Abstract
In mammals, leptin regulates food intake and energy balance mainly through the activation of LepRb in the hypothalamus, and estrogen has a leptin-like effect in the hypothalamic control of metabolism. However, it remains to be elucidated how estrogen signaling is intertwined with the leptin pathway. We show here that Shp2, a nonreceptor tyrosine phosphatase, acts to integrate leptin and estrogen signals. The expression of a dominant-active mutant (Shp2D61A) in forebrain neurons conferred female, but not male, transgenic mice resistance to high-fat diet (HFD)-induced obesity and liver steatosis, accompanied by improved insulin sensitivity and glucose homeostasis. Fed with either HFD or regular chow food, Shp2D61A female mice showed dramatically enhanced leptin sensitivity. Microinjection of Shp2D61A-expressing adeno-associated virus into mediobasal hypothalamus elicited a similar antiobese effect in female mice. Biochemical analyses showed a physical association of Shp2 with estrogen receptor alpha, which is necessary for the synergistic and persistent activation of Erk by leptin and estrogen. Together, these results elucidate a mechanism for the direct cross talk of leptin and estrogen signaling and offer one explanation for the propensity of postmenopausal women to develop obesity.
INTRODUCTION
In mammals, leptin regulates food intake and energy balance mainly through activation of the leptin receptor long form (LepRb) and downstream signaling pathways in the hypothalamus, and leptin deficiency causes obesity and diabetes as well as impaired reproductive functions (14, 15, 31). The SH2-tyrosine phosphatase Shp2 was shown to bind directly to activated LepRb by docking on p-Tyr985 in the intracellular domain (23, 37). Genetic ablation of Shp2 in the central nervous system resulted in gross obesity associated with leptin resistance and decreased p-Erk, suggesting a positive role of Shp2 in amplifying leptin signal in the hypothalamus (2, 22, 37).
Estradiol-17β (E2), a reproductive hormone, also plays a vital role in energy metabolism and body weight control (13). The deletion of aromatase that catalyzes the formation of estrogen or disruption of estrogen receptor α (ERα) leads to more severe age-dependent obesity in females than in males, indicating that estrogen deficiency is responsible for the progression of sexually dimorphic obesity (19, 21). In postmenopausal women or ovariectomized rodents, estrogen deficiency is associated with increased probability of obesity and type 2 diabetes (7, 32). Estrogen replacement in ovariectomized animals suppresses obese development by decreasing food intake and increasing energy expenditure (17). Hormone treatment in postmenopausal women prevents obesity progression and enhances insulin sensitivity and glucose tolerance (33). In contrast, deletion or silencing of ERα in the hypothalamus leads to obesity and metabolic defects in rodents (19, 25). Estrogen has a leptin-like effect in activation of intracellular signals in hypothalamic melanocortin cells (16). However, it remains to be determined how estrogen signaling is intertwined with the leptin pathway.
The positive role of Shp2 in mediating leptin signal in the brain predicted that pharmaceutical enhancement of Shp2 activity locally in the brain would overcome leptin resistance and alleviate obesity. To test this hypothesis, we generated a transgenic mouse line with expression in forebrain neurons of a dominantly activating (gain-of-function) mutant Shp2D61A, which was shown to exhibit dramatically increased phosphatase activity without affecting its SH2-binding activity (26). Characterization of the transgenic mice revealed an unexpectedly critical role of Shp2 in coupling leptin and estrogen signaling. These data fill in a gap in our knowledge about the overlapping function of these two hormones in metabolism and help show why postmenopausal women tend to develop obesity.
MATERIALS AND METHODS
Generation of Shp2D61A transgenic mice.
Shp2D61A mutant was created by site-directed mutagenesis using mouse Shp2 cDNA as a template. The mutant Shp2D61A fragment with an hemagglutinin (HA) tag sequence at the C terminus was subcloned into pcDNA3.1 vector. The cytomegalovirus (CMV) promoter in pcDNA3.1 plasmid was replaced by CaMKIIα promoter (12) to drive the Shp2D61A-HA mutant expression. The engineered construct was injected into the fertilized oocytes of C57BL/6 mice to generate transgenic mouse line in the animal facility of the Sanford/Burnham Medical Research Institute (SBMRI). All animal procedures were approved by the University of California, San Diego, and the SBMRI Institutional Animal Care and Use Committees.
AAV injection.
The adeno-associated virus (AAV) expression system (Stratagene) was used to produce AAV-green fluorescent protein (GFP) and AAV-Shp2D61A-GFP virus. AAV viruses were injected into the mediobasal hypothalamus of mice as previously described (38). Briefly, bilateral injection into the mediobasal hypothalamus was directed by using an ultraprecise stereotax with a resolution of 10 μm (Kopf Instruments) to the coordinates of 1.5 mm posterior to the bregma, 5.8 mm below the bregma, and 0.3 mm lateral to the midline. Viruses suspended in cerebrospinal fluid were injected over 10 min through a 26-gauge guide cannula and a 33-gauge injector (Plastics One) connected to a Hamilton Syringe and an infusion pump (WPI Instruments).
HFD feeding and ovariectomy.
