Abstract
Retinol-binding protein 4 (RBP4), the sole retinol transporter in blood, is secreted from adipocytes and liver. Serum RBP4 levels correlate highly with insulin resistance, other metabolic syndrome factors, and cardiovascular disease. Elevated serum RBP4 causes insulin resistance, but the molecular mechanisms are unknown. Here we show that RBP4 induces expression of proinflammatory cytokines in mouse and human macrophages and thereby indirectly inhibits insulin signaling in cocultured adipocytes. This occurs through activation of c-Jun N-terminal protein kinase (JNK) and Toll-like receptor 4 (TLR4) pathways independent of the RBP4 receptor, STRA6. RBP4 effects are markedly attenuated in JNK1−/− JNK2−/− macrophages and TLR4−/− macrophages. Because RBP4 is a retinol-binding protein, we investigated whether these effects are retinol dependent. Unexpectedly, retinol-free RBP4 (apo-RBP4) is as potent as retinol-bound RBP4 (holo-RBP4) in inducing proinflammatory cytokines in macrophages. Apo-RBP4 is likely to be physiologically significant since RBP4/retinol ratios are increased in serum of lean and obese insulin-resistant humans compared to ratios in insulin-sensitive humans, indicating that higher apo-RBP4 is associated with insulin resistance independent of obesity. Thus, RBP4 may cause insulin resistance by contributing to the development of an inflammatory state in adipose tissue through activation of proinflammatory cytokines in macrophages. This process reveals a novel JNK- and TLR4-dependent and retinol- and STRA6-independent mechanism of action for RBP4.
INTRODUCTION
Obesity is a major risk factor for insulin resistance, which is a critical pathogenic factor in type 2 diabetes (50). Determination of the physiologic and cellular mechanisms linking obesity to type 2 diabetes could lead to development of new prevention and treatment approaches. Multiple mechanisms may contribute, including abnormal production of adipocyte-secreted proteins (adipokines) (1, 15, 29), infiltration of white adipose tissue (WAT) with proinflammatory macrophages (42), and aberrant lipid deposition in tissues such as muscle and liver (51). These mechanisms are not mutually exclusive. For example, adipokines can affect inflammation and lipid deposition in tissues (15).
Serum retinol-binding protein 4 (RBP4) is an adipokine and is also secreted by liver. RBP4 levels are increased in obese and insulin-resistant humans and mouse models, and genetic or pharmacologic elevation of serum RBP4 causes insulin resistance in normal mice (19, 31, 65). Although many studies show strong correlations of serum RBP4 levels with obesity and the severity of insulin resistance (9, 16, 27, 35), others do not (8, 17, 32, 46), as reviewed in reference 32. This may result from the use of different populations of human subjects or from methodological issues with RBP4 assays (18, 32, 64). Many studies also show that serum RBP4 levels correlate with other components of the metabolic syndrome in humans, including hypertension (47, 54, 64), dyslipidemia (41, 47, 64, 67), waist/hip ratio (31, 47, 64), cardiovascular disease (26), and intra-abdominal fat mass (9, 31, 36, 56). Strong associations have been demonstrated in large-scale population studies in several ethnic backgrounds (38, 47). In addition, genetic studies support a potential role for RBP4 in causing insulin resistance in humans. A study in approximately 6,500 aging adults showed that a gain-of-function single nucleotide polymorphism (SNP) in the RBP4 promoter is associated with a 2-fold-increased risk of type 2 diabetes (58). This SNP increases RBP4 promoter activity and is positively associated with RBP4 expression in adipose tissue and with body mass index (BMI) (40).
While elevation of serum RBP4 levels is sufficient to cause insulin resistance in mice (45, 65, 68), the molecular mechanism is not understood. Insulin signaling is impaired in skeletal muscle of mice with transgenic or pharmacologic elevation of RBP4, whereas insulin signaling is increased in mice with genetic or pharmacologic lowering of RBP4 (65, 68). In addition, RBP4 treatment increases phosphoenolpyruvate carboxykinase (PEPCK) expression and glucose production in hepatoma cells (65) and PEPCK expression is elevated in the livers of RBP4-injected mice (65). Incubation of isolated adipocytes with RBP4 reduces the sensitivity to insulin-stimulated extracellular signal-regulated kinase (ERK) phosphorylation (43). These data provide some mechanistic insights into RBP4-mediated insulin resistance, but the underlying cellular mechanisms are not known.
Obesity is a state of chronic, low-grade inflammation, and macrophages are thought to play an important role in maintaining this state in adipose tissue (42, 61, 63). Many molecules secreted by adipose tissue promote adipose tissue inflammation (33, 49, 53). Emerging evidence suggests a possible role for proinflammatory pathways in RBP4-induced insulin resistance. RBP4 expression in adipose tissue (66) and serum RBP4 levels (3) strongly correlate with subclinical inflammation, including serum levels (3) and adipose tissue expression (66) of proinflammatory cytokines. Lifestyle intervention can reduce serum RBP4 levels in parallel with improvement in markers for subclinical inflammation (3, 22). At the cellular level, a recent study showed that RBP4 stimulates cytokine secretion from mouse macrophages (11). Another study showed that the RBP4/retinol complex stimulates JAK2/STAT5 signaling and expression of suppressor of cytokine signaling 3 (SOCS3) (4), which has been implicated in insulin resistance (34, 52). Together, these studies support the notion that RBP4-induced insulin resistance may involve inflammatory pathways.
It is not known whether RBP4's effects on insulin action are retinol (vitamin A) dependent. RBP4 delivers retinol to target tissues, where it is metabolized to retinoic acid. Retinoids have many fundamental effects on cellular function, including gene transcription (37), differentiation, proliferation (20), and immune function (44). Retinol deficiency, which is accompanied by low RBP4 levels, results in impaired immunity (60). The majority of RBP4 in serum is retinol bound (holo-RBP4), although a small amount is not bound to retinol (apo-RBP4). While the contribution of each form to insulin resistance is unknown, the proportion of serum RBP4 that is apo-RBP4 is increased in obese people (39) and the ratio of RBP4 to retinol is increased in people with type 2 diabetes (12).
