Abstract
Cotia virus (COTV) SPAn232 was isolated in 1961 from sentinel mice at Cotia field station, São Paulo, Brazil. Attempts to classify COTV within a recognized genus of the Poxviridae have generated contradictory findings. Studies by different researchers suggested some similarity to myxoma virus and swinepox virus, whereas another investigation characterized COTV SPAn232 as a vaccinia virus strain. Because of the lack of consensus, we have conducted an independent biological and molecular characterization of COTV. Virus growth curves reached maximum yields at approximately 24 to 48 h and were accompanied by virus DNA replication and a characteristic early/late pattern of viral protein synthesis. Interestingly, COTV did not induce detectable cytopathic effects in BSC-40 cells until 4 days postinfection and generated viral plaques only after 8 days. We determined the complete genomic sequence of COTV by using a combination of the next-generation DNA sequencing technologies 454 and Illumina. A unique contiguous sequence of 185,139 bp containing 185 genes, including the 90 genes conserved in all chordopoxviruses, was obtained. COTV has an interesting panel of open reading frames (ORFs) related to the evasion of host defense, including two novel genes encoding C-C chemokine-like proteins, each present in duplicate copies. Phylogenetic analysis revealed the highest amino acid identity scores with Cervidpoxvirus, Capripoxvirus, Suipoxvirus, Leporipoxvirus, and Yatapoxvirus. However, COTV grouped as an independent branch within this clade, which clearly excluded its classification as an Orthopoxvirus. Therefore, our data suggest that COTV could represent a new poxvirus genus.
INTRODUCTION
Poxviruses are brick-shaped viruses with a DNA-containing biconcave core surrounded by one or more envelopes (17). The nine genera within the subfamily Chordopoxvirinae are distinguished partially by the different host ranges and geographic distributions of their members but mainly by absent or diminished immune cross-reaction. On the other hand, members of the same genus are genetically related and show strong cross-neutralization (27). During the past 2 decades, the genome sequences of several poxviruses have been elucidated, shedding light on the phylogenetic relationships among family members and providing a genetic basis for classification within distinct genera (1–6, 11, 14, 15, 30, 31, 34, 38, 39, 41, 56, 64, 65, 71). Although most known poxviruses have been grouped within a recognized genus, a few isolates remain unclassified. Unclassified poxviruses include crocodilepox virus, which infects Nile crocodiles (2), squirrelpox virus, which infects squirrels (46), the recently characterized Yoka poxvirus, isolated from mosquitoes in Africa (71), and Cotia virus (COTV), isolated in Brazil (28, 66, 67).
COTV was isolated from 1961 to 1963 from sentinel suckling mice in Cotia field station, São Paulo, Brazil, during an arbovirus surveillance program coordinated by the Instituto Adolfo Lutz, São Paulo (42). The first isolate collected, on 3 March 1961, was designated strain SPAn232 and has been referred to as the COTV prototype (L. E. Pereira and T. L. Coimbra, Section of Arthropod-Transmitted Viruses, personal communication). Strain SPAn232 has not been reisolated, and the natural host for COTV remains unknown. Based on current reports, the assignment of COTV SPAn232 to a recognized poxvirus genus is still controversial. Antibodies against COTV were not able to neutralize infection by vaccinia virus (VACV), myxoma virus (MYXV), goatpox virus (GTPV), or tanapox virus (TANV), suggesting that COTV could not be classified within any poxvirus genus known in the 1970s (66). Further serological tests and analysis of viral proteins showed some similarity between COTV and leporipoxviruses, such as MYXV, but a unique restriction endonuclease profile was reported for the COTV genome (28). In 1995, Ueda and coworkers reported the relatedness of COTV to swinepox virus (SWPV) (Suipoxvirus) based on the physical map of the COTV genome and the nucleotide sequence of the thymidine kinase (TK) gene (J2R ortholog in VACV strain Copenhagen [VACV-Cop]) (67). In contrast, later studies based mainly on the sequences of the TK and vaccinia virus growth factor (VGF) (C11R ortholog in VACV-Cop) genes characterized COTV SPAn232 as a VACV strain, which was renamed SAV (19). Further studies confirmed the phylogenetic relationship between SAV and VACV strain WR (24).
Beyond these conflicting results, no information regarding the COTV replicative cycle is available. In this work, we have analyzed the biology and genomics of COTV. Our results show that cultured cells infected with COTV reached a maximum of virus production within 24 to 48 h after infection. Viral proteins and DNA accumulated progressively within this period of infection. Nevertheless, the detection of a typical poxvirus cytopathic effect (CPE), as well as virus plaque formation, was delayed compared to that with VACV infection. We have also determined the complete genomic sequence of COTV by using two high-throughput sequencing strategies, revealing a 185,139-bp genome containing 185 open reading frames (ORFs). COTV has an interesting set of genes related to immunomodulatory functions, including novel ORFs absent in other poxviruses. Phylogenetic analysis of the 90 proteins conserved in all Chordopoxvirinae revealed >65% amino acid identity scores with members of the Capripoxvirus, Suipoxvirus, and Cervidpoxvirus genera. Despite this relatedness, COTV grouped as a distinct branch, suggesting that it probably represents a member of a novel poxvirus genus.
MATERIALS AND METHODS
Cells and viruses.
BSC-40 (African green monkey kidney), Vero (African green monkey kidney), Hep-2 (human cervical carcinoma [HeLa contaminant]), C6 (rat glioma), RK-13 (rabbit kidney), L-929 (mouse fibroblast), MEF (mouse embryo fibroblast), CEF (chicken embryo fibroblast), PK-15 (pig kidney), Rat-2 (rat fibroblast), and LLC-MK2 (rhesus monkey kidney) cells were propagated at 37°C in Dulbecco modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 5% heat-inactivated fetal bovine serum (Invitrogen), 500 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 2.5 μg/ml amphotericin B (Fungizone), and 0.1 mM nonessential amino acids.
Frozen suckling mice infected with COTV SPAn232 (passage 35 from 19 October 1987) were kindly provided to our laboratory in 1998 by Akemi Suzuki (Instituto Adolfo Lutz, São Paulo, Brazil). Brains were homogenized in phosphate-buffered saline (PBS) supplemented with 1,000 U/ml penicillin, 200 μg/ml streptomycin, and 100 μg/ml gentamicin and were clarified by centrifugation at 600 × g for 10 min at 4°C; the supernatant was then used to inoculate BSC-40 cells. The crude stock was subsequently passaged four times, in Vero cells, BSC-40 cells, chorioallantoic membrane (CAM), and BSC-40 cells, and was then subjected to three cycles of plaque purification in BSC-40 cells.
Vaccinia virus (VACV) strain WR was propagated in BSC-40 cells as described elsewhere (22). Myxoma virus (strain Lausanne) and swinepox virus (strain Kasza) were kindly provided by Richard Moyer (University of Florida, Gainesville) and were propagated in RK-13 and PK-15 cells, respectively. Intracellular VACV and COTV were purified from lysates of infected cells by high-speed centrifugation through a 36% sucrose cushion, followed by sedimentation in 25 to 40% sucrose gradients, as described previously (22).
COTV infection and determination of the yield.
All infection assays were carried out at 34°C using semiconfluent monolayers infected at a multiplicity of infection (MOI) of 1. After 1 h (adsorption period), the inocula were removed and were replaced with fresh medium for the times indicated in the figure and table legends. Virus yield was determined by a plaque assay in semiconfluent BSC-40 monolayers, and viral plaques were visualized after 9 to 10 days at 34°C following 0.1% crystal violet staining.
Transmission electron microscopy.
C6 and BSC-40 cells were infected with COTV for 24 h or 96 h and were processed for transmission electron microscopy analysis as described by Damaso et al. (20). Monolayers were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) and were postfixed in 1% osmium tetroxide. Ultrathin sections of the Epon-embedded material were stained using uranyl acetate and lead citrate and were observed using a Zeiss 900 (at the Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro [IBCCF-UFRJ]) or Morgagni 268 (FEI) (at the Instituto de Microbiologia Prof. Paulo de Góes, UFRJ) electron microscope.
Analysis of COTV DNA replication.
BSC-40 and C6 cells in 35-mm-diameter dishes were infected with COTV as described above. Viral DNA accumulation was investigated essentially as described previously (21, 52). Briefly, at various times postinfection, cells were harvested in TLD buffer (10× SSC [1.5 M NaCl, 0.15 M sodium citrate] [pH 7.0], 1 M ammonium acetate), and samples (40 μl) were applied in triplicate to nylon membranes for slot blot DNA hybridization. COTV DNA isolated from purified virions and radiolabeled by nick translation was used as a probe (21). Densitometry analysis of the X-ray films was performed using Scion Image (beta release 4; Scion Corporation).
Analysis of viral proteins. (i) [35S]methionine incorporation.
BSC-40 and C6 cells in 35-mm-diameter dishes were infected with COTV. At multiple times of infection, cells were pulse-labeled for 1 h with 80 μCi/ml [35S]methionine-[35S]cysteine (Perkin-Elmer Life Sciences) in methionine-free medium (Invitrogen). Cells were then harvested in sodium dodecyl sulfate (SDS) sample buffer, and samples were subjected to electrophoresis in a 12% SDS-polyacrylamide gel electrophoresis (PAGE) gel, as described previously (22). The gels were dried and exposed to X-ray films.
(ii) Detection of viral proteins by immunoblotting.
A hyperimmune antiserum against COTV proteins was obtained by inoculating two male rabbits with SDS-solubilized proteins of purified COTV, as described elsewhere for anti-VACV serum preparation (21). A rabbit anti-MYXV serum was kindly provided by Grant McFadden (University of Florida, Gainesville). For Western blot assays, BSC-40 cells were infected with COTV or VACV-WR. PK-15 or RK-13 cells were infected with SWPV or MYXV, respectively. At the times of infection indicated in the figure legends, samples were processed for SDS-PAGE, followed by Western blotting essentially as described by Damaso et al. (20). Primary antibody dilutions were 1:2,000 for 1 h (anti-VACV and anti-COTV) and 18 h (anti-MYXV). Viral proteins were detected by incubation with rabbit anti-IgG conjugated to horseradish peroxidase, followed by Western blotting Luminol reagent (Santa Cruz Biotechnology, Santa Cruz, CA).
(iii) Detection of viral proteins by immunofluorescence assay.
BSC-40 and C6 cells were grown in 13-mm-diameter round glass coverslips in 24-well plates and were infected with COTV at an MOI of 1. After 48 h, the monolayers were fixed in 4% paraformaldehyde-PBS, permeabilized using 0.5% Triton X-100 as described previously (16), and stained with anti-COTV for 1 h, followed by Alexa 488-conjugated rabbit anti-IgG (Invitrogen). DNA was detected by staining with 4′,6-diamidino-2-phenylindole (DAPI). Samples were analyzed with a Zeiss Axio Observer Z1 inverted microscope, and confocal images were acquired with a Zeiss LSM 510 META confocal microscope.
Genomic library construction and DNA sequencing.