Wild-type (wt) and transgenic mice at age of 8 to 10 weeks were fed with either a 58% kcal high-fat diet (HFD; catalog no. D12331; Research Diets) or a 6% kcal chow diet (catalog no. 8664; Harlan Teklad) for 20 weeks (24). The body weights of mice were monitored weekly. To measure the food intake, the mice were separated into individual cages, and changes in food amounts were recorded daily. Ovariectomy was performed on wt and transgenic female mice at age of 10 to 12 weeks. At 1 week after surgery, the mice were fed either HFD or regular chow diet for 8 weeks, and their body weights were monitored biweekly.
Metabolic assays.
Serum insulin and leptin levels were measured using commercial kits (catalog no. 90030 and catalog no. INSKR020; Crystal Chem). Estradiol (Cayman), testosterone (Cayman), and corticosterone (Enzo Life Science) were measured by enzyme immunoassay. An insulin tolerance test (ITT) and a glucose tolerance test (GTT) were performed on Shp2D61A and wt mice at age of 24 to 26 weeks, after HFD feeding for 16 to 18 weeks. For the ITT, insulin (Humulin R; Eli Lilly) was injected intraperitoneally (1 U/kg [body weight]), and the blood glucose levels were measured with a glucometer (Lifescan One-Touch Basic) at 0, 15, 30, 60, and 120 min. For the GTT, the animals were fasted for 12 to 16 h and injected with glucose (1.5 g/kg [body weight]), and then the blood glucose levels were measured at 0, 15, 30, 60, and 120 min.
For leptin sensitivity, mice fed with HFD or chow diet for 16 weeks were intraperitoneally injected with leptin (1.5 μg/kg [body weight]; National Hormone and Peptide Program) twice daily for 3 days (7:30 a.m. and 6:30 p.m.; dose, 1.5 μg/kg [body weight]) (24). The mice were separated into individual cages and placed on chow diet. Body weight and food intake were monitored daily for 7 days. O2 consumption and CO2 production were measured by using the Oxymax System (Columbus Instruments).
Cell signaling studies.
Mice at the age of 30 weeks fed a HFD for 20 weeks were fasted for 12 to 16 h. Leptin (1.5 μg/kg [body weight]), estradiol-17β (catalog no. 3301 [Calbiochem], 50 μM/kg [body weight]), and saline were administered intraperitoneally 1 h before sacrifice. Brain was carefully isolated and homogenized with tissue lysis buffer, and then the lysate was centrifuged at 14,000 rpm for 30 min twice. Lysates containing 50 μg of protein were immunoblotted for pY-Stat3 (catalog no. 9131; Cell Signaling), Stat3 (catalog no. 9132; Cell Signaling), pAkt-s473 (catalog no. 9271; Cell Signaling), pAkt-t308 (catalog no. 9275; Cell Signaling), Akt (catalog no. 9272; Cell Signaling), pY-Src and Src (Cell Signaling), pErk1/2 (catalog no. 9101; Cell Signaling), adiponectin (Millipore), and HA (Update). Mouse brain was isolated after perfusion by 4% paraformaldehyde (PFA) for frozen sectioning. Shp2 antibody (syp homemade or sc-280; Santa Cruz Biotechnology), ERα antibody (sc-542; Santa Cruz Biotechnology), and pY-Stat3 antibody were used for immunostaining. Liver was collected for frozen sectioning and then stained by O oil-red or with hematoxylin and eosin (H&E) (4). Adipose tissue was fixed by Z-Fix solution for 24 h to performe H&E staining. Brain samples were collected for frozen sectioning and stained by antibodies.
MCF-7 cells were cultured in Dulbecco modified Eagle medium with 10% fetal bovine serum. PC12LepRb cells were cultured in F-12K medium with 12% horse serum and 3% fetal bovine serum (20). Cells were fixed by 4% PFA for 5 to 10 min. Shp2 primary antibody (sc-7384; Santa Cruz Biotechnology) and ERα primary antibody (sc-542; Santa Cruz Biotechnology) were used to perform immunostaining and immunoblotting. Human ERα small interfering RNA (siRNA; sc-29305; Santa Cruz Biotechnology) and human Shp2 siRNA (catalog no. 1027020; Qiagen) were used to knockdown target gene expression in MCF-7 cells by the Amaxa nucleofector method. After 3 days, the cells were stimulated with hormones, and the cell lysates were analyzed by immunoblotting. pcDNA-Shp2D61A construct was transiently transfected into MCF-7 cells. C57BL/6 mice at age of 10 weeks were sacrificed to collect uterus and ovary, and the tissue lysates (1 mg of total proteins) were prepared for immunoprecipitation with antibodies for Shp2 and ERα, followed by immunoblotting with specific antibodies as indicated. Mouse pCMV5-Shp2 and pGEM-ERα were used to express protein by TNT coupled reticulocyte lysate systems (cell-free protein expression, catalog nos. L5061 and L5020; Promega). The translation products were mixed with or without estrodial-17β and subjected to immunoprecipitation assay.
Statistical analysis.
Data are expressed as means ± the standard errors of the mean (SEM). Statistical significance was determined using an unpaired two-tailed Student t test and analysis of variance.
RESULTS
Shp2D61A mice are resistant to HFD-induced obesity in a sex-dimorphic manner.