Determination of the role of retinol in the effects of RBP4 on insulin resistance is critical for discerning the mechanisms of RBP4 action. Here we show that RBP4 can act independently of retinol to impair insulin signaling in adipocytes indirectly, by inducing proinflammatory cytokine production from macrophages. This is mediated, in part, by the Toll-like receptor 4 (TLR4) cell surface receptor and not by the RBP4 receptor, STRA6, and involves the c-Jun N-terminal protein kinase (JNK) signaling pathway. These studies enhance our understanding of the mechanisms involved in RBP4-induced insulin resistance and establish that RBP4, independent of retinol, has important biologic effects.
MATERIALS AND METHODS
Recombinant RBP4 preparation and treatment.
Mouse and human retinol-bound RBP4 (holo-RBP4) was expressed in Escherichia coli and purified as described previously (65). The endotoxin level of this recombinant protein was less than 0.001 endotoxin unit per μg, which is the same as the ambient endotoxin levels in reverse-osmosis double-deionized water as quantitatively measured by the Limulus amoebocyte lysate test (Lonza Limulus amoebocyte lysate QCL-1000; catalog no. 50-647 U). To generate retinol-free (apo-RBP4) or retinol-reconstituted (add-back) RBP4, holo-RBP4 was first incubated with 40% butanol–60% diisopropyl ether at 30°C overnight to remove retinol and centrifuged at 5,000 rpm for 5 min, and the bottom phase containing recombinant RBP4 was collected. This step was repeated twice more with 1-h incubations of 40% butanol–60% diisopropyl ether. The resulting retinol-stripped RBP4 (apo-RBP4) was then incubated at 30°C overnight with either 100 mM retinol in ethanol to generate add-back RBP4 or with an equal volume of ethanol to generate apo-RBP4. The holo, apo, and add-back forms of RBP4 were purified by high-performance liquid chromatography (HPLC), and both the protein quality and the efficiency of retinol binding were measured by Western blotting after separation by nondenaturing gel electrophoresis and by fluorescent spectrometry. After HPLC purification, RBP4 was dialyzed against 1× phosphate-buffered saline (PBS) for 4 h using a Slide A Lyzer dialysis cassette (Thermo Scientific, catalog no. 66370). The dialysate buffer was used as a vehicle control in experiments where indicated. The protein was stored at −80°C and protected from exposure to light.
Culture and coculture of 3T3L1 adipocytes and RAW264.7 macrophages.
3T3L1 preadipocyte culture and differentiation were performed as described previously (25). RAW264.7 macrophages were maintained in Dulbecco's modified Eagle's medium (DMEM), which was free of retinol and other retinoids and contained 10% fetal bovine serum (FBS) and antibiotics. Incubations with RBP4 were done under serum-free conditions, except for specific experiments to compare RBP4 effects with and without serum. Coculture of 3T3L1 adipocytes and RAW264.7 macrophages was performed as described previously with slight modifications (55). In the direct contact system, 3T3L1 adipocytes were cultured in a 6-well dish and 2 × 105 RAW264.7 macrophages were plated directly onto 3T3L1 adipocytes. Cells were cultured overnight prior to treatment with RBP4, inhibitors, or antibodies. In the noncontact Transwell system, 3T3L1 adipocytes were plated in the lower wells, RAW264.7 macrophages were plated in the upper Transwell inserts containing a 0.4-μm porous membrane (Corning), and the cells were cultured overnight and then used for the experiments.
Generation of primary mouse macrophages.
To generate peritoneal macrophages, PBS was injected into the peritoneal cavity. After massage of the abdomen, the PBS was collected and then centrifuged at 1,000 rpm for 5 min, and the cells were resuspended in RPMI supplemented with 10% FBS and antibiotics. Cells were plated at a density of 5 × 105 cells per well of a 24-well dish. The medium was changed 24 h after plating, and the macrophages were used for further studies.
Bone marrow-derived macrophages (BMDMs) were harvested from the femur and tibia. The bones were first washed in 70% ethanol, and then the bone marrow was flushed from the bone with DMEM supplemented with 10% FBS. The bone marrow was resuspended into a single-cell suspension, centrifuged for 5 min at 1,000 rpm, and then washed twice in DMEM–10% FBS. Cells were then resuspended in DMEM supplemented with 20% FBS and 30% L929 supernatant, plated at 107 cells per 10-cm plate, and cultured for 3 days. After 3 days, fresh DMEM–20% FBS–30% L929 was added to the cells. After 5 days, the plate was washed once with PBS and the macrophages were used for further study.
Primary human macrophage differentiation.
Primary human monocytes were isolated by Ficoll gradient centrifugation and adhered to 6-well plates in RPMI 1640 medium supplemented with glutamine and sodium pyruvate. After 1 h of incubation at 37°C, nonadherent cells were removed by repeated washings. Adherent cells were incubated with macrophage-specific medium (Invitrogen) containing glutamine, PEST and granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems) for 3 days, after which the medium was replaced with fresh macrophage-specific serum-free medium (SFM) without GM-CSF. The cells were then incubated for additional 3 days to generate well-differentiated macrophages.
Inhibitors.
JNK and IKK inhibitors were obtained from EMD (catalog no. 420119 and 401489). Cocultured 3T3L1 adipocytes and RAW264.7 macrophages were serum starved for 2 h, pretreated with 1 or 10 μM each inhibitor for 30 min, and then incubated with RBP4 for 24 h before insulin stimulation. For quantitative PCR analysis of cytokine expression in RAW264.7 macrophages, these cells were pretreated with 1 μM or 5 μM JNK inhibitor for 30 min and then incubated with RBP4 for the indicated times.
Neutralizing antibodies.
Neutralizing antibodies for tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein 1 (MCP-1) were obtained from R&D Systems (catalog no. AB-410-NA, AB406-NA, and AB-479-NA). Cocultured 3T3L1 adipocytes and RAW264.7 macrophages were serum starved for 2 h, pretreated with 1 μg/ml or 5 μg/ml of each neutralizing antibody or with combination of 5 μg/ml of neutralizing antibodies for TNF-α, IL-6, and MCP-1 for 30 min, and then incubated with RBP4 for 24 h before insulin stimulation.
Western blotting.
Cells were lysed with 20 mM Tris-HCl (pH 7.5), 137 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 1% NP-40, 10% glycerol, 1 mM sodium orthovanadate, 5 mM NaF, 5 mM β-glycerophosphate, and protease inhibitor, including 1 mM phenylmethylsulfonyl fluoride (PMSF) and 10 μg/ml aprotinin. Lysates were subjected to immunoblotting with the indicated antibody. The following antibodies from Cell Signaling were used: Akt (catalog no. 9272), P-Akt Ser473 (catalog no. 9271), P-Akt Thr308 (catalog no. 9275), IKKα (catalog no. 2682), IKKβ (catalog no. 2370), P-IKKα/β (catalog no. 2078), JNK (catalog no. 9252), P-JNK (catalog no. 9251), P-p38 (catalog no. 9211), p38 (catalog no. 9212), P-ERK (catalog no. 9101), and ERK (catalog no. 9102).