COTV genomic DNA was isolated from purified particles by using the DNeasy Blood & Tissue kit (Qiagen, Valencia, CA) as suggested by the manufacturer and was subjected to high-throughput sequencing using the Genome Sequencer FLX (454 Life Sciences, Branford, CT) and Genome Analyzer IIx (Illumina, San Diego, CA) at the Interdisciplinary Center for Biotechnology Research (ICBR), University of Florida. Sanger sequencing was also performed for confirmatory purposes and for DNA walking on genome ends at ICBR and at the Unidade Multidisciplinar de Genômica (Instituto de Biofísica Carlos Chagas Filho, UFRJ). In preparation for 454 sequencing, a single-strand template DNA (sstDNA) library was constructed using a GS FLX Titanium general library preparation kit and was amplified by emulsion PCR (emPCR) using the GS FLX Titanium SV and LV emPCR kits. A picotiter plate with the beads was loaded onto a 454 instrument along with the reagents, and sequences were obtained according to the manufacturer's protocol. For Illumina sequencing, COTV DNA was sheared using sonication and was blunt ended with T4 DNA polymerase and Klenow DNA polymerase. After the ligation of adapters to both ends, DNA fragments within the range of 250 to 400 bp were gel purified and PCR amplified with 18 cycles. The resulting libraries were gel purified and were quantified using the KAPA Library Quant Kit (Kapa Biosystems) on an ABI 7900HT real-time PCR system. Libraries were diluted to 10 pM for cluster generation on cBOT. The 2 × 100 cycle paired-end sequencing run was performed on an Illumina GA platform (running SCS 2.8) using a single lane of an 8-lane flow cell according to the manufacturer's instructions.
Sequence assembly, genome analysis, and phylogenetic inference.
An initial assembly of the 454 sequences was performed with the Newbler assembler, version 2.3 (454 Life Sciences), with masking and trimming sequencing repeats, primers, and/or adapters used in sequencing library preparation and normalization. Illumina data were assembled de novo using Velvet with a k-mer of 55 and a short paired insert length of 225 (70), after removal of adapters using fastx_clipper (FASTX-Toolkit, version 0.0.6). Any orphan reads were removed using an in-house Perl script. Combined assembly of 454 and Illumina contigs together with Sanger reads resulting from walking on the genome was performed using SeqMan (DNAStar package; Lasergene Inc.), with default parameters. Open reading frames (ORFs) longer than 30 amino acids were detected by FGENESV (Softberry), Vector NTI (Invitrogen), and CLC DNA Workbench (CLC bio, Aarhus, Denmark). Predicted ORFs containing poxvirus promoter elements and/or transcription termination signals were annotated. A total of 185 ORFs were identified by a homology search using BLASTP, available through the NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Tandem repeats were identified by Tandem Repeat Finder (12).
Phylogeny inference was performed by aligning the predicted amino acid sequences obtained for each of the 90 conserved genes with ortholog sequences from 21 different chordopoxviruses by use of Clustal X, version 1.81 (60), and MUSCLE, version 3.8 (25). After visual inspection of the alignments, the external gaps were excluded, and a concatenated data set was obtained by combining the alignments for each gene into a single alignment of 30,717 amino acids. All sequence manipulation steps were carried out twice as independent events. The combined data sets were used to generate neighbor-joining phylogenetic trees using MEGA, version 4 (59), opting for the JTT model of substitution, and 2,500 bootstrap replicates. Alternatively, maximum-likelihood trees were inferred using Puzzle, version 5.2 (54), opting for the WAG correction for multiple substitutions, a neighbor-joining input tree, 10,000 quartet puzzling steps, and an eight-category discrete gamma model. DNA sequences were aligned using KALIGN (37) and were adjusted by visual inspection.
The GenBank accession numbers of the poxvirus DNA genome sequences used for the multiple alignments were as follows: canarypox virus (CNPV) strain ATCC VR111, NC_005309; fowlpox virus (FWPV) strain Iowa, NC_002188; deerpox virus (DPV) strains W-848-83 and W-1170-84, NC_006966 and AY689437; swinepox virus strain Nebraska, NC_003389; myxoma virus strain Lausanne, NC_001132; rabbit fibroma virus (RFV) strain Kasza, NC_001266; goatpox virus strain Pellor, NC_004003; lumpy skin disease virus (LSDV) strain Neethling Warmbaths, AF409137; sheeppox virus (SPPV) strain Niskhi, AY077834; Yaba-like disease virus (YLDV) strain Davis, NC_002642; Yaba monkey tumor virus (YMTV) strain Amano, NC_005179; cowpox virus (CPXV) strain Brighton Red, NC_003663; monkeypox virus (MPXV) strain Liberia_1970_184, DQ011156; vaccinia virus strain WR (VACV-WR), NC_006998; vaccinia virus strain Copenhagen (VACV-Cop), M35027; variola minor virus (VARV) strain Garcia, Y16780; molluscum contagiosum virus (MOCV), NC_001731; bovine papular stomatitis virus (BPSV), NC_005337; orf virus (ORFV) strain NZ2, DQ184476; and crocodilepox virus (CRV) strain Zimbabwe, NC_008030.
Nucleotide sequence accession number.
The COTV genome sequence has been deposited in GenBank under accession number HQ647181.
RESULTS
Virus morphology and host range.
After the isolation of COTV SPAn232 from sentinel mice in 1961, the Instituto Adolfo Lutz propagated virus samples only by sequential intracranial passage of brain suspensions in 3-day-old mice. The last passage dates from 5 May 2003 and was derived from passage 34, which was used to generate passage 35 in two independent inoculation events: in 1987 and 2003 (T. L. Coimbra, personal communication). Passage 35 from 1987 was sent to our laboratory in 1998.
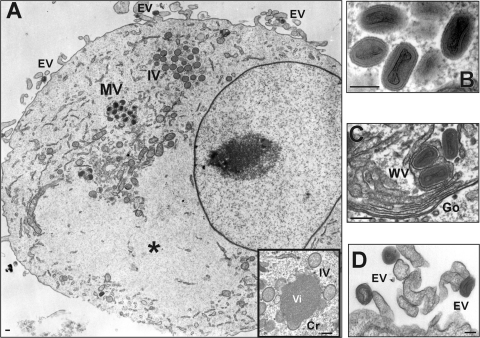
After adaptation to cell culture and three consecutive cycles of plaque purification, crude stocks of COTV were prepared and were used to infect C6 and BSC-40 cells in order to evaluate virus morphogenesis and to confirm the typical poxvirus morphology of the isolate. We observed all stages of poxvirus morphogenesis in the cell cytoplasm (Fig. 1): crescents (Cr), spherical immature particles (IV), mature particles (MV), wrapped virus (WV), and extracellular virus (EV). Inclusions devoid of cellular organelles similar to the granular Cotia bodies described by Ueda et al. (66) were also visualized (Fig. 1A, asterisk). These inclusions were less electron dense than typical viroplasm (Fig. 1A, inset).
Fig 1.
Electron microscopy analysis of COTV-infected cells. C6 (A, B, and D) and BSC-40 (C) cells were infected with COTV at an MOI of 1. The monolayers were fixed and processed for transmission electron microscopy at 24 h (A) or 96 h (B to D) postinfection. Representative fields are shown. (A) Mature viruses (MV) and immature spherical particles (IV) are visualized in the cytoplasm outside areas devoid of cellular organelles resembling Cotia bodies (asterisks). Extracellular viruses (EV) are found associated with the cell membrane. (Inset) High-magnification image of viroplasm (Vi) surrounded by membrane crescents (Cr) and IV. (B) High-magnification image of MV. (C) MV are enveloped by additional membranes derived from the trans-Golgi complex, generating wrapped virions (WV). The Golgi apparatus (Go) is shown. (D) High-magnification image of EVs associated with the cell membrane. Bars, 200 nm.
The production of COTV progeny during the infection of several cell types was analyzed 52 h postinfection by a plaque assay in BSC-40 cells (Table 1). We observed that distinct cell types produced similar virus yields, but C6 and BSC-40 cells generated higher titers than other cell types. In PK-15, LLC-MK2, and Rat-2 cells, specifically, progeny production was negligible. It is worth noting that all cell types tested in this assay were fully permissive to VACV (data not shown).
Table 1.
Production of COTV progeny in different cell typesa
| Cell line (species) | PFU per cell |
|---|---|
| BSC-40 (Cercopithecus aethiops) | 20 ± 3 |
| C6 (Rattus norvegicus) | 17 ± 1 |
| RK-13 (Oryctolagus cuniculus) | 9 ± 4 |
| L-929 (Mus musculus) | 7 ± 4 |
| Hep-2 (Homo sapiens) | 7 ± 1 |
| MEF (Mus musculus) | 5 ± 4 |
| Vero (Cercopithecus aethiops) | 4 ± 2 |
| CEF (Gallus gallus domesticus) | 2 ± 2 |
| PK-15 (Sus scrofa) | 0 |
| LLC-MK2 (Macaca mulatta) | 0 |
| Rat-2 (Rattus norvegicus) | 0 |
Cells were infected with COTV at an MOI of 1 and were collected at 52 h postinfection for virus titration by plaque assay in BSC-40 cells, as described in Materials and Methods. Results are means (± standard deviations) for three assays titrated in duplicate.
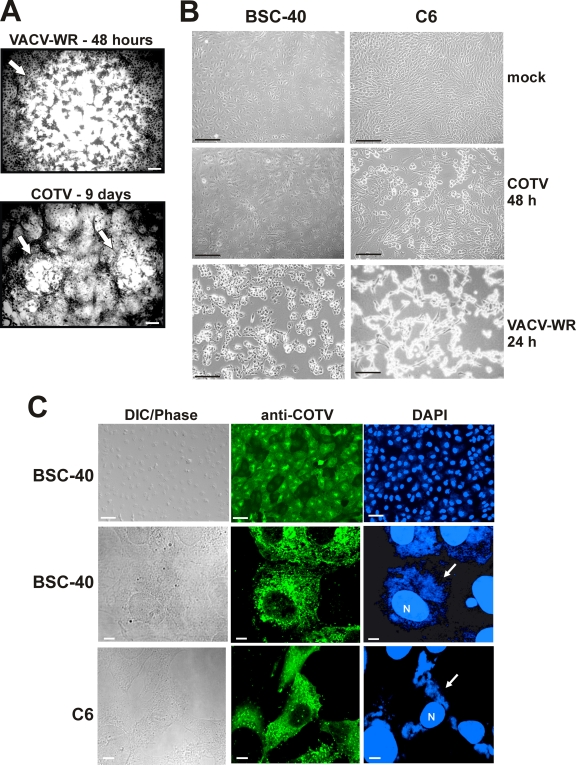
During the course of these assays, we observed that COTV generated tiny plaques detectable only after 8 days of infection. Plaque formation was dependent on monolayer confluence and incubation temperatures of 33°C to 34.5°C. Figure 2A shows the COTV plaque phenotype in BSC-40 cells at 9 days postinfection. Plaques were significantly smaller than VACV-WR plaques visualized at 48 h postinfection. We have also observed that the virus-induced cytopathic effect (CPE) progressed quite slowly in BSC-40 cells compared with that in C6 cells infected with COTV. Figure 2B shows that no apparent CPE was detected in BSC-40 cells at 48 h postinfection, while in C6 monolayers, typical cell rounding induced by poxvirus infection could be observed. CPE in BSC-40 cells was noticed only after 4 days of infection at an MOI of 1. In both cell lines, however, these effects proceeded much more slowly than the morphological changes induced by VACV-WR infection (Fig. 2B). It is noteworthy that viral DNA and COTV structural proteins could be detected in BSC-40 monolayers presenting normal cellular morphology, indicating active infection in these cells (Fig. 2C, top). Cells stained with anti-COTV contained viral factories characteristic of late-stage infection (Fig. 2C, center). A similar pattern was observed in C6 cells (Fig. 2C, bottom).
Fig 2.
Virus plaque phenotype and CPE progression in COTV-infected cells. (A) Monolayers of BSC-40 cells were infected with 300 PFU of VACV-WR or COTV for 2 or 9 days, respectively, at which time the cells were stained with 0.1% crystal violet. Arrows indicate viral plaques. Bars, 100 μm. (B) BSC-40 and C6 cells were either mock infected or infected with COTV or VACV-WR at an MOI of 1, and CPE was visualized at 24 (VACV-WR) or 48 (COTV) h postinfection. Bars, 100 μm. (C) BSC-40 and C6 cells were infected with COTV at an MOI of 1 for 48 h and were processed for immunofluorescence assays using an antiserum against COTV structural proteins (anti-COTV). DNA was stained with DAPI. N, nucleus. Arrows point to virus factories in the cell cytoplasm. Representative fields are shown. Bars, 50 μm (top) and 5 μm (center and bottom).