A dominant-active mutant of tyrosine phosphatase Shp2 (HA-tagged Shp2D61A) under the control of CaMKIIα promoter was selectively expressed in forebrain neurons, including the hypothalamus, and the expression levels were similar in male and female mice, as demonstrated by immunostaining and immunoblotting analyses (Fig. 1A and B). When maintained on regular chow food, the transgenic mice did not show any obvious abnormal phenotype (Fig. 1C). Both wt and transgenic mice, at ages of 8 to 10 weeks, were fed either HFD (58% Kcal) or chow diet (24). After 20 weeks, wt mice of both genders on HFD exhibited significantly increased body weight compared to those fed regular food (Fig. 1D). However, we observed a gender effect of Shp2D61A expression in transgenic mice fed with HFD. Shp2D61A male mice displayed a dramatic increase of body weight when on HFD compared to chow food, findings very similar to those for the wt controls (Fig. 1D). In contrast, Shp2D61A female mice showed only modest levels of body weight increase when placed on HFD (Fig. 1D), suggesting a more prominent effect of Shp2D61A expression in female mice for protection against body weight gain/obesity. Ovariectomization of Shp2D61A female mice disrupted the resistance to HFD-induced obesity, suggesting that estrogen signal plays an important role in the progression of sex-dimorphic obesity (Fig. 1E). Shp2D61A female mice had significantly lower indices of white fat (gonadal) pad to body weight than the wt controls, fed with either HFD or chow diet, although their indices of liver or brown fat versus body weight were normal (Fig. 1F). The indices of male mice did not show significant difference between control and transgenic mice with HFD or chow diet (Fig. 1G). Under the HFD condition, Shp2D61A female mice had smaller adipocyte sizes than controls (Fig. 1H), indicating that Shp2D61A expression in the brain inhibits obese development. Control and male transgenic mice showed severe hepatic steatosis when fed with HFD, as revealed by Oil Red and H&E staining (Fig. 2A to C). Shp2D61A female mice displayed fewer fat droplets in liver than controls on HFD (Fig. 2D to F). Interestingly, even on chow food, Shp2D61A female mice showed lower basal levels of liver steatosis than did wt animals (Fig. 2D to F).
Fig 1.
Shp2D61A expression confers resistance to HFD-induced obesity in female mice. (A and B) The expression of Shp2D61A in the hypothalamus area of transgenic mice was examined by anti-HA and anti-Shp2 immunoblotting (A) and immunostaining (B). Similar levels of HA-tagged Shp2D61A were detected in the brains of male and female mice. D61A: Shp2D61A. Scale bar, 50 μm. (C) Representatives of wt or transgenic female and male mice that were fed on HFD or chow diet for 20 weeks. (D) Body weights of wt versus Shp2D61A mice on either HFD or chow diet. The data are expressed as means ± the SEM (n = 10 to 15; *, P < 0.05; **, P < 0.01; ***, P < 0.001). (E) Female mice at the ages of 10 to 12 weeks were ovariectomized. After 1 week, the mice were fed on either HFD or regular chow food. The body weights were monitored biweekly. The percentages of weight gain are shown. The data are expressed as means ± the SEM (n = 4 to 6; *, P < 0.05; **, P < 0.01). (F and G) Index of gonadal fat pad to body weight. The ratios of brown adipose tissue or liver to body weight were compared between control and transgenic male or female mice on chow diet or HFD. The data are expressed as means ± the SEM (n = 11 to 15; *, P < 0.05; ***, P < 0.001). (H) Representative image of adipocyte sizes on HFD, with H&E staining. Scale bar, 100 μm.
Fig 2.
Shp2D61A expression suppresses liver steatosis. (A and B) Liver steatosis of male mice shown using Oil Red and H&E staining, respectively. (C and D) Fatty livers of female mice are shown using Oil Red and H&E staining, respectively. (E and F) Hepatic lipid accumulation was quantified for male and female mice on chow or HFD. Scale bar, 50 μm. (n = 3 to 5; *, P < 0.05; **, P < 0.01). D61A, Shp2D61A.
Shp2D61A expression reduces food intake and enhanced energy expenditure.
To examine the energy balance, we monitored food intake and found a significant decrease of food consumption by Shp2D61A female mice, compared to controls, when fed HFD (Fig. 3A). After being placed on a HFD for 4 weeks, Shp2D61A female mice showed increased heat production compared to wt mice in a HFD (Fig. 3B), and Shp2D61A female mice showed significantly more O2 consumption and CO2 release than did controls (Fig. 3C to F). These data suggest that Shp2D61A expression in female mice attenuated HFD-induced obesity due to enhanced energy expenditure and decreased food intake.
Fig 3.
Shp2D61A female mice displayed reduced food intake and increased energy expenditure. (A) The daily food intake of mice on HFD. The data are expressed as means ± the SEM. *, P < 0.05. (B) Heat production was measured for wt and Shp2D61A female mice on HFD. The data are expressed as means ± the SEM. (C) O2 consumption and CO2 production of wt versus Shp2D61A female mice on HFD. Total O2 consumption and CO2 production data were shown without normalization. (D to F) O2 consumption and CO2 production of wt versus Shp2D61A female mice on HFD. The data normalized by the whole body weight are expressed as means ± the SEM. *, P < 0.05.