Measurement of cytokine secretion.
Cytokines (IL-2, IL-8, Il-12p70, IL-1β, GM-CSF, gamma interferon [IFN-γ], IL-6, IL-10, and TNF-α) secreted into conditioned medium from human macrophages were measured by a human proinflammatory 9-plex assay (Meso Scale Discovery). In addition, MCP-1 secreted into conditioned medium was measured by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturers' instructions (Biosource).
The levels of secreted cytokines in culture medium of RAW264.7 macrophages or mouse peritoneal macrophages were measured by ELISAs from Biosource (TNF-α, catalog no. KMC3011; IL-6, catalog no. KMC0061; and MCP-1, catalog no. KMC1011).
Luciferase assay.
The pNFκB-Luc vector was purchased from Clontech (catalog no. 631743). RAW264.7 macrophages were transfected with the pNFκB-Luc vector, along with a β-galactosidase expression vector as a control for transfection efficiency, using Fugene6 transfection reagent from Roche (catalog no. 1815091). Luciferase activity in medium was measured according to the manufacturer's instructions and normalized to β-galactosidase activity.
Generation of JNK1−/− JNK2−/− knockout macrophages.
Male C57 Mx1-Cre or C57 Mx1-Cre JNK1fl/fl JNK2−/− mice (10) 4 weeks of age were treated with 5 intraperitoneal injections of 20 μg/g body weight poly(I-C) over 5 to 7 days. Four weeks after treatment, bone marrow-derived macrophages (BMDMs) were harvested and cultured. JNK1 deletion was confirmed by Western blotting. BMDMs were serum starved for 2 h prior to treatment with 50 μg/ml RBP4 for 24 h. The culture medium was then collected, and the levels of secreted cytokines were measured by ELISA.
Generation of TLR4−/− macrophages.
TLR4−/− peritoneal macrophages were harvested as described above from male C57/Bl TLR4−/− mice (Jackson Laboratory stock no. 007227; gift from Katherine Fitzgerald at the University of Massachusetts Medical School).
Human samples.
All subjects included in this study were healthy nondiabetic offspring of one parent with type 2 diabetes and one nondiabetic parent, as determined by oral glucose tolerance test (OGTT) (6). Height and weight were measured to the nearest centimeter and 0.1 kg, respectively, and body mass index (BMI) was calculated as kg body weight divided by height (m) squared. Fasting blood samples were drawn after an overnight fast before an OGTT (75 g glucose) to evaluate glucose tolerance (6). Circulating glucose and insulin concentrations were determined by standard laboratory methods. At 60 min after a glucose bolus, a euglycemic-hyperinsulinemic clamp was initiated and carried out for the next 120 min (insulin infusion of 40 mU m−2 min−2) to evaluate insulin sensitivity (6). Blood glucose was clamped at 5 mmol/liter by infusion of 20% glucose at various rates according to the blood glucose measurements performed at 5-min intervals. The mean amount of glucose infused during the last hour was used to calculate the rate of whole-body glucose uptake. Lean body mass was calculated from bioimpedance analysis (BIA-101; Akern SRI, Florence, Italy).
Serum retinol and RBP4 measurements.
Serum retinol was extracted as previously described (48) with minor modifications. Briefly, 50 μl of serum was mixed with 450 μl of PBS, and 0.5 ml ethanol was spiked with approximately 50 pmol of internal standard (IS) (2.5 μl of 20 μM retinyl acetate in acetonitrile). Retinol was extracted twice in 2.5 ml hexane. The organic solvent was evaporated under a stream of nitrogen, and retinol extracts were resuspended in 50 μl of acetonitrile.
Serum retinol and the internal standard were separated as previously described (28). Briefly, we used reverse-phase chromatography (Zorbax SB-C18, 2.1 by 50 mm, 1.8 μm) under isocratic conditions (89% acetonitrile [ACN]–H2O–0.01% formic acid, 0.5 ml/min) on an Agilent 1100/1200 high-performance liquid chromatograph. Retinol and retinyl acetate were measured by UV absorbance at 325 nm and quantified using a standard curve. The injection volume was 10 μl, and all samples were measured in duplicate. Retinol eluted at 1.14 min, and retinyl acetate (IS) eluted at 1.94 min.
Total serum RBP4 was measured by mass spectrometry immunoassay (MSIA) from 20 μl of serum, as previously described (30, 64). Serum retinol and RBP4 measurements were done in duplicate.
Quantitative PCR.
An RNeasy kit (Qiagen) was used for RNA isolation from human macrophages. Gene expression was analyzed with the ABI Prism sequence detection system (TaqMan; Applied Biosystems). Gene-specific primers and probes were designed using the Primer Express software (Applied Biosystems) (see Table S1 in the supplemental material). Each sample was run in duplicate, and the quantity of a particular gene in each sample was normalized to ribosomal 18S RNA.
For studies of mouse macrophages, RNA was extracted using Tri-Reagent (MRC, catalog no. TR 118) and cDNA was generated with random hexamers (Clontech, catalog no. 639506). Quantitative real-time PCR was performed with the ABI Prism sequence detection system. All primers and probes used were obtained from Applied Biosystems, and identification (ID) numbers for each gene are listed as follows: TNF-α, Mm00443258-m1; IL-6, Mm99999064-m1; MCP-1, Mm00441242-m1; and GAPDH (glyceraldehyde-3phosphate dehydrogenase), 4352339E-0902020. Expression levels of mRNA were normalized to those of GAPDH.
RESULTS
RBP4 induces expression of proinflammatory cytokines in macrophages.