Analysis of the COTV replicative cycle.
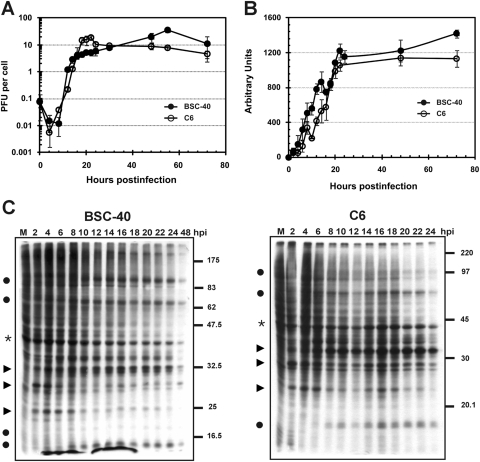
To further investigate COTV infection in BSC-40 and C6 cells, we analyzed virus progeny production and DNA replication in time course assays. As shown in Fig. 3A, the infection of the two cell lines generated similar kinetics of virus growth, which reached a plateau at approximately 48 h in BSC-40 cells and 24 h in C6 cells. The time course profile of progeny yield in C6 cells was similar to that reported previously for VACV-infected BSC-40 cells, although virus production by VACV is at least 5 times greater than that by COTV (23).
Fig 3.
Time course analysis of progeny production, DNA replication, and protein synthesis in COTV-infected cells. Semiconfluent BSC-40 and C6 monolayers were infected with COTV at an MOI of 1, and at the indicated times postinfection, cells were either harvested for virus titration by plaque assay (A), processed for detection of viral DNA by slot blot hybridization (B), or pulse-labeled with [35S]methionine followed by 12% SDS-PAGE analysis (C). (A) Values represent the means for three assays titrated in duplicate. (B) The autoradiograms obtained for BSC-40 and C6 cells were scanned, and densitometry analysis was performed. The numbers (arbitrary units) express the mean values for nine autoradiograms in which samples were applied in triplicate. (C) Representative autoradiograms are shown. Filled circles indicate viral late proteins; arrowheads indicate viral early proteins; asterisks indicate a host protein. M, mock-infected cells. Molecular size markers (in kilodaltons) are given on the right.
A time course assay of COTV DNA accumulation was evaluated by slot blot hybridization of infected-cell extracts. As shown in Fig. 3B, the viral DNA contents in both cell lines increased with time, reaching a plateau after 24 h of infection. The time course profiles were similar to those observed for progeny production assays, and similar kinetics of DNA accumulation have been observed in VACV-infected cells (23).
We also analyzed viral protein synthesis during the course of infection. BSC-40 and C6 cells infected with COTV were pulse-labeled with [35S]methionine at the time points indicated in Fig. 3C, and samples were resolved by SDS-PAGE, followed by autoradiography. In both cell lines, the analysis revealed the typical pattern of pre- and postreplicative polypeptides observed for other poxviruses (Fig. 3C). Viral early proteins were initially detected at 2 h postinfection (Fig. 3C, arrowheads), followed by the onset of late protein synthesis at 8 h, progressing up to 24 h postinfection (Fig. 3C, circles). Interestingly, although we observed the inhibition of cellular protein synthesis as infection developed (Fig. 3C, asterisks), this effect was not as dramatic as the typical shutoff of host translation observed during infection with VACV (9, 22).
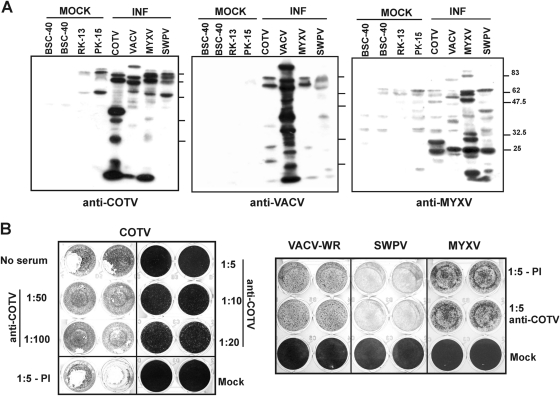
Together, these data indicate that COTV successfully developed all stages of the replicative cycle within 24 h of infection, with equivalent kinetics in C6 and BSC-40 cells, despite the formation of relatively small plaques and the delay in the development of CPE observed in both cell lines. Therefore, these data support the conclusion that COTV is clearly distinct from VACV. In addition, we investigated the immunological relationships of COTV with VACV, MYXV, and SWPV. Western blot analysis of cells infected with COTV, VACV-WR, MYXV, and SWPV revealed that anti-COTV primarily detected several COTV proteins, cross-reacting slightly, but equally, with VACV, MYXV, and SWPV high-molecular weight polypeptides (Fig. 4A, left). Similarly, no significant cross-reactivity was detected with antisera against other viruses (Fig. 4A). In addition, an anti-COTV antiserum failed to neutralize VACV, MYXV, or SWPV infection but efficiently neutralized COTV infection (Fig. 4B). These data suggested that COTV does not belong to the Orthopoxvirus, Leporipoxvirus, or Suipoxvirus genus.
Fig 4.
Analysis of COTV cross-reactivity and virus neutralization. (A) The indicated cells were infected (INF) with COTV (BSC-40), VACV (BSC-40), MYXV (RK-13), or SWPV (PK-15) at an MOI of 5 and were harvested after reaching intense CPE (for COTV, 4 days; for VACV, 24 h; for MYXV, 48 h; for SWPV, 3 days). Samples were analyzed by Western blotting using an antiserum against COTV (left), VACV (center), or MYXV (right). (B) A total of 10,000 PFU of COTV, VACV, MYXV, or SWPV was incubated with the indicated dilutions of anti-COTV antiserum or the preimmune serum (PI) for 1 h at 37°C. The mixtures were then placed on monolayers of BSC-40 (COTV and VACV), RK-13 (MYXV), or PK-15 (SWPV) cells for 9 days (COTV) or for 48, 52, or 96 h postinfection (VACV, MYXV, or SWPV, respectively), after which virus-induced CPE was visualized by crystal violet staining.
Sequencing and structure of the COTV genome.
To proceed with COTV characterization in detail, we determined the complete nucleotide sequence of the COTV genome by using a combination of two high-throughput sequencing strategies, 454 GS-FLX Titanium and Illumina GAII, plus traditional Sanger sequencing (the latter used mainly to primer-walk on the ends of the genome). The 454 sequencing of the COTV genome yielded 41,387 raw reads (average length, 371 nucleotides [nt]), which were assembled into 550 contigs (average length, 1,110 nt). Two contigs of 157,656 nt and 13,281 nt were used for further assembly (average 60× genome coverage). The Illumina sequencing produced 27,415,784 raw read pairs of 105 nt, which were used by the Velvet assembler to generate 6,624 small contigs of variable lengths. The Illumina reads resulted in an average 962× coverage of the COTV genome. Nucleotide mismatches and possible errors in homopolymeric regions of 454 contigs not corrected by Illumina were solved by Sanger sequencing. Assembly of Illumina contigs with the two 454 contigs and the Sanger reads resulting from primer walking on the inverted terminal repeat (ITR) regions generated a final contiguous sequence of 185,139 bp.
The COTV genome has an average A+T content of 76.4% and a central genomic region of 157,703 bp flanked by two identical ITRs of 13,718 bp. The ITR contains 16 copies of a 17-bp tandem repeat and 11 copies of a 24-bp tandem repeat, which are partially inserted into 2 distinct 47-bp tandem repeats present in 6 copies each. These repeats are also present in 2 copies of a 132-bp element. As with other poxviruses, the leftmost nucleotide of the final assembled sequence was arbitrarily set to nucleotide number 1.
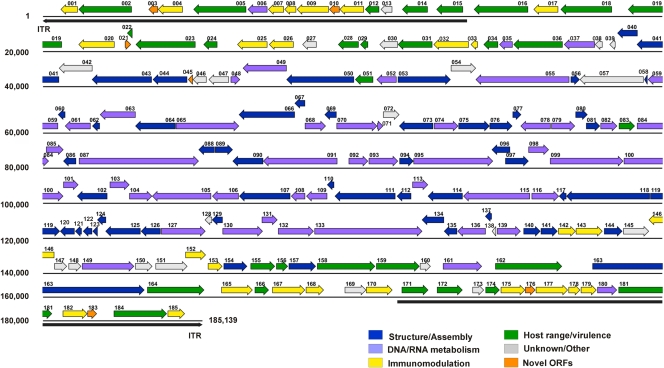
COTV contains 185 ORFs, which account for a coding density of 91.5% (Fig. 5; Table 2). Fifteen ORFs are duplicated, with one copy located in each ITR. As with other poxviruses, ORFs situated in the middle region of the COTV genome encode proteins related to viral DNA and RNA metabolism, the structure of virus particles, and virion morphogenesis. Within this region, we identified the 90 conserved genes present in all chordopoxviruses, located between VACV-Cop orthologs F9L and A34R (COTV040 to COTV141). Gene synteny in this region is conserved overall in COTV relative to members of the Chordopoxvirinae (Table 2). The ends of the genome encode proteins involved in the modulation of host immune response, virulence, and host range, as well as some proteins of unknown function (Fig. 5).
Fig 5.
ORF map of the COTV genome. The annotated ORFs are represented by arrows color coded according to their functional categories. The arrows representing ORFs point left or right, indicating the direction of transcription. The inverted terminal repeat (ITR) regions are indicated by long black arrows (shown below the sequence) at the ends of the genome.
Table 2.