Shp2D61A female mice show improved insulin sensitivity and glucose homeostasis.
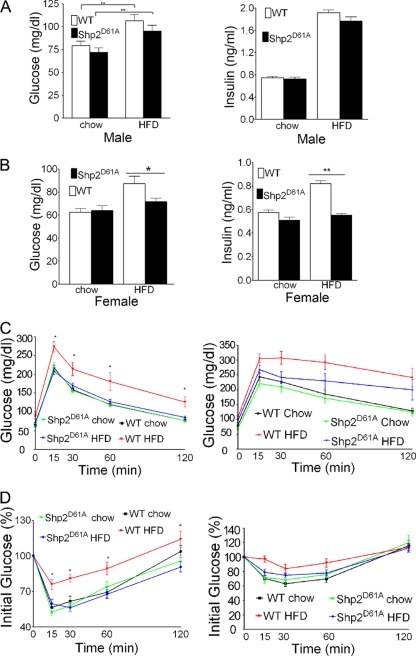
Blood insulin and fasting glucose levels were similarly elevated in wt and Shp2D61A male mice fed with HFD compared to mice fed regular food (Fig. 4A), suggesting the development of obesity-related hyperglycemia and insulin resistance. In contrast, Shp2D61A female mice on HFD exhibited normal or slightly increased insulin and glucose concentrations (Fig. 4B). We performed GTTs and ITTs on mice at the ages of 26 to 28 weeks. The wt mice on HFD showed a decreased response to a glucose load (Fig. 4C and D), indicating the status of HFD-induced glucose intolerance. In contrast, Shp2D61A female mice on either a regular diet or HFD showed normal glucose tolerance levels compared to wt controls on chow diet (Fig. 4C). After being paced on HFD, Shp2D61A female mice were more insulin sensitive than wt mice (Fig. 4D). The insulin sensitivity was similar between Shp2D61A female mice fed a HFD or chow diet and wt controls on regular food (Fig. 4D). After on HFD for 16 weeks, Shp2D61A male mice also exhibited mildly improved glucose and insulin sensitivity compared to wt male mice (Fig. 4C and D). Thus, Shp2D61A expression in forebrain neurons prevents obesity-related insulin resistance and glucose intolerance.
Fig 4.
Shp2D61A expression improves metabolic parameters. (A) Serum insulin and blood glucose levels were compared between Shp2D61A and wt male mice, fed HFD or chow diet. The data are expressed as the means ± the standard deviation (SD). (B) Blood glucose and serum insulin levels were measured for control and transgenic female mice on HFD compared to chow diet. The data are expressed as means ± the SD. (C) GTT in wt versus Shp2D61A mice on HFD or chow diet. The data are expressed as means ± the SD. Left panel, female mice; right panel, male mice. (D) ITT in wt versus Shp2D61A mice on HFD or chow diet. The data are expressed as means ± the SD. Left panel, female; right panel, male mice. (n = 11 to 15, *, P < 0.05; **, P < 0.01).
Shp2D61A enhances leptin sensitivity.
We investigated whether the antiobese phenotype is due to increased leptin sensitivity in Shp2D61A transgenic mice. As shown in Fig. 5A and B, the leptin levels of control and male transgenic mice on HFD were significantly elevated compared to the same groups on a chow diet, indicating a hyperleptinemia status. Shp2D61A female mice showed significantly lower leptin levels than wt female mice on HFD (Fig. 5B), suggesting an increased leptin sensitivity in female transgenic animals. To further determine leptin sensitivity, we performed intraperitoneal injection of leptin twice daily for 3 days and monitored the body weight changes continuously for 7 days (24). Either on HFD or chow diet, wt mice underwent similar percentages of body weight loss upon leptin injection (Fig. 5C and D). However, Shp2D61A transgenic female mice displayed more significant weight loss after leptin administration compared to female control mice for the first 3 days while receiving leptin (Fig. 5C). Over the 3 days after leptin injection, the body weight of Shp2D61A female mice on HFD remained at low levels compared to wt female mice on HFD (Fig. 5C). The same dose of leptin administration caused a similar weight loss between wt and Shp2D61A male mice fed with HFD or chow diet (Fig. 5D). Thus, Shp2D61A expression enhanced the leptin sensitivity in female mice, resulting in protection from HFD-induced obesity and associated hyperglycemia, hyperinsulinemia, and hyperleptinemia.
Fig 5.
Expression of Shp2D61A increases leptin sensitivity. After mice were fed a HFD or a chow diet for 20 weeks, the serum leptin levels were measured. (A) Serum leptin levels were compared between wt and Shp2D61A male mice on a HFD or chow diet. (B) Serum leptin amounts were compared between wt and Shp2D61A female mice on a HFD or chow diet. (C) Leptin was injected intraperitoneally twice daily for 3 days. The body weights of female mice were measured daily for 7 days. The data are expressed as means ± the SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (D) Leptin was injected intraperitoneally twice daily for 3 days. Body weight of male mice was measured daily for 7 days. The data are expressed as means ± the SD. (E) Immunoblotting analysis was performed to evaluate serum levels of adiponectin after 20 weeks on HFD. The lower panel gives the relative intensities of the adiponectin bands in the immunoblot. (F to H) Serum levels of estrogen, testosterone, and corticosterone measured after 20 weeks on a HFD.