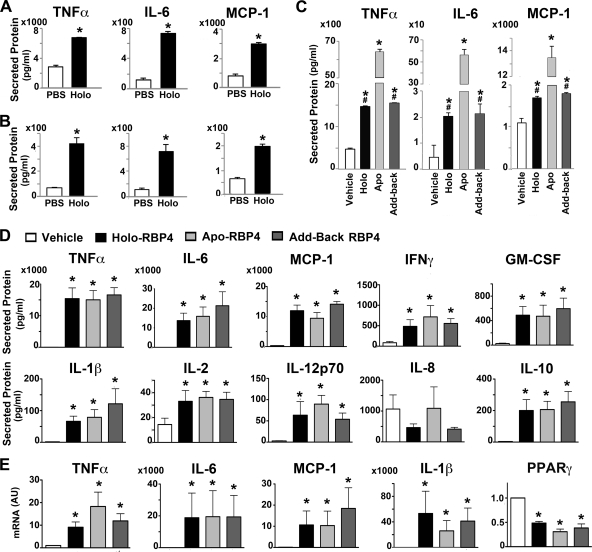
We first examined whether RBP4 induces proinflammatory cytokines in macrophages. RAW264.7 macrophages (Fig. 1A) or primary mouse peritoneal macrophages (Fig. 1B) were incubated with recombinant mouse holo-RBP4 (50 μg/ml for 24 h) at a concentration present in serum of some insulin-resistant humans (19, 31), and the levels of cytokines secreted into culture medium were measured. RBP4 treatment markedly increased TNF-α, IL-6, and MCP-1 secretion in both types of macrophages (Fig. 1A and B). Maximal effects were achieved with 50 μg/ml RBP4 (data not shown). The very low endotoxin concentration in the incubation medium using this concentration of RBP4 was tested as a separate control and showed no effects on cytokine expression or secretion (data not shown).
Fig 1.
RBP4 induces cytokines in mouse and human macrophages. (A and B) Holo-RBP4 induces TNF-α, IL-6, and MCP-1 secretion from cultured RAW264.7 macrophages (A) and primary mouse macrophages (B). (C) Mouse apo-RBP4 (Apo) has a greater effect than holo-RBP4 (Holo) on secretion of TNF-α, IL-6, and MCP-1 from RAW264.7 macrophages. Retinol was rebound to apo-RBP4 as an additional control (add-back). (D) Induction of cytokine secretion in primary human macrophages after stimulation with human holo- or apo-RBP4 or add-back RBP4 relative to vehicle control. (E) Induction of TNF-α, IL-6, MCP-1, IL-1β, and PPARγ gene expression in primary human macrophages after treatment with holo- or apo-RBP4 or add-back RBP4 relative to the vehicle control. All RBP4 incubations were with 50 μg/ml for 24 h. Either PBS or vehicle (dialysate) was used as a control. AU, arbitrary units. Data are expressed as means ± standard errors (SE). For panels A and B, n = 3 per group; *, P < 0.05 versus vehicle by t test. For panel C, n = 3 per group; *, P < 0.05 versus vehicle; #, P < 0.05 versus apo-RBP4 by ANOVA with post hoc analysis. For panels D and E, n = 7 per group. *, P < 0.05 versus vehicle by ANOVA with Wilcoxon analysis.
Since retinol has many roles in cellular function, determination of whether the effect of RBP4 to induce cytokines is retinol dependent is mechanistically important. To investigate this, we incubated macrophages with either mouse holo- or apo-RBP (Fig. 1C). As a control to ensure that the retinol-stripping process did not damage the RBP4 protein, we also added back retinol to an aliquot of apo-RBP4 (add-back RBP). The presence or absence of retinol in all forms of RBP4 was verified by fluorimetry (see Fig. S1A in the supplemental material). We confirmed that apo-RBP4 remained free of retinol at the end of the incubation of the cells with RBP4 by nondenaturing gel electrophoresis and Western blotting (see Fig. S1B). Mouse holo-RBP4 increased the production of TNF-α and IL-6 by 3- to 5-fold and MCP-1 by nearly 2-fold. Surprisingly, mouse apo-RBP4 increased the secretion of TNF-α and MCP-1 by greater than 10-fold while increasing IL-6 secretion by greater than 100-fold (Fig. 1C). Apo-RBP4 with retinol added back (add-back RBP4) had similar effects to the original holo-RBP4, confirming that the retinol-stripping process used to generate apo-RBP4 did not alter the function of the protein.
We next examined whether RBP4 induces cytokine production in human macrophages and if this effect is retinol dependent. Incubation of primary human macrophages with human holo- or apo-RBP4 or add-back RBP4 (50 μg/ml for 24 h) strongly induced secretion of TNF-α, IL-6, and MCP-1 as well as the cytokines IFN-γ, GM-CSF, IL-1β, IL-2, IL-12p70, and IL-10 (Fig. 1D). IL-8 was the only cytokine we tested which was not induced by RBP4 treatment. To test whether increased cytokine secretion in response to RBP4 reflects increased production, we measured expression of a subset of cytokines. In agreement with increased cytokine secretion, both holo- and apo-RBP4 induced expression of TNF-α, IL-6, MCP-1, and IL-1β (Fig. 1E). Since peroxisome proliferator-activated receptor γ (PPARγ) is a negative regulator of proinflammatory pathways in macrophages (23), we also examined if PPARγ expression is altered with RBP4 stimulation. PPARγ expression was decreased by 50 to 70% after treatment with holo- or apo-RBP4 or add-back RBP4 (Fig. 1E). One interpretation of these data is that reduced PPARγ expression may be involved in the induction of a proinflammatory state by RBP4.
These experiments were done under serum-free cell culture conditions. Since serum is present in vivo, we repeated the experiments in the presence of serum and obtained similar results (see Fig. S1C in the supplemental material). Thus, the absence or presence of serum in the cell culture medium does not affect RBP4 stimulation of cytokine secretion from macrophages.
RBP4 inhibits insulin signaling in 3T3L1 adipocytes cocultured with RAW264.7 macrophages.