ORFs encoded by COTV
| ORFa | Position (bp) | Length (aa) | Best matchb |
Description/putative functiond | VACV-Cop orthologe | ||
|---|---|---|---|---|---|---|---|
| Species, gene | Length (aa) | % IDc | |||||
| COTV001 | 1122-571 | 183 | CPXV-GER1980-EP4, 205 | 188 | 32 | Serine protease inhibitor-like protein | C13L/C14L |
| COTV002 | 2864-1152 | 570 | DPV-W1170-84, 019 | 643 | 25 | Ankyrin repeat protein | B4R |
| COTV003 | 3713-3411 | 100 | Mustela putorius furo, gene CCL13 | 70 | 35 | C-C motif chemokine protein | — |
| COTV004 | 4511-3717 | 264 | CPXV-NOR1994-MAN, 002 | 245 | 28 | Chemokine binding protein | C23L/B29R |
| COTV005 | 6560-4860 | 566 | DPV-W848-83, 019 | 643 | 25 | Ankyrin repeat protein/PRANC domain | B4R |
| COTV006 | 7245-6613 | 210 | Bos taurus, locus BOS-3916/CNPV-VR111, 170* | 212/212 | 50/55 | Thymidylate kinase | A48R |
| COTV007 | 7777-7274 | 167 | CPXV-UK2000-K2984, 003 | 355 | 25 | TNF receptor-like protein (CrmB) | C22L/B28R |
| COTV008 | 8175-7801 | 124 | YKV, 178 | 127 | 42 | TNF receptor-like protein (CrmE) | — |
| COTV009 | 9230-8199 | 343 | MPXV-ZAR-1979, 005/187 | 354 | 37 | Soluble IFN-α/β receptor | B19R |
| COTV010 | 9577-9245 | 110 | Mus musculus, gene Scya2 | 148 | 25 | C-C motif chemokine protein | — |
| COTV011 | 10363-9581 | 260 | CPXV-GER91, 001/219 | 252 | 26 | C-C chemokine binding protein (35-kDa major secreted virus protein) | B29R |
| COTV012 | 10856-10404 | 150 | LSDV-LW1959, 001/161 | 159 | 47 | NF-κB inhibitor/virulence factor | B15R |
| COTV013 | 11278-10910 | 122 | RFV-Kas, 004/163 | 125 | 49 | Unknown | — |
| COTV014 | 12422-11601 | 273 | CPXV-UK2000-K2984, 009 | 273 | 31 | Kelch-like protein | A55R |
| COTV015 | 13562-12708 | 284 | ECTV-Mos, 004 | 273 | 29 | Kelch-like protein | A55R |
| COTV016 | 15664-13919 | 581 | MYXV-Lau, 152 | 675 | 24 | Ankyrin repeat protein | — |
| COTV017 | 16637-15846 | 263 | DPV-W1170-84, 010 | 272 | 35 | Soluble IFN-γ receptor | B8R |
| COTV018 | 18372-16720 | 550 | LSDV-NW-LW, 148 | 550 | 24 | Kelch-like protein | F3L |
| COTV019 | 20606-18897 | 569 | YMTV-Amano, 007 | 637 | 26 | Ankyrin repeat protein/PRANC domain | B4R |
| COTV020 | 22308-21148 | 386 | VACV-Cop, 254 | 353 | 36 | Soluble IFN-α/β-receptor | B19R |
| COTV021 | 22645-22839 | 64 | — | — | — | Hypothetical protein | — |
| COTV022 | 22886-22716 | 56 | VARV-BEN68-59, 008 | 58 | 76 | Ankyrin repeat protein/orthopoxvirus C10L protein | WR-018 |
| COTV023 | 24921-22996 | 641 | CPXV-BR, 027 | 632 | 62 | Ankyrin repeat protein | C9L |
| COTV024 | 25628-25185 | 147 | VACV-WR, 021 | 150 | 56 | Host range virulence factor | C7L |
| COTV025 | 27243-26275 | 322 | CPXV-FIN2000-MAN, 031 | 321 | 68 | IL-1 receptor antagonist | C10L/C4L |
| COTV026 | 28091-27303 | 262 | CPXV-UK2000-K2984, 032 | 262 | 65 | Complement binding protein | C3L |
| COTV027 | 28833-28378 | 151 | CPXV-GER1990-2, 036 | 176 | 70 | Alpha-amanitin sensitivity protein | N2L |
| COTV028 | 30193-29531 | 220 | CPXV-NOR1994-MAN, 038 | 220 | 72 | NF-κB inhibitor | M2L |
| COTV029 | 30495-30247 | 82 | CPXV-NOR1994-MAN, 041 | 88 | 64 | IFN resistance/eIF2α-like PKR inhibitor | K3L |
| COTV030 | 31457-30870 | 195 | AMEV-Moyer, 173‡ | 209 | 23 | Hypothetical protein | — |
| COTV031 | 32571-31462 | 369 | VACV-GLV-1h68, 037 | 284 | 27 | Ankyrin repeat protein/host range protein/NF-κB and PKR inhibitor | K1L |
| COTV032 | 33750-32614 | 378 | DPV-W848-83, 018 | 382 | 42 | Serine protease inhibitor-like (SPI-3)/fusion regulatory protein | K2L |
| COTV033 | 34038-33814 | 74 | Anopheles darlingi, locus AND-04657/SPPV-A, 013 | 241/100 | 43/34 | Epidermal growth factor-like domain | C11R |
| COTV034 | 34694-34230 | 154 | YMTV-Amano, 011 | 172 | 28 | Apoptosis inhibitor | F1L |
| COTV035 | 35163-34738 | 141 | MYXV-Lau, 016 | 148 | 64 | dUTPase | F2L |
| COTV036 | 36780-35194 | 528 | DPV-W848-83, 025 | 529 | 34 | Kelch-like protein | F3L |
| COTV037 | 37822-36827 | 331 | DPV-W848-83, 026 | 321 | 74 | Ribonucleotide reductase small subunit | F4L |
| COTV038 | 38067-37825 | 80 | SPPV-TU, 018‡ | 79 | 34 | Hypothetical protein | — |
| COTV039 | 38487-38296 | 63 | YLDV-Davis, 024 | 50 | 68 | Unknown | F8L |
| COTV040 | 39200-38550 | 216 | DPV-W848-83, 030 | 215 | 56 | S-S bond formation pathway protein | F9L |
| COTV041 | 40515-39178 | 445 | SWPV-Neb, 022 | 440 | 76 | Serine/threonine kinase | F10L |
| COTV042 | 41592-40516 | 358 | DPV-W1170-84, 032 | 381 | 40 | RhoA inhibitor/cell motility | F11L |
| COTV043 | 43510-41579 | 643 | DPV-W1170-84, 033 | 651 | 45 | WV-associated protein | F12L |
| COTV044 | 44654-43545 | 369 | DPV-W848-83, 034 | 375 | 69 | Palmitylated EV envelope protein | F13L |
| COTV045 | 44835-44686 | 49 | — | — | — | Hypothetical protein | — |
| COTV046 | 45278-44838 | 146 | DPV-W848-83, 036 | 148 | 56 | Unknown | F15L |
| COTV047 | 46000-45350 | 216 | SWPV-Neb, 028 | 217 | 36 | Unknown | F16L |
| COTV048 | 46060-46359 | 99 | YMTV-Amano, 025 | 104 | 74 | Nucleic acid binding phosphoprotein | F17R |
| COTV049 | 47865-46453 | 470 | SPPV-TU, 029 | 474 | 74 | Poly(A) polymerase large subunit | E1L |
| COTV050 | 50042-47862 | 726 | SWPV-Neb, 031 | 732 | 54 | WV assembly/interaction with F12 | E2L |
| COTV051 | 50659-50081 | 192 | SPPV-TU, 031 | 177 | 43 | IFN resistance/dsRNA-binding protein/PKR inhibitor | E3L |
| COTV052 | 51414-50776 | 212 | RFV-Kas, 035 | 222 | 60 | RNA polymerase 30-kDa subunit | E4L |
| COTV053 | 51458-53161 | 567 | DPV-W848-83, 044 | 566 | 70 | Core protein/virus morphogenesis | E6R |
| COTV054 | 53171-53977 | 268 | DPV-W848-83, 045 | 267 | 80 | Unknown | E8R |
| COTV055 | 56994-53974 | 1006 | LSDV-LW1959, 041 | 1006 | 70 | DNA polymerase | E9L |
| COTV056 | 57041-57328 | 95 | RFV-Kas, 040 | 96 | 76 | Sulfhydryl oxidase | E10R |
| COTV057 | 59394-57337 | 685 | DPV-W1170-84, 048 | 678 | 41 | Unknown | O1L |
| COTV058 | 59521-59417 | 34 | MYXV-Lau, 042 | 32 | 68 | MV entry-fusion complex | O3L |
| COTV059 | 60481-59537 | 314 | DPV-W848-83, 049 | 313 | 69 | DNA-binding protein | I1L |
| COTV060 | 60715-60482 | 77 | SWPV-Neb, 040 | 75 | 56 | Virus assembly, crescent formation | I2L |
| COTV061 | 61532-60708 | 274 | DPV-W848-83, 051 | 272 | 60 | DNA-binding phosphoprotein | I3L |
| COTV062 | 61830-61588 | 80 | DPV-W848-83, 053 | 78 | 39 | MV membrane protein | I5L |
| COTV063 | 62986-61838 | 382 | DPV-W848-83, 054 | 389 | 59 | Telomere binding protein | I6L |
| COTV064 | 64277-62979 | 432 | DPV-W848-83, 055 | 432 | 76 | Virion core protease | I7L |
| COTV065 | 64287-66326 | 679 | LSDV-LW1959, 051 | 683 | 67 | RNA helicase/NPH-II | I8R |
| COTV066 | 68128-66323 | 601 | SWPV-Neb, 047 | 593 | 63 | Metalloprotease | G1L |
| COTV067 | 68460-68125 | 111 | MYXV-Lau, 051 | 111 | 51 | MV entry-fusion complex | G3L |
| COTV068 | 68454-69128 | 224 | SPPV-TU, 049 | 222 | 52 | Viral late transcription elongation factor | G2R |
| COTV069 | 69475-69092 | 127 | SPPV-TU, 050 | 126 | 78 | Glutaredoxin protein | G4L |
| COTV070 | 69478-70788 | 436 | DPV-W1170-84, 061 | 434 | 55 | Flap endonucleases (FEN-1) | G5R |
| COTV071 | 70791-70982 | 63 | LSDV-Nee, 056 | 63 | 79 | RNA polymerase subunit RPO7 | G5.5R |
| COTV072 | 70982-71503 | 173 | RFV-Kas, 056 | 174 | 63 | NlpC/P60 superfamily | G6R |
| COTV073 | 72597-71500 | 365 | DPV-W848-83, 064 | 375 | 60 | Assembly/seven-protein complex | G7L |
| COTV074 | 72627-73406 | 259 | LSDV-Nee, 059 | 260 | 81 | Viral late transcription factor VLTF-1 | G8R |
| COTV075 | 73416-74420 | 334 | DPV-W1170-84, 066 | 335 | 61 | MV entry-fusion complex | G9R |
| COTV076 | 74421-75152 | 243 | DPV-W1170-84, 067 | 249 | 79 | Myristylated MV membrane protein | L1R |
| COTV077 | 75164-75439 | 91 | DPV-W848-83, 068 | 95 | 46 | Virus assembly, crescent formation | L2R |
| COTV078 | 76390-75422 | 322 | LSDV-Nee, 063 | 318 | 72 | Core protein, early transcription | L3L |
| COTV079 | 76415-77176 | 253 | DPV-W848-83, 070 | 252 | 80 | DNA-binding virion protein VP8 | L4R |
| COTV080 | 77195-77581 | 128 | SPPV-TU, 061 | 132 | 56 | MV entry-fusion complex | L5R |
| COTV081 | 77538-77984 | 148 | LSDV-LW1959, 067 | 148 | 72 | Assembly/seven-protein complex | J1R |
| COTV082 | 78003-78539 | 178 | SWPV-Neb, 063 | 180 | 68 | Thymidine kinase | J2R |
| COTV083 | 78596-79108 | 170 | YLDV-Davis, 069 | 178 | 43 | Host range virulence factor | C7L |
| COTV084 | 79181-80182 | 333 | DPV-W1170-84, 075 | 374 | 76 | Poly(A) polymerase small subunit | J3R |
| COTV085 | 80097-80654 | 185 | SWPV-Neb, 066 | 185 | 75 | RNA polymerase subunit RPO22 | J4R |
| COTV086 | 81064-80651 | 137 | SWPV-Neb, 067 | 134 | 59 | MV entry-fusion complex | J5L |
| COTV087 | 81158-85024 | 1288 | DPV-W1170-84, 078 | 1286 | 82 | RNA polymerase subunit RPO147 | J6R |
| COTV088 | 85536-85021 | 171 | DPV-W1170-84, 079 | 172 | 80 | Serine/tyrosine dual phosphatase | H1L |
| COTV089 | 85551-86123 | 190 | DPV-W1170-84, 080 | 190 | 67 | MV entry-fusion complex | H2R |
| COTV090 | 87106-86120 | 328 | SWPV-Neb, 071 | 324 | 56 | MV heparin binding surface protein | H3L |
| COTV091 | 89500-87107 | 797 | SWPV-Neb, 072 | 801 | 75 | RNA polymerase-associated protein RAP94 | H4L |
| COTV092 | 89869-90492 | 207 | SWPV-Neb, 073 | 181 | 41 | Viral late transcription factor VLTF-4 | H5R |
| COTV093 | 90516-91460 | 314 | SWPV-Neb, 074 | 320 | 68 | DNA topoisomerase type I | H6R |
| COTV094 | 91507-91962 | 151 | SWPV-Neb, 075 | 149 | 55 | Virus assembly, crescent formation | H7R |