Under HFD conditions, the level of adiponectin was higher in female than in male animals (Fig. 5E). There was no difference between wt and Shp2D61A groups in both genders, suggesting that adiponectin does not play a role in resistance to HFD-induced obesity in this model. To investigate whether sex hormones are involved in obesity development, we examined the serum levels of estrogen and testosterone. No significant difference was observed between wt and Shp2D61A mice (Fig. 5F and G). However, the level of corticosterone was significantly decreased in the Shp2D61A group of both genders, compared to the wt control (Fig. 5H), suggesting that corticosterone is not a major factor in the sex-dimorphic development of obesity.
A Shp2/ERα complex orchestrates leptin and estrogen signals.
The results described above suggest a biochemical connection of Shp2 with an estrogen signaling component downstream of the leptin receptor in control of energy metabolism in the hypothalamus (Fig. 1E). Consistently, it has been shown previously that estrogen has a leptin-like effect in the hypothalamus for the control of energy balance, although the mechanism is not fully understood (16). To probe the underlying mechanism for the gender effect of the Shp2 transgene expression, we explored a putative role of Shp2 in coupling leptin and estrogen signals. We used a leptin-stimulated pY-Stat3 signal to locate LepRb-positive cells. Intracellular colocalization of Shp2, ERα, and pY-Stat3 was detected in the hypothalamus by immunofluorescent staining (Fig. 6A). Using ovary and uterus tissue or brain lysates, we demonstrated that Shp2 and ERα were coimmunoprecipitated with each other in both ways (Fig. 6B and C), indicating the formation of a Shp2/ERα complex in vivo. Immunostaining analysis also showed colocalization of Shp2 and ERα in MCF-7 breast cancer cells (Fig. 6D). To test whether Shp2 association with ERα is stimulated by estrogen, we performed an immunoprecipitation assay in vitro. Treatment of cells with E2 enhanced the binding of Shp2 with ERα, suggesting a stimulatory effect by estrogen (Fig. 6E). In MCF-7 cells, the binding of Shp2 and ERα was greatly enhanced 30 min after E2 treatment (Fig. 6F). Therefore, Shp2/ERα is linked with leptin and estrogen signaling.
Fig 6.
Shp2 couples leptin and estrogen signaling in hypothalamus. (A) Shp2, ERα, and pY-STAT3 signals were detected by immunostaining with specific antibodies in the hypothalamus area after leptin injection. Scale bar, 50 μm. (B and C) Brain (B) and uterus (C) tissues were used to perform coimmunoprecipitation assay with specific antibodies to Shp2 and ERα. (D) MCF-7 cell lysate was prepared for coimmunoprecipitation with specific antibodies to ERα or Shp2 and subjected to immunoblotting as shown. Immunofluorescent staining for Shp2 and ERα in MCF-7 cells. (E) In vitro translation system using estradiol-17β (E2) enhanced binding of Shp2 and ERα in vitro. (F) E2-induced association of Shp2 with ERα in MCF-7 cells.
Shp2 enhances leptin and estrogen action.
We determined whether leptin and estrogen operate in concert to activate intracellular signaling pathways. Stimulation by leptin or estrogen induced rapid phosphorylation of Erk, Src, Stat3, and Akt (Fig. 7A), and combined treatment with leptin and estrogen dramatically enhanced the phosphorylation signal of Erk, indicating a synergistic effect in cell signaling (Fig. 7A). No dramatic change in the phosphorylation levels of Src were detected after leptin or estrogen stimulation (Fig. 7A). We injected leptin and/or estrogen into wt and Shp2D61A mice placed on HFD for 20 weeks. Phosphorylation of Erk, Akt, and Stat3 was significantly increased after 1 h of leptin or estrogen administration compared to mice given an saline injection (Fig. 7B). Compared to controls, transgenic mice of both genders displayed higher basal level of p-Erk, p-Akt, and p-Stat3. Consistently, the transgenic mice exhibited higher sensitivity to leptin and estrogen than wt animals and possessed lower circulating levels of leptin (Fig. 5A). Moreover, the overall phosphorylation levels of Erk, Akt, and Stat3 were higher in female than in male mice, suggesting that leptin and estrogen operate in concert in regulating Erk, Akt, and Stat3 pathways in vivo (1, 5, 17, 18, 36, 37).
Fig 7.
Shp2 enhances leptin and estrogen signals. (A) Leptin and E2 induced phosphorylation of Erk1/2, Src, Stat3, and Akt in PC12LepRb cells. The right panel shows p-Erk quantification (n = 3). (B) Leptin and E2 stimulated phosphorylation of Erk1/2, Akt, and Stat3 in the brains of Shp2D61A mice. The right panel shows p-Erk quantification (n = 4 to 5). (C) Knockdown of Shp2 or ERα inhibited p-Erk1/2 induction by leptin or estrogen. Cells incubated in medium with 1 to 2% serum were treated with either or both of the two hormones. The right panel shows p-Erk quantification (n = 3). (D) Cells starved in serum-free medium were treated with leptin and/or estrogen for the induction of p-Erk1/2 signals.