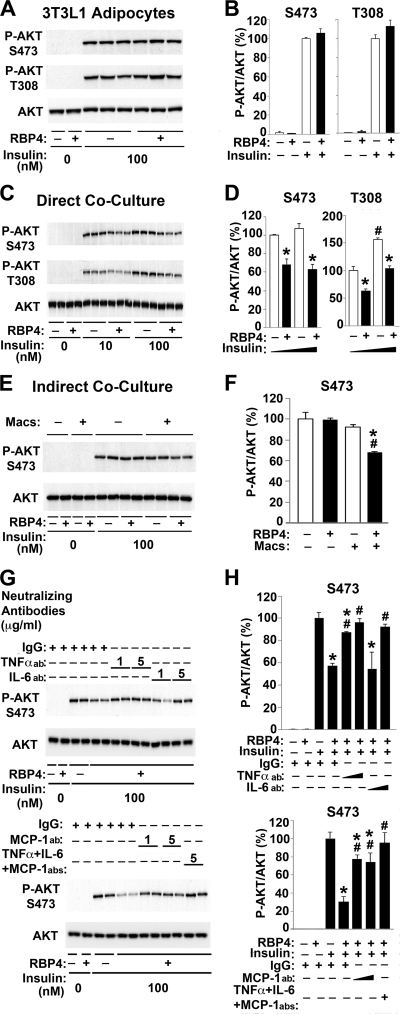
Macrophages can play a critical role in the development of insulin resistance through interacting with adipocytes (61, 63). We next tested whether RBP4 affects communication between adipocytes and macrophages. In adipocytes cultured without macrophages, RBP4 did not suppress insulin-dependent AKT phosphorylation (Fig. 2A and B). In contrast, when 3T3L1 adipocytes were cocultured in a contact system intermingled with macrophages, RBP4 treatment suppressed Akt phosphorylation (S473 and T308) by 30 to 40% at both submaximal and maximal insulin concentrations (Fig. 2C and D). These results suggest that macrophages are required for RBP4-mediated inhibition of insulin-stimulated Akt phosphorylation in adipocytes.
Fig 2.
RBP4 indirectly inhibits insulin signaling in adipocytes by stimulating proinflammatory cytokine secretion from macrophages. (A) Representative Western blot of total Akt and phosphorylated Akt (S473 and T308) in 3T3L1 adipocytes after direct treatment with holo-RBP4 (50 μg/ml for 24 h) and insulin stimulation (100 nM for 10 min). (B) Quantification of experiments shown in panel A (n = 4). All conditions were normalized to cells treated with PBS and stimulated with insulin. (C) Representative Western blot of total Akt and phosphorylated Akt (S473 and T308) from contact coculture of 3T3L1 adipocytes and RAW264.7 macrophages after treatment with holo-RBP4 (50 μg/ml for 24 h) and insulin stimulation (10 nM or 100 nM for 10 min). (D) Quantification of experiments shown in panel C (n = 3). All conditions were normalized to cells treated with PBS and stimulated with 10 nM insulin. *, P < 0.05 versus vehicle under the same insulin conditions; #, P < 0.05 versus other insulin conditions for no RBP4 by two-way analysis of variance (ANOVA) with post hoc analysis. (E) Representative Western blot of total Akt and phosphorylated Akt (S473) from noncontact Transwell coculture of 3T3L1 adipocytes and RAW264.7 macrophages after treatment with holo-RBP4 (50 μg/ml for 24 h) and insulin (100 nM for 10 min). (F) Quantification of exper-iments shown in panel E (n = 4). All conditions were normalized to cells treated with PBS and then stimulated with insulin. Macs, macrophages. *, P < 0.05 versus no RBP4 in the absence or presence of macrophages; #, P < 0.05 versus absence of macrophages with same RBP4 treatment by two-way ANOVA with post hoc analysis. (G) Representative Western blot of total Akt and phosphorylated Akt (S473) from contact coculture of 3T3L1 adipocytes and RAW264.7 macrophages after treatment with neutralizing antibodies (abs) (1 or 5 μg/ml as indicated) for 30 min prior to holo-RBP4 (50 μg/ml for 24 h) treatment and then insulin (100 nM for 10 min). (H) Quantification of experiments shown in panel G (n = 2). All conditions were normalized to cells treated with PBS and then stimulated with insulin. *, P < 0.05 versus no RBP4 with control IgG; #, P < 0.05 versus RBP4 treatment with control IgG. Data are expressed as means ± SE except for panel H, in which n = 2.
The signaling results in Fig. 2C and D are from the mixture of adipocytes and macrophages, although the macrophages contribute relatively little to the total cellular protein. However, to definitively show that the effect on Akt phosphorylation occurs in adipocytes and not in macrophages and that it results from a secreted protein, we cocultured adipocytes with macrophages in a noncontact, Transwell system. RBP4 treatment suppressed insulin-dependent Akt phosphorylation by 30% in adipocytes cocultured with macrophages in the noncontact system, similar to the results in the direct contact system, but not in adipocytes alone (Fig. 2E and F). These results imply that macrophage-derived proinflammatory cytokines induced by RBP4 may mediate the impairment in insulin signaling. To test this, we next investigated the effect of neutralizing antibodies against proinflammatory cytokines on RBP4-mediated inhibition of insulin signaling in adipocytes cocultured with macrophages. Pretreatment with neutralizing antibodies for TNF-α, IL-6, or MCP-1 (1 or 5 μg/ml) for 30 min prior to RBP4 treatment prevented RBP4-dependent inhibition of insulin-stimulated Akt phosphorylation compared to the level with control IgG (Fig. 2G and H). Each of these antibodies had a significant effect, and treatment with all three neutralizing antibodies completely reversed RBP4-dependent inhibition of insulin signaling (Fig. 2G and H). These findings indicate that RBP4 can inhibit insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages.
RBP4 activates signaling pathways in macrophages.
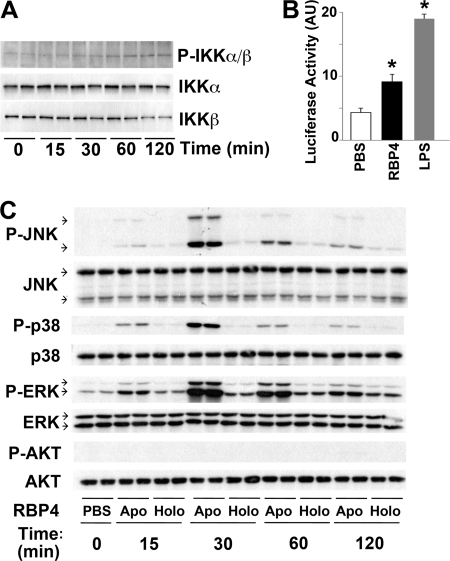
Next we sought to determine which pathways RBP4 activates in macrophages. We first tested the IκB kinase (IKK)/NF-κB pathway, which plays a central role in cytokine production in macrophages (57). RBP4 increased IKKα/β phosphorylation in cultured macrophages (Fig. 3A) and NF-κB transcriptional activity, as determined by a 2-fold increase in NF-κB luciferase activity (Fig. 3B). Lipopolysaccharide (LPS) served as a positive control.
Fig 3.