| COTV095 | 91963-94527 | 854 | DPV-W848-83, 086 | 843 | 70 | Capping enzyme large subunit | D1R |
| COTV096 | 95079-94489 | 196 | SWPV-Neb, 077 | 146 | 45 | Assembly/seven-protein complex | D2L |
| COTV097 | 94925-95677 | 250 | SWPV-Neb, 078 | 244 | 41 | Assembly/seven-protein complex | D3R |
| COTV098 | 95674-96330 | 218 | DPV-W848-83, 089 | 218 | 78 | Uracil DNA glycosylase | D4R |
| COTV099 | 96372-98735 | 787 | DPV-W1170-84, 090 | 786 | 69 | DNA-independent NTPase | D5R |
| COTV100 | 98755-100647 | 630 | LSDV-Nee, 085 | 635 | 86 | Viral early transcription factor (VETF) small subunit | D6R |
| COTV101 | 100650-101135 | 161 | SWPV-Neb, 082 | 161 | 81 | RNA polymerase subunit RPO18 | D7R |
| COTV102 | 102071-101097 | 324 | MYXV-Lau, 088 | 286 | 31 | Carbonic anhydrase-like protein | D8L |
| COTV103 | 102152-102784 | 210 | DPV-W848-83, 093 | 211 | 59 | Decapping enzyme/Nudix hydrolase motif | D9R |
| COTV104 | 102781-103518 | 245 | TANV-KEN, 091 | 255 | 58 | Decapping enzyme/Nudix hydrolase motif | D10R |
| COTV105 | 105424-103520 | 634 | DPV-W1170-84, 095 | 635 | 72 | NPH-I; transcription elongation, termination, release factor | D11L |
| COTV106 | 106314-105460 | 284 | SWPV-Neb, 086 | 287 | 73 | Capping enzyme, small subunit | D12L |
| COTV107 | 107980-106331 | 549 | DPV-W848-83, 097 | 550 | 80 | Crescent/IV scaffold protein; rifampin resistance | D13L |
| COTV108 | 108460-108008 | 150 | DPV-W848-83, 098 | 151 | 64 | Viral late transcription factor VLTF-2 | A1L |
| COTV109 | 109173-108499 | 224 | MYXV-Lau, 095 | 224 | 87 | Viral late transcription factor VLTF-3 | A2L |
| COTV110 | 109397-109170 | 75 | MYXV-Lau, 096 | 75 | 68 | Thioredoxin-like protein | A2.5L |
| COTV111 | 111374-109419 | 651 | SWPV-Neb, 091 | 652 | 76 | Core wall protein | A3L |
| COTV112 | 111885-111424 | 153 | DPV-W1170-84, 102 | 151 | 42 | Core wall protein | A4L |
| COTV113 | 111925-112428 | 167 | YMTV-Amano, 089 | 165 | 57 | RNA polymerase subunit RPO19 | A5R |
| COTV114 | 113546-112425 | 373 | DPV-W848-83, 104 | 374 | 74 | Virion morphogenesis | A6L |
| COTV115 | 115720-113570 | 716 | DPV-W1170-84, 105 | 715 | 78 | Viral early transcription factor (VETF) large subunit | A7L |
| COTV116 | 115777-116555 | 292 | MYXV-Lau, 102 | 286 | 66 | Viral intermediate transcription factor VITF-3 | A8R |
| COTV117 | 116896-116660 | 78 | DPV-W1170-84, 107 | 81 | 76 | MV membrane protein | A9L |
| COTV118 | 119593-116897 | 898 | DPV-W1170-84, 108 | 915 | 64 | Core wall protein | A10L |
| COTV119 | 119608-120537 | 309 | RFV-Kas, 105 | 314 | 69 | Virus assembly, crescent formation | A11R |
| COTV120 | 121013-120546 | 155 | YMTV-Amano, 096 | 167 | 61 | Core structural protein | A12L |
| COTV121 | 121243-121031 | 70 | LSDV-Nee, 105 | 67 | 47 | MV maturation protein | A13L |
| COTV122 | 121579-121286 | 97 | MYXV-Lau, 108 | 96 | 52 | MV membrane protein | A14L |
| COTV123 | 121757-121596 | 53 | LSDV-Nee, 107 | 53 | 79 | MV membrane protein, virulence factor | A14.5L |
| COTV124 | 122028-121747 | 93 | DPV-W848-83, 114 | 94 | 57 | Assembly/seven-protein complex | A15L |
| COTV125 | 123142-122012 | 376 | DPV-W848-83, 115 | 380 | 60 | MV entry-fusion complex | A16L |
| COTV126 | 123789-123157 | 210 | DPV-W848-83, 116 | 197 | 57 | MV membrane protein | A17L |
| COTV127 | 123804-125240 | 478 | DPV-W1170-84, 117 | 482 | 60 | DNA helicase, transcription elongation factor | A18R |
| COTV128 | 125451-125221 | 76 | DPV-W848-83, 118 | 75 | 66 | Unknown | A19L |
| COTV129 | 125795-125454 | 113 | DPV-W848-83, 119 | 115 | 61 | MV entry-fusion complex | A21L |
| COTV130 | 125794-127089 | 431 | DPV-W1170-84, 120 | 428 | 51 | DNA polymerase processivity factor | A20R |
| COTV131 | 127061-127564 | 167 | DPV-W848-83, 121 | 181 | 79 | Holliday junction resolvase | A22R |
| COTV132 | 127587-128735 | 382 | RFV-Kas, 118 | 385 | 59 | Viral intermediate transcription factor VITF-3 | A23R |
| COTV133 | 128762-132241 | 1159 | DPV-W1170-84, 123 | 1155 | 83 | RNA polymerase subunit RPO132 | A24R |
| COTV134 | 132944-132231 | 237 | DPV-W848-83, 124 | 137 | 45 | MV attachment protein | A27L |
| COTV135 | 133367-132945 | 140 | DPV-W848-83, 125 | 140 | 69 | MV entry-fusion complex | A28L |
| COTV136 | 134290-133382 | 302 | DPV-W848-83, 126 | 300 | 64 | RNA polymerase subunit RPO35 | A29L |
| COTV137 | 134486-134274 | 70 | DPV-W848-83, 127 | 75 | 67 | Assembly/seven-protein complex | A30L |
| COTV138 | 134625-134494 | 43 | LSDV-Nee, 122‡ | 41 | 44 | Hypothetical protein | — |
| COTV139 | 134659-135417 | 252 | DPV-W848-83, 129 | 254 | 82 | DNA packaging protein | A32L |
| COTV140 | 135513-136058 | 181 | CMLV-M96, 154 | 184 | 33 | EV envelope glycoprotein | A33R |
| COTV141 | 136071-136610 | 179 | SWPV-Neb, 121 | 169 | 37 | EV envelope glycoprotein | A34R |
| COTV142 | 136636-137196 | 186 | SWPV-Neb, 122 | 185 | 42 | MHC class II inhibitor | A35R |
| COTV143 | 137204-138061 | 285 | SWPV-Neb, 123 | 314 | 30 | Concanavalin-like precursor | — |
| COTV144 | 138116-138703 | 195 | SWPV-Neb, 124‡ | 199 | 26 | WV transmembrane phosphoprotein | A36R |
| COTV145 | 138735-139562 | 275 | SWPV-Neb, 125 | 280 | 40 | Unknown | A37R |
| COTV146 | 140356-139559 | 265 | MYXV-Lau, 133 | 299 | 29 | CD47-like protein | A38L |
| COTV147 | 140358-140765 | 135 | DPV-W1170-84, 137 | 138 | 39 | Unknown | E7R |
| COTV148 | 140819-141226 | 135 | SPPV-TU, 129 | 161 | 47 | Superoxide dismutase-like protein | A45R |
| COTV149 | 141254-142933 | 559 | DPV-W848-83, 143 | 562 | 58 | DNA ligase | A50R |
| COTV150 | 142968-143522 | 184 | DPV-W848-83, 146 | 188 | 37 | Unknown | — |
| COTV151 | 143628-144653 | 341 | SPPV-TU, 134 | 335 | 36 | Unknown | A51R |
| COTV152 | 144592-145248 | 218 | MYXV-Lau, 144 | 192 | 45 | Toll/IL-1-receptor protein | A52R |
| COTV153 | 145319-145783 | 154 | LSDV-Nee, 138 | 186 | 32 | Ig domain OX-2-like protein | A56R |
| COTV154 | 145827-146585 | 252 | YLDV-Davis, 145 | 309 | 62 | Serine/threonine kinase | B1R |
| COTV155 | 146697-147482 | 261 | MYXV-Lau, 148 | 234 | 35 | Ubiquitin ligase/host defense modulator | — |
| COTV156 | 147526-147900 | 124 | MYXV-SG33, 150 | 138 | 42 | Apoptosis inhibitor/NF-κB inhibitor | N1L |
| COTV157 | 147929-148804 | 291 | SPPV-TU, 140 | 302 | 39 | Tyrosine kinase-like protein | — |
| COTV158 | 148846-150717 | 623 | DPV-W848-83, 159 | 641 | 38 | Ankyrin repeat protein/PRANC domain | B4R |
| COTV159 | 150755-152152 | 465 | LSDV-Nee, 150 | 447 | 25 | Ankyrin repeat protein | — |
| COTV160 | 152167-152511 | 114 | DPV-W1170-84, 166 | 94 | 39 | Unknown | — |
| COTV161 | 152910-154166 | 418 | CPXV-GER91, 042 | 424 | 51 | Phospholipase-D-like protein/nicking-joining enzyme | K4L |
| COTV162 | 154599-156752 | 717 | MYXV-Lau, 152 | 675 | 21 | Ankyrin repeat protein | B4R |
| COTV163 | 157738-163266 | 1842 | CPXV-AUS1999-867, 206 | 1924 | 57 | Surface glycoprotein | — |
| COTV164 | 163359-165197 | 612 | LSDV-LW1959, 150 | 636 | 25 | Ankyrin repeat protein/PRANC domain | — |
| COTV165 | 165751-166758 | 335 | CPXV-GER91-3, 195 | 346 | 55 | Serine protease inhibitor (SPI-2/CrmA) | B13R/B14R |
| COTV166 | 166831-167283 | 150 | CPXV-GER91-3, 196 | 149 | 65 | NF-κB inhibitor/virulence factor | B15R |
| COTV167 | 167398-168453 | 351 | CPXV-AUS1999-867, 204 | 373 | 57 | Serine protease inhibitor-like SPI-1 | C12L |
| COTV168 | 168481-169059 | 192 | CPXV-BR, 014 | 202 | 42 | TNF-like receptor protein (CrmB) | C22L/B28R |
| COTV169 | 169738-170427 | 229 | DPV-W1170-84, 009 | 244 | 45 | Alpha-amanitin sensitivity protein | N2L |
| COTV170 | 170429-171274 | 281 | Leopardus pardalis, gene FLA-I/SQPV-I2L* | 338/344 | 26/22 | MHC class I antigen | — |
| COTV171 | 171578-172432 | 284 | ECTV-Mos, 004 | 273 | 29 | Kelch-like protein | A55R |
| COTV172 | 172718-173539 | 273 | CPXV-UK2000-K2984, 009 | 273 | 31 | Kelch-like protein | A55R |
| COTV173 | 173862-174230 | 122 | RFV-Kas, 004/163 | 125 | 49 | Unknown | — |
| COTV174 | 174284-174736 | 150 | LSDV-LW1959, 001/161 | 159 | 47 | NF-κB inhibitor/virulence factor | B15R |
| COTV175 | 174777-175559 | 260 | CPXV-GER91, 001/219 | 252 | 26 | C-C chemokine binding protein (35-kDa major secreted virus protein) | B29R |
| COTV176 | 175563-175895 | 110 | Mus musculus, gene Scya2 | 148 | 25 | C-C motif chemokine protein | — |
| COTV177 | 175910-176941 | 343 | MPXV-ZAR-1979, 005/187 | 354 | 37 | Soluble IFNα/β receptor | B19R |
| COTV178 | 176965-177339 | 124 | YKV, 178 | 127 | 42 | TNF receptor-like protein (CrmE) | — |
| COTV179 | 177363-177866 | 167 | CPXV-UK2000-K2984, 003 | 355 | 25 | TNF receptor-like protein (CrmB) | C22L/B28R |
| COTV180 | 177895-178527 | 210 | Bos taurus, locus BOS_3916/CNPV-VR111, 170* | 212/212 | 55/50 | Thymidylate kinase | A48R |
| COTV181 | 178580-180280 | 566 | DPV-W848-83, 019 | 643 | 25 | Ankyrin repeat protein/PRANC domain | B4R |
| COTV182 | 180629-181423 | 264 | CPXV-NOR1994-MAN, 002 | 245 | 28 | Chemokine binding protein | C23L/B29R |
| COTV183 | 181427-181729 | 100 | Mustela putorius furo, gene CCL13 | 70 | 35 | C-C motif chemokine protein | — |
| COTV184 | 182276-183988 | 570 | DPV-W1170-84, 019 | 643 | 25 | Ankyrin repeat protein | B4R |
| COTV185 | 184018-184569 | 183 | CPXV-GER1980-EP4, 205 | 188 | 32 | Serine protease inhibitor -like protein | C13L/C14L |
ORFs in boldface are conserved in all chordopoxviruses and encode proteins used for analysis of phylogeny.