We further investigated the cross talk of leptin and estrogen signaling in MCF-7 cells that express both LepRb and ERα. Knockdown of Shp2 or ERα expression by RNA interference significantly decreased the basal levels of p-Erk (Fig. 7C). Shp2 knockdown delayed induction of p-Erk from 15 to 30 min by estrogen stimulation (Fig. 7C). Combined stimulation of estrogen with leptin partially restored the reaction time of p-Erk to 15 min. Interestingly, ERα knockdown also caused a delayed induction of p-Erk signal by leptin, similar to Shp2 knockdown; the combined treatment of leptin and estrogen did not rescue the delay in p-Erk induction. In contrast, expression of Shp2D61A in MCF-7 cells resulted in sustained Erk activation by leptin and estrogen (Fig. 7D), indicating a coordinated regulation by Shp2 of the leptin and estrogen signaling pathways.
Hypothalamic injection of AAV-Shp2D61A causes a similar phenotype as the transgenic mice.
As described above, Shp2D61A was expressed in forebrain neurons in the transgenic mice generated in the present study. To determine whether hypothalamus is indeed the target site for Shp2D61A, we generated AAV-Shp2D61A virus and specifically administered the AAV-GFP control or AAV-Shp2D61A virus into the mediobasal hypothalamus of wt C57BL/6 mice at age of 8 weeks (38) (Fig. 8A). The female mice injected with AAV-Shp2D61A had body weights similar to those of the control group on chow diet. Fed with HFD, the body weights of AAV-Shp2D61A female mice were significantly decreased compared to those for the controls (Fig. 8B and C). Consistent with the transgenic male animals, the antiobese effect was not detected in male mice that received AAV-Shp2D61A when placed on an HFD. AAV-Shp2D61A injection suppressed animal food intake compared to the control group (Fig. 8D). AAV-Shp2D61A-injected female mice had less fat mass than control mice injected with AAV-GFP (Fig. 8E and F). AAV-Shp2D61A female mice displayed a higher rate of O2 consumption and CO2 production (whether or not normalized by lean mass weight) (Fig. 8G and H). Furthermore, the injection of AAV-Shp2D61A also enhanced glucose handling capability of experimental animals (Fig. 8I). Thus, hypothalamic expression of Shp2D61A attenuated HFD-induced obese development by increasing energy expenditure and improving glucose homeostasis in a sex-dimorphic manner.
Fig 8.
Hypothalamic injection of AAV-Shp2D61A prevents HFD-induced obesity by inhibiting food intake and increasing energy expenditure. C57BL/6 mice (chow-fed, ∼3 months old) with matched sex and body weight were randomized and bilaterally injected with AAV-Shp2D61A or the control AAV-GFP into the mediobasal hypothalamus. After viral injection, the mice were maintained on chow feeding for 3 to 4 weeks and then switched to HFD feeding. (A) Brain sections across the mediobasal hypothalamus were prepared from AAV-GFP injected mice, and the GFP distribution in hypothalamus sections was examined by GFP immunostaining. DAPI (4′,6′-diamidino-2-phenylindole) staining revealed the nuclei of all cells in the sections. 3V, third ventricle. Scale bar, 50 μm. (B) Body weights of AAV-Shp2D61A-injected female mice versus AAV-GFP-injected female mice. (C) Body weight gains of AAV-Shp2D61A-injected mice versus AAV-GFP-injected mice on HFD. The data are expressed as means ± the SD. *, P < 0.05. (D) Average daily HFD intake of AAV-Shp2D61A-injected mice versus AAV-GFP-injected mice. The data are expressed as means ± the SD. *, P < 0.05. (E) Fat and lean masses of AAV-Shp2D61A-injected mice versus AAV-GFP-injected female mice. (F) Fat percentages of AAV-Shp2D61A-injected female mice versus AAV-GFP-injected female mice with HFD. The data are expressed as means ± the SD. *, P < 0.05. (G and H) O2 consumption and CO2 production of AAV-Shp2D61A-injected female mice versus AAV-GFP-injected female mice on HFD. O2 consumption and CO2 production data were normalized by lean mass of mice. The data are expressed as means ± the SD. *, P < 0.05. (I) GTT in AVV-Shp2D61A-injected female mice versus AAV-GFP-injected female mice on HFD. The data are expressed as means ± the SD. *, P < 0.05.
DISCUSSION
In previous experiments, we showed that selective ablation of Shp2 in the brain resulted in leptin resistance and early onset obesity, suggesting a positive role of this enzyme in leptin signal relay in the hypothalamus (22, 37). In the present study, we have demonstrated that enhancing Shp2 activity locally in the hypothalamus increases leptin activity in the control of energy metabolism. As expected, expression of a dominant-active mutant of Shp2 in forebrain neurons decreases adiposity, increases energy expenditure, and impairs food intake in transgenic mice. Although the Shp2D61A mutant is more widely expressed in forebrain neurons, including the hypothalamus in the transgenic mouse, we further demonstrate that a similar antiobese phenotype is induced by the injection of AAV-Shp2D61A virus into mediobasal hypothalamus. Together, these data strongly suggest that the hypothalamus is indeed the primary acting site for Shp2 in the transgenic mouse line for the regulation of leptin signal.