RBP4 activates proinflammatory signaling pathways in macrophages. (A) Western blot of total and phosphorylated IKKα/IKKβ from RAW264.7 macrophages treated with holo-RBP4 (50 μg/ml) for the indicated times. (B) Quantification of NF-κB promoter activity in RAW264.7 macrophages transfected with pNFκB-Luc reporter plasmid and then treated with holo-RBP4 (50 μg/ml for 18 h). LPS (10 ng/ml) was used as a positive control. AU, arbitrary units. Data are expressed as the mean ± SE and normalized to β-galactosidase (β-Gal) expression. *, P < 0.05 versus PBS. (C) Western blot of total and phosphorylated Akt, ERK, p38, and JNK from RAW264.7 cells that were treated with either apo- or holo-RBP4 (50 μg/ml) for the indicated times.
We next examined whether RBP4 activates other proinflammatory pathways. Both apo-RBP4 and holo-RBP4 treatment of macrophages activated the JNK, p38, and ERK pathways, with a peak effect at 30 min (Fig. 3C). Similar to the results with cytokine release in RAW264.7 macrophages (Fig. 1C), mouse apo-RBP4 had a much greater effect than holo-RBP4. In contrast, neither apo-RBP4 nor holo-RBP4 activated the Akt pathway in macrophages (Fig. 3C). These results suggest that both holo- and apo-RBP4 activate proinflammatory pathways at several steps.
RBP4 induces proinflammatory cytokines through the JNK pathway in macrophages.
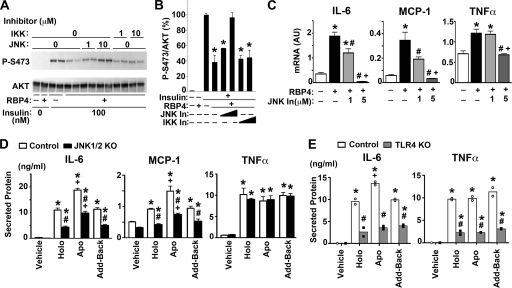
We next sought to determine which pathway is responsible for RBP4-mediated induction of proinflammatory cytokines in macrophages and for RBP4-mediated inhibition of insulin signaling in adipocytes that are cocultured with macrophages. IKK/NF-κB and JNK play central roles in inflammatory pathways leading to insulin resistance (2, 7, 24). We therefore tested the effect of specific inhibitors for JNK or IKK on RBP4-mediated inhibition of insulin signaling in 3T3L1 adipocytes cocultured with macrophages. Insulin-dependent Akt phosphorylation was again inhibited by RBP4 treatment (Fig. 4A and B). A JNK-specific inhibitor reversed this RBP4-dependent inhibition of insulin signaling in a concentration-dependent manner, while an IKK-specific inhibitor did not (Fig. 4A and B). The JNK inhibitor also blocked RBP4-mediated induction of proinflammatory cytokines TNF-α, IL-6, and MCP-1 in a concentration-dependent manner (Fig. 4C).
Fig 4.
JNK and TLR4 at least partially mediate the effects of RBP4 on insulin stimulation and cytokine production. (A) Western blot of total and phosphorylated Akt in 3T3L1 adipocytes from direct contact coculture with RAW264.7 macrophages. Cells were pretreated with JNK or IKK inhibitor (1 or 10 μM for 30 min), and then human holo-RBP4 was added (50 μg/ml for 24 h) prior to insulin (100 nM for 10 min). (B) Quantification of experiments shown in panel A. All conditions were normalized to cells treated with PBS and then stimulated with insulin. Data are means ± SE. *, P < 0.05 versus insulin-stimulated (+) with no RBP4. (C) mRNA expression of TNF-α, IL-6, and MCP-1 in RAW264.7 macrophages treated with JNK inhibitor (1 or 5 μM) and RBP4 as in panel A. The control for RBP4 was dialysate, and the control for JNK inhibitor was dimethyl sulfoxide (DMSO). AU, arbitrary units. Data are means ± SE and normalized to GAPDH expression (n = 3 to 4). *, P < 0.05 versus no RBP4; #, P < 0.05 versus no JNK inhibitor; +, P < 0.05 versus RBP4 plus 1 μM JNK inhibitor, one-way ANOVA. In, inhibitor. (D) TNF-α, IL-6, and MCP-1 secretion from primary mouse macrophages stimulated with holo- or apo-RBP4 or add-back RBP4 (50 μg/ml for 24 h). Macrophages were harvested from either control mice with functional JNK1 and JNK2 or mice with JNK1 and JNK2 deleted (JNK1/2 KO). Data are means ± SE (n = 4). *, P < 0.05 versus vehicle (dialysate) treatment within the same genotype; #, P < 0.05 for JNK1/2 KO versus control with same treatment; +, P < 0.05 versus holo-RBP4 and add-back RBP4 treatment within same genotype by two-way ANOVA with post hoc analysis. (E) TNF-α and IL-6 secretion from primary mouse macrophages stimulated with human holo- or apo-RBP4 or add-back RBP4 (50 μg/ml for 24 h). Macrophages were harvested and pooled from either two wild-type mice (open circles) or two mice with TLR4 deleted (TLR4 KO) (closed circles), and the experiments were done in duplicate. The bars show the mean with individual values indicated by circles (n = 2). *, P < 0.05 versus vehicle (dialysate) treatment within the same genotype; #, P < 0.05 for TLR4 KO versus control with same treatment; +, P < 0.05 versus holo- and add-back RBP4 treatment within same genotype by two-way ANOVA with post hoc analysis.
To further determine the role of JNK in RBP4 induction of cytokines, we studied the effects in primary mouse macrophages lacking JNK1 and JNK2. In control macrophages with functional JNK1/2, holo-RBP4 stimulated IL-6, MCP-1, and TNF-α secretion and apo-RBP4 had a greater effect on IL-6 and MCP-1 (Fig. 4D), consistent with effects in cultured macrophages (Fig. 1C). In the JNK1−/− JNK2−/− macrophages, the induction of IL-6 and MCP-1 secretion by both holo- and apo-RBP4 was reduced by 35 to 75% relative to control macrophages, demonstrating a JNK-dependent effect for both holo- and apo-RBP4. TNF-α induction was also retinol independent and was independent of JNK in these cells. Taken together, these results suggest that RBP4 inhibits insulin signaling in adipocytes (Fig. 4A and B) by inducing proinflammatory cytokines in macrophages, in part through the JNK pathway (Fig. 4C and D) and that this does not require that RBP4 be bound to retinol.