Best-matching protein sequences obtained by BLASTP-NCBI. YKV, Yoka poxvirus; VARV, variola virus; ECTV, ectromelia virus; AMEV, Amsacta moorei entomopoxvirus; SPPV, sheeppox virus; TANV, tanapox virus; SQPV, squirrel poxvirus; CMLV, camelpox virus. Symbols: *, the highest score obtained with a cellular protein, but a significant match also with a poxvirus protein, which is indicated after a slash; ‡, no significant match was obtained using BLASTP-NCBI, and scores were obtained by searching the poxvirus database; —, no significant match.
% ID, percentage of amino acid identity.
PRANC, poxvirus protein repeats of ankyrin, C-terminal; TNF, tumor necrosis factor; Crm, cytokine response modifier; IL-1, interleukin-1; eIF2α, α subunit of eukaryotic initiation factor 2; dsRNA, double-stranded RNA; NPH, nucleoside triphosphate phosphohydrolase; NTPase, nucleoside triphosphatase.
—, no VACV-Cop ortholog. In the absence of an ortholog in VACV-Cop, an ortholog in VACV-WR is indicated if available.
Notable genes in the COTV genome possibly related to immunomodulation and virulence.
COTV has an interesting panel of ORFs possibly related to immunomodulation, virulence, and host range (Table 2; Fig. 5). It encodes 5 serine protease inhibitor (serpin)-like proteins, 6 kelch-like proteins, 4 C-C chemokine binding proteins, 4 C-C chemokine-like proteins, and 13 proteins with ankyrin repeats, 5 of which possess C-terminal PRANC domains (F-box-like domains). COTV also codes for proteins related to the evasion of the interferon (IFN) response, such as protein kinase R (PKR) inhibitors, encoded by COTV029 and COTV051 (orthologs of VACV-Cop K3L and E3L), IFN-γ receptor-like protein (COTV017), and three IFN-α/β receptor-like proteins (COTV009, COTV020, and COTV177). Interestingly, COTV009 and COTV177 are duplicated copies of the same gene located in the ITRs, but COTV020 is a different ortholog, sharing 29% amino acid identity with COTV009 and COTV177. Other proteins are complement binding protein (COTV026), major histocompatibility complex class II (MHC-II) inhibitor (COTV142), concanavalin-like precursor (COTV143), CD47-like protein (COTV146), Ig domain OX-2-like protein (COTV153), and MHC-I antigen-like protein (COTV170).
Also present in the COTV genome are genes related to NF-κB inhibition, such as orthologs of VACV-Cop M2L (COTV028), K1L (COTV031), B15R (COTV166 and COTV174), and N1L (COTV156), and ORFs involved in the modulation of apoptosis: COTV034 and COTV165 are predicted to encode orthologs of the apoptosis regulatory protein of YMTV (VACV-Cop F1L) and the serpin CrmA/SPI-2 of CPXV, respectively. We also identified five tumor necrosis factor (TNF) receptor-like proteins: COTV007 and COTV179 (orthologs of CPXV CrmB), COTV008 and COTV178 (orthologs of CPXV CrmE), and COTV168 (ortholog of a CrmB pseudogene of CPXV). Of these, only the orthologs of CrmE have typical TNF-binding domains and are likely to be functional.
Notably, the COTV genome lacks an ortholog of VACV-Cop B5R, which is involved in the wrapping of intracellular MV to generate WV and in the formation of EV (26, 53). Nevertheless, COTV encodes all other proteins involved in these final stages of WV and EV formation, i.e., orthologs of VACV-Cop F12L, F13L, E2L, A33R, A34R, and A36R (COTV043, COTV044, COTV050, COTV140, COTV141, and COTV144), as well as of F11L (COTV042), recently shown to be involved in VACV release (18).
Novel genes unique to the COTV genome.
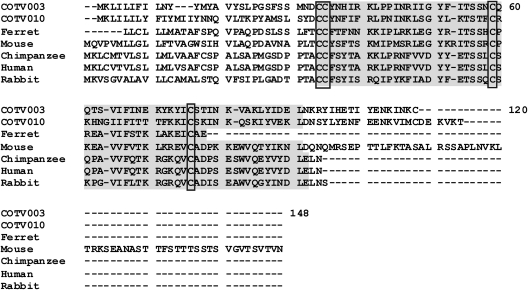
COTV contains 6 genes that are predicted to encode proteins with no homologs within poxviruses (Table 2). COTV021 and COTV045 encode proteins with no significant homology to any sequences currently in the databases. On the other hand, COTV003/COTV183 and CTOV010/COTV176 are duplicated genes located in the ITRs and are predicted to encode C-C chemokine-like proteins of 11.9 kDa and 13.16 kDa, respectively. COTV003/COTV183 are similar to C-C chemokine ligand 13 (CCL13) of the ferret (Mustela putorius furo) (BLASTP E value, 9e−06). The predicted COTV protein has a secretory signal peptide, two putative glycosaminoglycan (GAG) binding sites, and one putative receptor-binding site, in addition to other domains characteristic of C-C chemokines, all inserted within the chemokine superfamily domain (Fig. 6). CTOV010/COTV176 are similar to the small inducible cytokine A2 precursor of the rodent Mus musculus (BLASTP E value, 8e−05), and although a secretory peptide signal is predicted and a chemokine superfamily domain is detected by the Pfam and Smart databases, no GAG-binding domains or receptor-binding domains were identified.
Fig 6.
Amino acid alignment of COTV C-C motif chemokine-like proteins (COTV003 and COTV010) with cellular homologs. The chemokine superfamily domain detected by Pfam, Smart, and InterPro is shaded. The four conserved cysteine residues involved in the formation of two disulfide bonds are boxed. Amino acid positions are indicated on the right. GenBank accession numbers are as follows: Mustela putorius furo (ferret) C-C motif chemokine ligand 13, ACJ54430.1; Mus musculus (mouse) small inducible cytokine A2 precursor, AAF15379.1; Pan troglodytes (chimpanzee) C-C motif chemokine 4 isoform 3, XP_001173914; Homo sapiens (human) Act-2 cytokine, AAB00790; Oryctolagus cuniculus (rabbit) C-C motif chemokine ligand 3-like, XP_002719292.
COTV may represent a novel poxvirus genus.
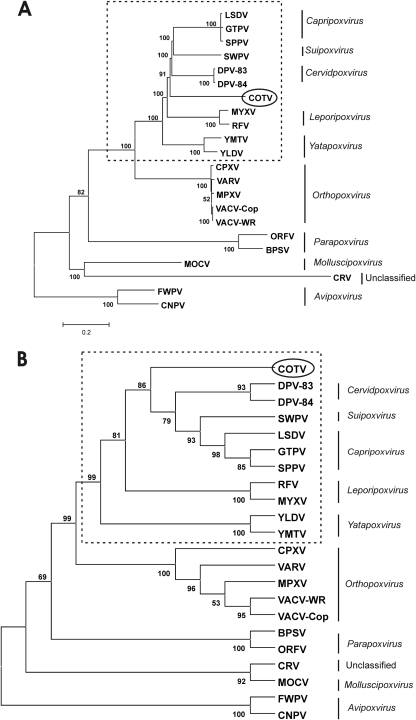
To infer COTV phylogeny, the predicted amino acid sequences of the 90 proteins conserved in all chordopoxviruses were aligned with their respective orthologs from the members of each genus within the Chordopoxvirinae. A concatenated alignment was constructed based on the individual alignments, comprising 30,717 amino acids. The overall identity scores of the concatenated sequence were highest with Cervidpoxvirus (66.1%), Capripoxvirus (65.2%), and Suipoxvirus (64.8%), although the individual scores for each protein differed considerably (Table 2). Figure 7 shows phylogenetic trees reconstructed from the concatenated amino acid data sets, using the neighbor-joining (Fig. 7A) and maximum-likelihood (Fig. 7B) methods. We observed that COTV grouped as an independent branch of the Chordopoxvirinae supported with high bootstrap values, clearly excluding its classification within established poxvirus genera. Nevertheless, COTV branched within a main clade that included Capripoxvirus, Suipoxvirus, Yatapoxvirus, Leporipoxvirus, and Cervidpoxvirus (the CSYLC clade) and distant from another chordopoxvirus clade corresponding to the Orthopoxvirus genus. Identical topology with high bootstrap value support was also obtained for most parsimony trees (data not shown). Maximum-likelihood distances were estimated for the nucleotide sequences comprising the region limited by VACV orthologs F9L to A34R, and the distances between COTV and cervidpoxviruses, capripoxviruses, and SWPV ranged from 0.869 to 1.088. They were comparable to the distances between distinct genera of the CSYLC clade (0.691 to 1.219) but higher than the estimates for species of the same genus (0.018 to 0.258).
Fig 7.
Phylogenetic inference of COTV. The concatenated data set was obtained by combining the individual alignments of the predicted amino acid sequences for 90 genes conserved in 22 chordopoxviruses (ORFs in boldface in Table 2). The combined alignment was used to construct the neighbor-joining tree, opting for the JTT model of substitution, with 2,500 bootstrap replicates, using MEGA 4 (A), and the maximum-likelihood tree, opting for WAG correction for multiple substitutions, 10,000 quartet puzzling steps, and the gamma heterogeneity model (B). The bar in panel A represents relative genetic distance. The poxvirus genera and subfamilies are given on the right. Dashed boxes enclose the poxvirus CSYLC clade, which includes Capripoxvirus, Suipoxvirus, Yatapoxvirus, Leporipoxvirus, and Cervidpoxvirus.
It is noteworthy that several COTV genes related to immunomodulation and virulence were more similar to their Orthopoxvirus counterparts than to those in the CSYLC clade (Table 2). Nevertheless, this fact did not alter the clustering of COTV within the CSYLC clade, as determined by the analysis of a phylogeny tree based on the genome nucleotide sequence (data not shown).
