal regulation of milk protein gene expression.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Harris A. W., Pinkert C. A., Corcoran L. M., Alexander W. S., Cory S., Palmiter R. D., Brinster R. L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985 Dec 12;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Andres A. C., Schönenberger C. A., Groner B., Hennighausen L., LeMeur M., Gerlinger P. Ha-ras oncogene expression directed by a milk protein gene promoter: tissue specificity, hormonal regulation, and tumor induction in transgenic mice. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1299–1303. doi: 10.1073/pnas.84.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché J. P. The effect of spermidine on endonuclease inhibition by agarose contaminants. Anal Biochem. 1981 Jul 15;115(1):42–45. doi: 10.1016/0003-2697(81)90519-4. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Hobbs A. A., Richards D. A., Kessler D. J., Rosen J. M. Complex hormonal regulation of rat casein gene expression. J Biol Chem. 1982 Apr 10;257(7):3598–3605. [PubMed] [Google Scholar]
- Jones W. K., Yu-Lee L. Y., Clift S. M., Brown T. L., Rosen J. M. The rat casein multigene family. Fine structure and evolution of the beta-casein gene. J Biol Chem. 1985 Jun 10;260(11):7042–7050. [PubMed] [Google Scholar]
- Kelly J. H., Darlington G. J. Hybrid genes: molecular approaches to tissue-specific gene regulation. Annu Rev Genet. 1985;19:273–296. doi: 10.1146/annurev.ge.19.120185.001421. [DOI] [PubMed] [Google Scholar]
- Khillan J. S., Schmidt A., Overbeek P. A., de Crombrugghe B., Westphal H. Developmental and tissue-specific expression directed by the alpha 2 type I collagen promoter in transgenic mice. Proc Natl Acad Sci U S A. 1986 Feb;83(3):725–729. doi: 10.1073/pnas.83.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Hurst J., deBoer E., Grosveld F. The human beta-globin gene contains a downstream developmental specific enhancer. Nucleic Acids Res. 1987 Jul 24;15(14):5739–5747. doi: 10.1093/nar/15.14.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R., Hammer R. E., Tilghman S. M., Brinster R. L. Developmental regulation of alpha-fetoprotein genes in transgenic mice. Mol Cell Biol. 1985 Jul;5(7):1639–1648. doi: 10.1128/mcb.5.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Lee W. H., Kaetzel C. S., Parry G., Bissell M. J. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Blattner F. R. Lambda Charon vectors (Ch32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene. 1983 Dec;26(2-3):171–179. doi: 10.1016/0378-1119(83)90187-7. [DOI] [PubMed] [Google Scholar]
- Lubon H., Hennighausen L. Nuclear proteins from lactating mammary glands bind to the promoter of a milk protein gene. Nucleic Acids Res. 1987 Mar 11;15(5):2103–2121. doi: 10.1093/nar/15.5.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin induction of casein mRNA in organ culture. A model system for studying peptide hormone regulation of gene expression. J Biol Chem. 1978 Apr 10;253(7):2343–2347. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello D., Moore G., Salmon A. M., Yaniv M., Babinet C. Studies on the expression of an H-2K/human growth hormone fusion gene in giant transgenic mice. EMBO J. 1986 Aug;5(8):1877–1883. doi: 10.1002/j.1460-2075.1986.tb04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Palmiter R. D., Hammer R. E., Brinster R. L., Swift G. H., MacDonald R. J. Specific expression of an elastase-human growth hormone fusion gene in pancreatic acinar cells of transgenic mice. Nature. 1985 Feb 14;313(6003):600–602. doi: 10.1038/313600a0. [DOI] [PubMed] [Google Scholar]
- Osborn L., Rosenberg M. P., Keller S. A., Meisler M. H. Tissue-specific and insulin-dependent expression of a pancreatic amylase gene in transgenic mice. Mol Cell Biol. 1987 Jan;7(1):326–334. doi: 10.1128/mcb.7.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek P. A., Chepelinsky A. B., Khillan J. S., Piatigorsky J., Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Transgenic mice. Cell. 1985 Jun;41(2):343–345. doi: 10.1016/s0092-8674(85)80004-0. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Shani M. Tissue-specific and developmentally regulated expression of a chimeric actin-globin gene in transgenic mice. Mol Cell Biol. 1986 Jul;6(7):2624–2631. doi: 10.1128/mcb.6.7.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M. Tissue-specific expression of rat myosin light-chain 2 gene in transgenic mice. Nature. 1985 Mar 21;314(6008):283–286. doi: 10.1038/314283a0. [DOI] [PubMed] [Google Scholar]
- Simons J. P., McClenaghan M., Clark A. J. Alteration of the quality of milk by expression of sheep beta-lactoglobulin in transgenic mice. Nature. 1987 Aug 6;328(6130):530–532. doi: 10.1038/328530a0. [DOI] [PubMed] [Google Scholar]
- Sinn E., Muller W., Pattengale P., Tepler I., Wallace R., Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987 May 22;49(4):465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens D., Park C. S., Stockdale F. E. Milk protein expression and ductal morphogenesis in the mammary gland in vitro: hormone-dependent and -independent phases of adipocyte-mammary epithelial cell interaction. Dev Biol. 1987 Mar;120(1):245–258. doi: 10.1016/0012-1606(87)90122-9. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Banerjee M. R., Oka T. Nucleotide sequence of a cDNA encoding mouse beta casein. Nucleic Acids Res. 1986 Oct 24;14(20):8224–8224. doi: 10.1093/nar/14.20.8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Richter-Mann L., Couch C. H., Stewart A. F., Mackinlay A. G., Rosen J. M. Evolution of the casein multigene family: conserved sequences in the 5' flanking and exon regions. Nucleic Acids Res. 1986 Feb 25;14(4):1883–1902. doi: 10.1093/nar/14.4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]