However, it is interesting that the antiobese effect of Shp2D61A mutant is much more prominent in female than in male mice. Consistent with the sex-dimorphic phenotype, we have found that Shp2 is physically associated with the estrogen receptor ERα and that the association is enhanced by estrogen stimulation. Thus, Shp2, a cytoplasmic signaling molecule, acts to mediate the direct coupling of signaling events triggered by a metabolic hormone leptin and a sex hormone estrogen in the hypothalamus (Fig. 9). Expression of Shp2D61A mutant in male mice induced a mild effect on insulin signaling and glucose homeostasis, suggesting that the HPA axis is involved in the control of glucose metabolism.
Fig 9.
Model for concerted leptin and estrogen signaling in hypothalamic regulation of energy balance. (A) Young women and girls have normal levels of leptin and estrogen that act in concert in the hypothalamus to regulate energy metabolism. (B) Even with normal leptin levels, postmenopausal women have increased risk for development of obesity and leptin resistance, due to estrogen deficiency. (C) Obese young women with normal estrogen production may develop obesity due to leptin resistance. (D) Obese postmenopausal women have defects in leptin and estrogen signaling due to leptin resistance and estrogen deficiency.
In addition to the well-known role in control of metabolism and energy balance, leptin also regulates reproductive functions (13), indicating the overlap of downstream signaling pathways elicited by leptin and sex hormones. Rodents lacking leptin (ob/ob) or leptin receptor (db/db) are infertile (34, 39). Leptin injection restores the fertility of ob/ob mice (3, 8). Characterization of genetically engineered mouse models also showed that leptin mediates reproductive function in the forebrain (11, 29). Deficient signaling downstream of leptin receptor leads to various obese phenotypes in a sex-biased manner. On the other hand, estrogen, a sex hormone, plays a critical role in gender development, reproductive physiology, and the female mammary gland. Importantly, experimental data in vivo and in vitro have provided evidence that estrogen signaling modulates fat accumulation and body weight by binding to ERα (2, 19, 27). In rodents, siRNA-mediated silencing of ERα in the hypothalamus area resulted in development of obesity and other metabolic disorders (17, 25). Consistently, ovariectomized rats deficient for estrogen develop obesity and leptin resistance, which can be reversed by E2 replacement therapy (9). A recent report indicates that a mutant ERα retaining the activity for noncanonical signaling (lacking the DNA-binding ability to the estrogen response element [ERE]) is sufficient to mediate the antiobese effect of ERα in energy homeostasis (28). However, it remains to be elucidated how the estrogen and leptin signals are coupled in regulation of energy metabolism.
Data presented here suggest that the Shp2/ERα complex, acting downstream of LepRb, mediates the concerted regulation of cytoplasmic signaling pathways by leptin and estrogen. Ovariectomization of Shp2D61A mice disrupted their resistance to HFD-induced obesity. Consistently, ablation of ERα or aromatase in mice leads to the development of age-dependent obesity and female animals displayed more severe weight gain, indicating that the estrogen signaling pathway is associated with sexually dimorphic obesity (19). The Shp2 effect was amplified in the Shp2D61A mutant molecule, since it has elevated catalytic activity due to the removal of the autoinhibitory mechanism. Therefore, expression of Shp2D61A allowed us to observe a dramatic difference between male and female mice in the present study. One prominent effect for the integration of leptin and estrogen signaling by Shp2 is to mediate stronger and more sustained activation of the Erk pathway, and it may also affect signaling events through Stat3 and Akt (Fig. 7B). Therefore, transgenic mice showed enhanced sensitivity to leptin and estrogen stimulation. Compared to wt controls, Shp2D61A mice exhibited lower circulating levels of leptin and higher basal levels of p-Erk, reinforcing the notion that Shp2 acts to amplify leptin signal. However, caution needs to be taken in interpretation of results from transgenic mice in which a constitutively active molecule is expressed in the forebrain region. In the present study, Shp2D61A was expressed in transgenic mice at lower level than the wt protein. Therefore, we are reasonably confident that the transgenic phenotype is not an artificial result but reveals a physiological function of Shp2.
Based on the experimental data presented here and previously known overlapping functions between leptin and estrogen, we propose a model illustrating the mechanism for development of obesity in postmenopausal women due to estrogen deficiency and leptin resistance (Fig. 9). Epidemiological data have also suggested a higher risk for the development of breast cancer in obese women because of an elevated estrogen level (6, 30). Increased serum levels of leptin were frequently detected in breast cancer patients (35), and oncogene-induced mammary tumorigenesis was suppressed in mice deficient for leptin or leptin receptor (10). Notably, overexpression of Shp2 has been detected in breast cancer cells (40). Elucidating Shp2 action in linking leptin and estrogen signaling in breast tumorigenesis may provide novel therapeutic target for improving prognosis of breast cancer patients worldwide.
ACKNOWLEDGMENTS
We thank our coworkers for helpful discussion.