In support of the results of the phylogenetic inference, a common genomic feature shared by all members of the CSYLC clade, which is also present in COTV, is worth noting. In this clade, the VACV-Cop C7L homolog (one or more copies in tandem) is inserted between homologs of the VACV-Cop J2R and J3R genes (www.poxvirus.org). COTV has one copy of the C7L homolog (COTV083) in this position, but it also contains a different ortholog of this gene at the 5′-terminal region of the genome (COTV024), unlike other members of the clade (Table 2). In addition, COTV and all clade members, except for DPV, lack orthologs of VACV-Cop A31R and A40R. Similarly, COTV and all CSYLC members but SWPV lack the large subunit of the ribonucleotide reductase (VACV-Cop I4L).
Nevertheless, unlike all members of the CSYLC clade, COTV does not encode a homolog of VACV-Cop B5R, a PHD finger protein, and unlike all CSYLC members except leporipoxviruses, COTV does not encode a G protein-coupled receptor (GPCR) protein (www.poxvirus.org). On the other hand, the COTV genome contains orthologs of VACV-Cop C3L, K1L, and A48R, which are not carried by members of the CSYLC clade (Table 2). Together, these data reinforce the relatedness of COTV to the members of the CSYLC clade but also emphasize the unfeasibility of grouping it within an established genus.
DISCUSSION
Novel poxvirus infections have been reported frequently in Brazil (49). Most agents have been characterized as VACV related to the Cantagalo strain (20, 47, 51, 61), whereas others, such as Cotia SPAn232/SAV, have been reported to be related to VACV strain WR (19). Nevertheless, COTV SPAn232 has been studied by different research groups with conflicting results, and its classification has been a matter of discussion (19, 28, 42, 66, 67). In this work, we present a comprehensive study of COTV SPAn232 biology, combining an evaluation of the virus replicative cycle with serologic analyses and high-throughput genome sequencing. Together, these data suggest the classification of COTV as a novel genus within the Poxviridae, closely related to Capripoxvirus, Suipoxvirus, Yatapoxvirus, Leporipoxvirus, and Cervidpoxvirus (the CSYLC clade).
Using a combination of two next-generation sequencing strategies, 454 pyrosequencing and Illumina sequencing, we determined the nucleotide sequence of the 185,139-bp genome. The COTV genome is the largest in the CSYLC clade and has the longest ITRs and the highest A+T content (1, 4, 14, 15, 38, 64, 65). COTV has 185 ORFs and an intriguing assortment of genes related to virulence, host range, and immunomodulation functions. A notable feature of COTV is that nearly 30 of these genes are similar to their Orthopoxvirus counterparts, whereas all COTV genes related to essential functions in virus replication/morphogenesis have the highest identity scores with orthologs in the CSYLC clade. Actually, some of these COTV ORFs related to host interaction do not have orthologs in the CSYLC clade, and until now, genes such as C3L and K1L were considered unique to the genus Orthopoxvirus (www.poxvirus.org).
C3L encodes a secreted virus complement control protein (VCP), which is also located on the cell surface. VCP has four SCR domains, binds C3b and C4b, and plays a major role in inhibiting complement-mediated neutralization of orthopoxviruses during infection (29, 35, 36). The VCP homolog in COTV (COTV026) is predicted to be secreted and has the four SCR domains necessary for its function. K1L, in turn, encodes an ankyrin repeat protein, which is an inhibitor of NF-κB and PKR activation during VACV infection (57, 68). K1 is a host range protein essential for VACV replication in human cells. However, in the absence of K1 expression, the product of the VACV host range gene C7L can rescue virus replication in human cells but not in rabbit kidney cells (50, 58). COTV031 encodes an ortholog of VACV-Cop K1L with predicted ankyrin repeat domains, which is likely to be functional. Unlike K1L, which was considered unique to Orthopoxvirus until now, C7L has orthologs in both Orthopoxvirus and the CSYLC clade, but in different genomic locations (www.poxvirus.org). In COTV, there are two distinct C7L orthologs. COTV083 is located between the thymidine kinase gene (COTV082) and the small subunit of the poly(A) polymerase gene (COTV084), as in all members of the CSYLC clade. The highest identity score matches YLDV-Davis gene 069. Nevertheless, the other C7L ortholog (COTV024) is located at the 5′ end of the genome, as in all members of the genus Orthopoxvirus, but in contrast to members of the CSYLC clade. In this case, the highest identity score matches VACV-WR gene 021.
Taken together, these intriguing features allow us to speculate whether COTV could have a wider tropism than other members of the CSYLC clade. These viruses, particularly members of the genera Capripoxvirus, Suipoxvirus, and Cervidpoxvirus, usually have a restricted host range in cell culture compared to that of Orthopoxvirus (7, 10, 32, 33, 43, 45). Nonetheless, in support of our assumption, our results show that COTV propagates in a relatively wide range of cell types. In BSC-40 (monkey) and C6 (rat) cells, virus yields increased >100-fold within the first 24 h of infection, as observed by time course analysis of progeny production. Interestingly, discrepant progeny yields were obtained in cells derived from the same species, such as C6 and Rat-2.
Nevertheless, analysis of the complete set of COTV genes is not sufficient to support an inference of its possible natural hosts. In this regard, COTV encodes two completely novel proteins within the Poxviridae. COTV003/183 and COTV010/176 encode C-C chemokine-like proteins similar to homologs in ferret and mouse, respectively. In addition, COTV also encodes an MHC class I heavy chain protein (COTV170), which is more similar to the MHC-I homolog of Leopardus pardalis (BLASTP E value, 2e−18) than to those of squirrelpox virus (SQPV) (E value, 7e−17) and MOCV (E value, 1e−12). Interestingly, L. pardalis is a wild cat typical of the neotropical forests of South and Central America. Although these three genes are thought to have been acquired from vertebrate hosts, the discrepant origins do not allow any further inference. Even so, the presence of two novel C-C chemokine-like genes in the genome highlights an important role of this pathway in COTV pathogenesis. This is particularly evident because COTV also encodes four poxvirus-like C-C chemokine-binding proteins. The chemokine network regulates several immune and inflammatory functions in vertebrates, such as cell recruitment, angiogenesis, and T-cell differentiation (13). Poxviruses have developed strategies to modulate this pathway, but only MOCV, Avipoxvirus, and COTV encode C-C chemokine-like proteins, although they do not share significant identity levels (3, 55, 63).
Despite the presence of virulence/immunomodulatory genes with high identity to orthologs in Orthopoxvirus, COTV is clearly less cytopathic than orthopoxviruses, developing noncytolytic patterns of infection similar to those of members of the CSYLC clade (8, 43, 48). Cells infected with COTV induced moderate inhibition of host translation and generated CPE much later than did VACV infection. In addition, COTV-induced CPE started at different times postinfection in BSC-40 and C6 cells, despite similar kinetics of virus production and gene expression. These results may suggest distinct interactions with host proteins involved in the process of cellular morphological changes during COTV infection. Indeed, different patterns of cytoskeleton rearrangement seem to occur in BSC-40- and C6-infected cells, and this issue is currently being investigated in detail (P. P. Afonso, C. Mermelstein, N. Cunha-e-Silva, and C. R. Damaso, unpublished data).
In addition, COTV produced tiny virus plaques only after 8 days of infection, suggesting a deficient mechanism of cell-to-cell spread compared to that of VACV. A notable feature of COTV that may be involved in this process is the lack of an ortholog of VACV-Cop B5R, which is present in all orthopoxviruses and in all members of the CSYLC clade. B5 is an EV membrane glycoprotein with four SCR domains. Together with A33, A34, A36, F12, F13, and E2, B5 plays an important role in the wrapping of MV through the trans-Golgi network or late endosomes to generate WV, as well in the production of EV, responsible for the cell-to-cell spread of infection (26, 53). In the absence of B5 expression, VACV forms less WV and EV, small plaques, and few actin tails, reducing virus spread (26, 69). Interestingly, despite the absence of an ortholog of B5R in COTV, we consistently detected WV and EV in infected cells by transmission electron microscopy. In addition, studies in progress in our laboratory show that COTV induces the formation of actin tails. Their involvement in the spread of virus infection in BSC-40 and C6 cells is currently being addressed (Afonso et al., unpublished). As mentioned above, it is also interesting that, unlike all members of the CSYLC clade, COTV encodes an ortholog of VACV C3L (COTV026), which, like B5R, encodes a protein containing four SCR domains. These domains in B5 are important for the formation of actin tails during VACV infection (44). Nevertheless, C3 does not replace B5 in Orthopoxvirus (26, 69). Whether the COTV ortholog of C3 could somehow participate in the process of virus release and spread has yet to be investigated.
Our phylogenetic inferences support the suggestion that COTV represents a novel genus within the CSYLC clade and is therefore distant from the Orthopoxvirus clade. Previous work from Ueda's and Esposito's laboratories had hypothesized that COTV could be a member of a novel poxvirus genus (28, 66, 67). Consistent with their findings, we did not observe significant cross-reaction or cross-neutralization of an anti-COTV antiserum with VACV, MYXV, or SWPV proteins. In addition, COTV orthologs of VACV J2R and G1L were 100% identical to those present in Ueda's (67) and Damon's (40) samples, respectively (the latter was the same sample used by Esposito's group at the CDC, Atlanta, GA). The results presented here particularly conflict with a previous report characterizing COTV SPAn232 as a VACV strain named SAV, closely related to VACV-WR (17). This noticeable divergence of data could be related to the possible presence of VACV in the COTV sample of Kroon's group (62). In this case, the selection of VACV plaques during the plaque purification procedure would have been favored because VACV plaques appear much earlier than COTV plaques, as shown here. Nevertheless, the hypothesis of COTV and VACV coinfection was ruled out for the samples sent to our laboratory. The original brain extracts of mice, as well as all COTV passages up to the last cycle of virus plaque purification, were tested by PCR and found positive for detection of the COTV TK gene but negative for amplification of the Orthopoxvirus hemagglutinin (HA) gene, even in seminested PCR assays (data not shown).
It is interesting to speculate about COTV infection in nature. Reisolation has not been reported following the initial virus isolation in the 1960s. Therefore, there is no evidence suggestive of COTV circulation in Brazil, although we cannot exclude the possibility of asymptomatic hosts carrying the virus in the wild. Serological and molecular surveys in Cotia field station, São Paulo, Brazil, could shed light on this subject and reveal asymptomatic carriers seropositive for COTV. In sum, we present evidence that COTV may represent a member of a new genus with a unique set of genes devoted to host range, virulence, and immunomodulatory functions. This study opens up the possibility of uncovering novel and unique biological features of the Poxviridae.
ACKNOWLEDGMENTS
We thank Akemi Suzuki, Luiz Eloy Pereira, Terezinha Lisieux Coimbra, and Marli Ueda (Instituto Adolfo Lutz, São Paulo, Brazil) for providing COTV SPAn232 samples and sharing unpublished data and essential information on COTV history; Richard Moyer and Grant McFadden for virus samples and antiserum; Lilian Ayres for sequencing support at UMG-IBCCF; Thaís Souto-Padrón for the use of the Morgani 268 electron microscope; and the late Ademilson Bizerra for technical assistance.
This work was supported by grants from CNPq, MAPA, INPeTAm, and Faperj to C.R.D. and by grant NIH R01 AI055560 to R.C.C. P.P.A. received fellowships from Faperj and CNPq. P.M.S. and L.C.S. were recipients of fellowships from Capes and Faperj. D.M.J. received a fellowship from CNPq.