To understand whether this effect was mediated by STRA6 (stimulated by retinoic acid 6), the only known specific receptor for RBP4 (5), we measured STRA6 expression by quantitative PCR in all of the types of macrophages used in our studies. We did not find expression of STRA6 in cultured or primary mouse macrophages from either the peritoneum or the bone marrow from several mouse strains (data not shown). Furthermore, we did not detect STRA6 in cultured or primary human macrophages (data not shown).
To determine whether another cell surface receptor could be involved, we tested the role of the TLR4 receptor since phosphorylation of JNK, ERK, and p38 and subsequent cytokine secretion can be initiated by stimulation of TLR4 (21). A previous publication indicated that TLR4 was involved in cytokine secretion from macrophages stimulated with holo-RBP4 (11). We sought to test whether TLR4 is necessary for the effects of both holo- and apo-RBP4 in primary macrophages (Fig. 4E). In wild-type primary mouse macrophages, holo- and apo-RBP4 and add-back RBP4 stimulate IL-6 and TNF-α secretion. However, in TLR4−/− macrophages, IL-6 and TNF-α secretion is attenuated by 60 to 80% (Fig. 4E). The partial suppression of IL-6 secretion in TLR4−/− macrophages is similar to that seen in the JNK1−/− JNK2−/− macrophages. In contrast, TNF-α secretion is also attenuated in the TLR4−/− macrophages but not in the JNK1−/− JNK2−/− macrophages, suggesting that another pathway downstream of TLR4 may also be involved.
The RBP4/retinol ratio is elevated in insulin-resistant humans independent of obesity.
Since the cytokine-induced impairment of insulin signaling by RBP4 is retinol independent, we wanted to know the potential physiological significance of apo-RBP4. Apo-RBP4 has been shown to be elevated in humans with obesity, type 2 diabetes, and/or renal failure (12, 13, 39). To determine whether apo-RBP4 is elevated in association with obesity per se or in insulin-resistant states regardless of BMI, we measured the ratio of apo- to holo-RBP4 in insulin-sensitive and insulin-resistant lean humans and in insulin-resistant obese subjects. The lean insulin-resistant and obese insulin-resistant subjects were matched for equivalent levels of insulin resistance, as determined by the glucose disposal rate during a euglycemic-hyperinsulinemic clamp (Table 1).
Table 1.
Anthropometric and metabolic characteristics of human subjects included in Fig. 5
| Characteristic | Result for groupa: |
||
|---|---|---|---|
| Lean insulin sensitive | Lean insulin resistant | Obese insulin resistant | |
| Gender (no. male/female) | 4/4 | 4/4 | 4/4 |
| Age (yr) | 42.2 ± 2.4 | 36.6 ± 2.3 | 39.0 ± 2.6 |
| BMI (kg/m2) | 22.3 ± 0.5 | 22.3 ± 0.5 | 31.1 ± 0.3 |
| GIR (mg/kg LBM × min)b | 19.3 ± 0.7 | 8.19 ± 0.5 | 8.09 ± 0.8 |
| HbA1c (%) | 4.14 ± 0.07 | 4.18 ± 0.05 | 4.09 ± 0.12 |
All subjects were nondiabetic. Except for gender, data are expressed as means ± SE. n = 8 per group.
The glucose infusion rate (GIR) was determined by euglycemic-hyperinsulinemic clamp as described previously (6). Lean body mass (LBM) was determined by bioimpedance analysis.
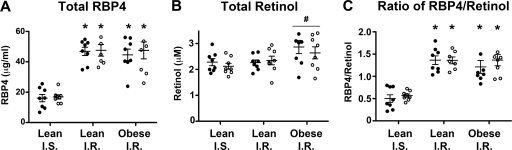
Our data show that total RBP4 levels are increased by 2.5- to 3-fold in both lean and obese insulin-resistant subjects (Fig. 5A). The magnitudes of elevation in lean and obese insulin-resistant subjects are similar. While total serum retinol levels do not vary significantly between insulin-sensitive and insulin-resistant lean subjects, retinol is slightly elevated in obese subjects relative to the level in the lean groups combined (Fig. 5B). We calculated the molar ratio of total RBP4 to total retinol and found that the ratios were equivalently elevated in lean and obese insulin-resistant subjects (Fig. 5C). This demonstrates that apo-RBP4 relative to holo-RBP4 is increased in the setting of insulin resistance even in lean subjects and that it does not require obesity for this elevation to occur. Infusion of insulin and glucose (euglycemic clamp) did not alter RBP4, retinol, or RBP4/retinol ratio in any group.
Fig 5.
The ratio of apo-RBP4 to holo-RBP4 is elevated in human subjects with insulin resistance but not altered by insulin-glucose infusion during a euglycemic-hyperinsulinemic clamp. Values in serum from lean insulin-sensitive (I.S.), lean insulin-resistant (I.R.), and obese insulin-resistant nondiabetic subjects before (closed circles) and after (open circles) euglycemic-hyperinsulinemic clamp are shown. RBP4 levels (μg/ml) were measured by mass spectrometry immunoassay (A), and total serum retinol (μM) was measured by HPLC (B). (C) The molar concentration of serum RBP4 and retinol was used to calculate the molar ratio of RBP4 to retinol in serum for each subject. Data are means ± SE, with individual values shown (n = 8 per group). *, P < 0.05 versus lean insulin-sensitive subjects both before and after euglycemic-hyperinsulinemic clamp, as determined by two-way ANOVA with post hoc analysis. #, P < 0.05 for retinol values in obese subjects versus the two lean groups combined. The lean groups were combined because there was no difference in retinol values between the lean groups. The retinol values for each subject before and after clamp were averaged for comparison of obese versus lean serum retinol values by t test.
DISCUSSION
Mounting evidence indicates that stimulation of proinflammatory macrophages is a critical component for maintaining chronic inflammation in adipose tissue in the setting of obesity-induced insulin resistance (42, 55, 62, 63). However, there is debate over what factors establish and maintain this inflammation. Our study provides evidence that the elevated RBP4 levels associated with insulin resistance and obesity (19, 31, 47, 65) may contribute to either initiating or sustaining this proinflammatory state by activating macrophages. Through this mechanism, RBP4 indirectly impairs insulin action in adipocytes. Furthermore, this effect of RBP4 is mediated, in part, through the JNK and TLR4 pathways and is independent of retinol binding to RBP4.