This study was supported by National Institutes of Health R01 grants DK073945, DK075916, and AR051300 to G.-S.F. and DK078750 to D.C. and by the CAS/SAFEA International Partnership Program for Creative Research Team (grant 20080491026).
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Al-Qassab H, et al. 2009. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 10:343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banno R, et al. 2010. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J. Clin. Invest. 120:720–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barash IA, et al. 1996. Leptin is a metabolic signal to the reproductive system. Endocrinology 137:3144–3147 [DOI] [PubMed] [Google Scholar]
- 4. Bard-Chapeau EA, et al. 2011. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell 19:629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bjorbaek C, et al. 2001. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 276:4747–4755 [DOI] [PubMed] [Google Scholar]
- 6. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. 2003. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 348:1625–1638 [DOI] [PubMed] [Google Scholar]
- 7. Carr MC. 2003. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 88:2404–2411 [DOI] [PubMed] [Google Scholar]
- 8. Chehab FF, Lim ME, Lu R. 1996. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 12:318–320 [DOI] [PubMed] [Google Scholar]
- 9. Chu SC, et al. 1999. Fluctuation of serum leptin level in rats after ovariectomy and the influence of estrogen supplement. Life Sci. 64:2299–2306 [DOI] [PubMed] [Google Scholar]
- 10. Cleary MP, et al. 2003. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res. Treat 77:205–215 [DOI] [PubMed] [Google Scholar]
- 11. de Luca C, et al. 2005. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J. Clin. Invest. 115:3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dragatsis I, Zeitlin S. 2000. CaMKIIα-Cre transgene expression and recombination patterns in the mouse brain. Genesis 26:133–135 [DOI] [PubMed] [Google Scholar]
- 13. Dubuc PU. 1985. Effects of estrogen on food intake, body weight, and temperature of male and female obese mice. Proc. Soc. Exp. Biol. Med. 180:468–473 [DOI] [PubMed] [Google Scholar]
- 14. Flier JS. 2004. Obesity wars: molecular progress confronts an expanding epidemic. Cell 116:337–350 [DOI] [PubMed] [Google Scholar]
- 15. Friedman JM, Halaas JL. 1998. Leptin and the regulation of body weight in mammals. Nature 395:763–770 [DOI] [PubMed] [Google Scholar]
- 16. Gao Q, Horvath TL. 2008. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am. J. Physiol. Endocrinol. Metab. 294:E817–E826 [DOI] [PubMed] [Google Scholar]
- 17. Gao Q, et al. 2007. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat. Med. 13:89–94 [DOI] [PubMed] [Google Scholar]
- 18. Gao Q, et al. 2004. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl. Acad. Sci. U. S. A. 101:4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. 2000. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U. S. A. 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang L, Li Z, Rui L. 2008. Leptin stimulates both JAK2-dependent and JAK2-independent signaling pathways. J. Biol. Chem. 283:28066–28073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones ME, et al. 2000. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. U. S. A. 97:12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krajewska M, et al. 2008. Development of diabesity in mice with neuronal deletion of Shp2 tyrosine phosphatase. Am. J. Pathol. 172:1312–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li C, Friedman JM. 1999. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc. Natl. Acad. Sci. U. S. A. 96:9677–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mori H, et al. 2004. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 10:739–743 [DOI] [PubMed] [Google Scholar]
- 25. Musatov S, et al. 2007. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. U. S. A. 104:2501–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Reilly AM, Pluskey S, Shoelson SE, Neel BG. 2000. Activated mutants of SHP-2 preferentially induce elongation of Xenopus animal caps. Mol. Cell. Biol. 20:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohlsson C, et al. 2000. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem. Biophys. Res. Commun. 278:640–645 [DOI] [PubMed] [Google Scholar]
- 28. Park CJ, et al. 2011. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese ERα-null mutant mice. J. Clin. Invest. 121:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quennell JH, et al. 2009. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150:2805–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. 2008. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371:569–578 [DOI] [PubMed] [Google Scholar]
- 31. Ring LE, Zeltser LM. 2010. Disruption of hypothalamic leptin signaling in mice leads to early onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J. Clin. Invest. 120:2931–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. 2009. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150:2161–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salpeter SR, et al. 2006. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obesity Metab. 8:538–554 [DOI] [PubMed] [Google Scholar]
- 34. Tartaglia LA, et al. 1995. Identification and expression cloning of a leptin receptor, OB-R. Cell 83:1263–1271 [DOI] [PubMed] [Google Scholar]
- 35. Tessitore L, et al. 2000. Leptin expression in colorectal and breast cancer patients. Int. J. Mol. Med. 5:421–426 [DOI] [PubMed] [Google Scholar]
- 36. Xu Y, et al. 2010. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 12:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang EE, Chapeau E, Hagihara K, Feng GS. 2004. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc. Natl. Acad. Sci. U. S. A. 101:16064–16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang X, et al. 2008. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, et al. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- 40. Zhou X, Coad J, Ducatman B, Agazie YM. 2008. SHP2 is upregulated in breast cancer cells and in infiltrating ductal carcinoma of the breast, implying its involvement in breast oncogenesis. Histopathology 53:389–402 [DOI] [PubMed] [Google Scholar]