Footnotes
Published ahead of print 15 February 2012
REFERENCES
- 1. Afonso CL, et al. 2005. Genome of deerpox virus. J. Virol. 79:966–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Afonso CL, et al. 2006. Genome of crocodilepox virus. J. Virol. 80:4978–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Afonso CL, et al. 2000. The genome of fowlpox virus. J. Virol. 74:3815–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Afonso CL, et al. 2002. The genome of swinepox virus. J. Virol. 76:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afonso CL, et al. 2002. The genome of camelpox virus. Virology 295:1–9 [DOI] [PubMed] [Google Scholar]
- 6. Antoine G, Scheiflinger F, Dorner F, Falkner FG. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365–396 [DOI] [PubMed] [Google Scholar]
- 7. Aspden K, Passmore JA, Tiedt F, Williamson AL. 2003. Evaluation of lumpy skin disease virus, a capripoxvirus, as a replication-deficient vaccine vector. J. Gen. Virol. 84:1985–1996 [DOI] [PubMed] [Google Scholar]
- 8. Babiuk S, et al. 2007. Evaluation of an ovine testis cell line (OA3Ts) for propagation of capripoxvirus isolates and development of an immunostaining technique for viral plaque visualization. J. Vet. Diagn. Invest. 19:486–491 [DOI] [PubMed] [Google Scholar]
- 9. Bablanian R. 1984. Comprehensive virology, vol 19. Poxvirus cytopathogenicity: effects on cellular macromolecular synthesis, p 391–402 Plenum, New York, NY [Google Scholar]
- 10. Barcena J, Blasco R. 1998. Recombinant swinepox virus expressing beta-galactosidase: investigation of viral host range and gene expression levels in cell culture. Virology 243:396–405 [DOI] [PubMed] [Google Scholar]
- 11. Bawden AL, et al. 2000. Complete genomic sequence of the Amsacta moorei entomopoxvirus: analysis and comparison with other poxviruses. Virology 274:120–139 [DOI] [PubMed] [Google Scholar]
- 12. Benson G. 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27:573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boomker JM, de Leij LF, The TH, Harmsen MC. 2005. Viral chemokine-modulatory proteins: tools and targets. Cytokine Growth Factor Rev. 16:91–103 [DOI] [PubMed] [Google Scholar]
- 14. Brunetti CR, et al. 2003. Complete genomic sequence and comparative analysis of the tumorigenic poxvirus Yaba monkey tumor virus. J. Virol. 77:13335–13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cameron C, et al. 1999. The complete DNA sequence of myxoma virus. Virology 264:298–318 [DOI] [PubMed] [Google Scholar]
- 16. Castro AP, Carvalho TM, Moussatche N, Damaso CR. 2003. Redistribution of cyclophilin A to viral factories during vaccinia virus infection and its incorporation into mature particles. J. Virol. 77:9052–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Condit RC, Moussatche N, Traktman P. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 66:31–124 [DOI] [PubMed] [Google Scholar]
- 18. Cordeiro JV, et al. 2009. F11-mediated inhibition of RhoA signalling enhances the spread of vaccinia virus in vitro and in vivo in an intranasal mouse model of infection. PLoS One 4:e8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. da Fonseca FG, et al. 2002. Characterization of a vaccinia-like virus isolated in a Brazilian forest. J. Gen. Virol. 83:223–228 [DOI] [PubMed] [Google Scholar]
- 20. Damaso CR, Esposito JJ, Condit RC, Moussatche N. 2000. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology 277:439–449 [DOI] [PubMed] [Google Scholar]
- 21. Damaso CR, Moussatche N. 1998. Inhibition of vaccinia virus replication by cyclosporin A analogues correlates with their affinity for cellular cyclophilins. J. Gen. Virol. 79(Pt 2):339–346 [DOI] [PubMed] [Google Scholar]
- 22. Damaso CR, Moussatche N. 1992. Protein synthesis in vaccinia virus-infected cells. I. Effect of hypertonic shock recovery. Arch. Virol. 123:295–308 [DOI] [PubMed] [Google Scholar]
- 23. Damaso CR, Oliveira MF, Massarani SM, Moussatche N. 2002. Azathioprine inhibits vaccinia virus replication in both BSC-40 and RAG cell lines acting on different stages of virus cycle. Virology 300:79–91 [DOI] [PubMed] [Google Scholar]
- 24. Drumond BP, et al. 2008. Brazilian vaccinia virus strains are genetically divergent and differ from the Lister vaccine strain. Microbes Infect. 10:185–197 [DOI] [PubMed] [Google Scholar]
- 25. Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engelstad M, Smith GL. 1993. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology 194:627–637 [DOI] [PubMed] [Google Scholar]
- 27. Esposito JJ, Fenner F. 2001. Poxviruses, p 2885–2921 In Knipe DM, et al. (ed), Fields virology, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 28. Esposito JJ, et al. 1980. Studies on the poxvirus Cotia. J. Gen. Virol. 47:37–46 [DOI] [PubMed] [Google Scholar]
- 29. Girgis NM, et al. 2008. Cell surface expression of the vaccinia virus complement control protein is mediated by interaction with the viral A56 protein and protects infected cells from complement attack. J. Virol. 82:4205–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gubser C, Hue S, Kellam P, Smith GL. 2004. Poxvirus genomes: a phylogenetic analysis. J. Gen. Virol. 85:105–117 [DOI] [PubMed] [Google Scholar]
- 31. Gubser C, Smith GL. 2002. The sequence of camelpox virus shows it is most closely related to variola virus, the cause of smallpox. J. Gen. Virol. 83:855–872 [DOI] [PubMed] [Google Scholar]
- 32. Hu Y, et al. 2001. Yaba-like disease virus: an alternative replicating poxvirus vector for cancer gene therapy. J. Virol. 75:10300–10308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnston JB, Nazarian SH, Natale R, McFadden G. 2005. Myxoma virus infection of primary human fibroblasts varies with cellular age and is regulated by host interferon responses. Virology 332:235–248 [DOI] [PubMed] [Google Scholar]
- 34. Kara PD, et al. 2003. Comparative sequence analysis of the South African vaccine strain and two virulent field isolates of lumpy skin disease virus. Arch. Virol. 148:1335–1356 [DOI] [PubMed] [Google Scholar]
- 35. Kotwal GJ, Isaacs SN, McKenzie R, Frank MM, Moss B. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250:827–830 [DOI] [PubMed] [Google Scholar]
- 36. Kotwal GJ, Moss B. 1988. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature 335:176–178 [DOI] [PubMed] [Google Scholar]
- 37. Lassmann T, Sonnhammer EL. 2005. Kalign—an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics 6:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee HJ, Essani K, Smith GL. 2001. The genome sequence of Yaba-like disease virus, a yatapoxvirus. Virology 281:170–192 [DOI] [PubMed] [Google Scholar]
- 39. Li G, et al. 2005. Complete coding sequences of the rabbitpox virus genome. J. Gen. Virol. 86:2969–2977 [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Meyer H, Zhao H, Damon IK. 2010. GC content-based pan-pox universal PCR assays for poxvirus detection. J. Clin. Microbiol. 48:268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Likos AM, et al. 2005. A tale of two clades: monkeypox viruses. J. Gen. Virol. 86:2661–2672 [DOI] [PubMed] [Google Scholar]
- 42. Lopes ODS, et al. 1965. Cotia virus: a new agent isolated from sentinel mice in São Paulo, Brazil. Am. J. Trop. Med. Hyg. 14:156–157 [DOI] [PubMed] [Google Scholar]
- 43. Massung RF, Moyer RW. 1991. The molecular biology of swinepox virus. II. The infectious cycle. Virology 180:355–364 [DOI] [PubMed] [Google Scholar]
- 44. Mathew E, Sanderson CM, Hollinshead M, Smith GL. 1998. The extracellular domain of vaccinia virus protein B5R affects plaque phenotype, extracellular enveloped virus release, and intracellular actin tail formation. J. Virol. 72:2429–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McFadden G. 2005. Poxvirus tropism. Nat. Rev. Microbiol. 3:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McInnes CJ, et al. 2006. Genomic characterization of a novel poxvirus contributing to the decline of the red squirrel (Sciurus vulgaris) in the UK. J. Gen. Virol. 87:2115–2125 [DOI] [PubMed] [Google Scholar]
- 47. Medaglia ML, Pessoa LC, Sales ER, Freitas TR, Damaso CR. 2009. Spread of Cantagalo virus to northern Brazil. Emerg. Infect. Dis. 15:1142–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mediratta S, Essani K. 1999. The replication cycle of tanapox virus in owl monkey kidney cells. Can. J. Microbiol. 45:92–96 [DOI] [PubMed] [Google Scholar]
- 49. Moussatche N, Damaso CR, McFadden G. 2008. When good vaccines go wild: feral orthopoxvirus in developing countries and beyond. J. Infect. Dev. Ctries. 2:156–173 [DOI] [PubMed] [Google Scholar]
- 50. Perkus ME, et al. 1990. Vaccinia virus host range genes. Virology 179:276–286 [DOI] [PubMed] [Google Scholar]
- 51. Quixabeira-Santos JC, Medaglia ML, Pescador CA, Damaso CR. 2011. Animal movement and establishment of vaccinia virus Cantagalo strain in Amazon biome, Brazil. Emerg. Infect. Dis. 17:726–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rempel RE, Anderson MK, Evans E, Traktman P. 1990. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J. Virol. 64:574–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roberts KL, Smith GL. 2008. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 16:472–479 [DOI] [PubMed] [Google Scholar]
- 54. Schmidt HA, Strimmer K, Vingron M, von Haeseler A. 2002. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18:502–504 [DOI] [PubMed] [Google Scholar]
- 55. Senkevich TG, et al. 1996. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science 273:813–816 [DOI] [PubMed] [Google Scholar]
- 56. Shchelkunov SN, et al. 2000. Alastrim smallpox variola minor virus genome DNA sequences. Virology 266:361–386 [DOI] [PubMed] [Google Scholar]
- 57. Shisler JL, Jin XL. 2004. The vaccinia virus K1L gene product inhibits host NF-κB activation by preventing IκBα degradation. J. Virol. 78:3553–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sutter G, Ramsey-Ewing A, Rosales R, Moss B. 1994. Stable expression of the vaccinia virus K1L gene in rabbit cells complements the host range defect of a vaccinia virus mutant. J. Virol. 68:4109–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 60. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Trindade GDS, et al. 2003. Aracatuba virus: a vaccinialike virus associated with infection in humans and cattle. Emerg. Infect. Dis. 9:155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Trindade GS, Emerson GL, Carroll DS, Kroon EG, Damon IK. 2007. Brazilian vaccinia viruses and their origins. Emerg. Infect. Dis. 13:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tulman ER, et al. 2004. The genome of canarypox virus. J. Virol. 78:353–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tulman ER, et al. 2001. Genome of lumpy skin disease virus. J. Virol. 75:7122–7130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tulman ER, et al. 2002. The genomes of sheeppox and goatpox viruses. J. Virol. 76:6054–6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ueda Y, Dumbell KR, Tsuruhara T, Tagaya I. 1978. Studies on Cotia virus—an unclassified poxvirus. J. Gen. Virol. 40:263–276 [DOI] [PubMed] [Google Scholar]
- 67. Ueda Y, Morikawa S, Watanabe T. 1995. Unclassified poxvirus: characterization and physical mapping of Cotia virus DNA and location of a sequence capable of encoding a thymidine kinase. Virology 210:67–72 [DOI] [PubMed] [Google Scholar]
- 68. Willis KL, Patel S, Xiang Y, Shisler JL. 2009. The effect of the vaccinia K1 protein on the PKR-eIF2α pathway in RK13 and HeLa cells. Virology 394:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wolffe EJ, Isaacs SN, Moss B. 1993. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J. Virol. 67:4732–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhao G, et al. 2011. The genome of Yoka poxvirus. J. Virol. 85:10230–10238 [DOI] [PMC free article] [PubMed] [Google Scholar]