We show that RBP4 induces expression and secretion of proinflammatory cytokines in primary human macrophages as well as in mouse macrophages. The robust RBP4-stimulated cytokine response in primary human cells results in production of many proinflammatory cytokines, including TNF-α, IL-6, MCP-1, IFN-γ, GM-CSF, IL-1β, IL-2, and IL-12p70. The concomitant decrease in PPARγ, a negative regulator of inflammation, indicates that reduction in PPARγ could be part of the mechanism for the proinflammatory effects of RBP4. Similar to the effects in obesity, whereby localized adipose tissue inflammation promotes insulin resistance (42), we find that RBP4-induced cytokine production from macrophages causes insulin resistance in adipocytes, and this is blocked by neutralizing antibodies for several cytokines. We also find that RBP4 strongly induces IL-10, which is typically considered an anti-inflammatory cytokine. This may be a compensatory response to the proinflammatory signals since the simultaneous induction of pro- and anti-inflammatory cytokines also occurs in the inflammation induced by a high-fat diet (14).
Multiple redundant and overlapping signaling pathways mediate proinflammatory effects in cells (21, 42). We show that RBP4 activates the p38, ERK, NF-κB, and JNK signaling pathways in macrophages. In our studies, blocking of JNK signaling caused the greatest decrease in RBP4-stimulated cytokine secretion and in the resulting insulin resistance in adipocytes. The reduced effect of RBP4 on stimulation of IL-6 and MCP-1 in JNK1−/− JNK2−/− macrophages strongly supports a critical role for JNK in RBP4-induced inflammation. The persistent effect on TNF-α production in these macrophages is not surprising because TNF-α production is stimulated by multiple signaling pathways, but IL-6 production is more indicative of JNK activation (59).
A recent report indicated that holo-RBP4 induces TNF-α and IL-6 through a TLR4-dependent pathway (11). We show that TLR4 is necessary for complete stimulation of cytokine release from macrophages treated with either holo- or apo-RBP4. The fact that RBP4-induced TNF-α secretion is attenuated in TLR4−/− macrophages but not in JNK1−/− JNK2−/− macrophages indicates another pathway downstream of TLR4 may be involved in the RBP4 effects. The fact that the RBP4 effects are not completely blocked in TLR4−/− macrophages suggests that additional parallel pathways may be involved.
Since RBP4's most well-defined function is to deliver retinol to tissues and retinol has many important effects on the immune system, it would not have been surprising if the action of RBP4 on adipose tissue inflammation was retinol dependent. However, we find that apo-RBP4 elicits a cytokine response as robust as that of holo-RBP4 in macrophages. While we observe a 10- to 100-fold-enhanced effect of mouse apo-RBP4 in the mouse macrophage cell line, RAW264.7, (Fig. 1C and 3C), we do not observe this with human apo-RBP4 in the same cell line or in primary human macrophages (Fig. 1D). The effect of human apo-RBP4 in primary mouse macrophages is only about 50 to 80% greater than that of holo-RBP4 (Fig. 4D and E). The reason for the differential effects of mouse RBP4 and human RBP4 is not known, but the important conclusion is that RBP4 can elicit cytokine secretion from macrophages in a retinol-independent manner.
Our data show an increase in serum apo-RBP4 (Fig. 5C) in both lean and obese insulin-resistant subjects. This implies that elevation of RBP4, and not a concordant increase in retinol, is associated with insulin resistance. It is not obesity per se that is associated with increased apo-RBP4 but rather insulin resistance. Interestingly, glucose-insulin infusion did not alter the levels of retinol, RBP4, or the RBP4/retinol ratio in lean or obese subjects, underscoring the relative stability of these levels.
The data in this paper demonstrate a novel alternative mechanism for RBP4 action and demonstrate that the RBP4 protein directly induces proinflammatory signaling pathways independent of retinol. Furthermore, we find that the effects of RBP4 to impair insulin signaling in adipocytes are indirect since they require the presence of macrophages (Fig. 2A to F). In contrast, a recent study reported that RBP4 inhibits insulin signaling directly in adipocytes by activating JAK2/STAT5 signaling, which induced SOCS3 expression (4). This mechanism is retinol-dependent and requires the membrane protein STRA6. The proinflammatory actions of RBP4 in macrophages are not STRA6 dependent since we did not detect STRA6 expression in any of the macrophages used in our studies. A STRA6-independent effect is not surprising since overall, the role of STRA6 in RBP4 actions and retinoid biology is controversial (5). The results presented here do not contradict the findings of Berry et al. (4) since the effects of the JAK/STAT pathway in adipocytes may be retinol and STRA6 dependent, whereas the effects of RBP4 on cytokine production in macrophages are not. Differences in RBP4 signaling in adipocytes and macrophages are also indicated by our finding that RBP4 increases ERK activation in macrophages, in contrast to a previous report showing that RBP4 impairs insulin-stimulated ERK signaling in adipocytes (43). Importantly, both direct and indirect effects of RBP4 on adipocyte insulin signaling may contribute to insulin resistance.
In conclusion, we have identified a novel mechanism for RBP4 action that does not require either retinol or STRA6. We show that RBP4 induces a proinflammatory response in macrophages through JNK- and TLR4-dependent pathways, and the resulting cytokine production causes insulin resistance in adipocytes. These findings provide insights into the cellular mechanisms by which RBP4 causes insulin resistance and could lead to new therapeutic approaches to reduce obesity-related inflammation and insulin resistance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Madhumita Das for providing Mx1-Cre JNK1fl/fl JNK2−/− mice, Katherine A. Fitzgerald for providing TLR4−/− mice, and Zhaozhao Jiang for assistance with TLR4−/− macrophage isolation. We also thank Simon Dillon at the Beth Israel Deaconess Medical Center Genomics and Proteomics Center and Edwin A. Homan for assistance with the measurement of serum retinol and Odile D. Peroni for critical assistance with experiments.
This work was supported by National Institutes of Health grants NIDDK F32 DK091041 (to J.N.) and NIDDK R37 DK43051 (to B.B.K). T.H. was supported by a Research Fellowship from the Manpei Suzuki Diabetes Foundation, Japan. B.J.K. was supported by a Research Fellowship from the German Cardiac Society. R.J.D. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Published ahead of print 19 March 2012
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1. Ahima RS, Osei SY. 2008. Adipokines in obesity. Front. Horm. Res. 36:182–197 [DOI] [PubMed] [Google Scholar]
- 2. Arkan MC, et al. 2005. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 11:191–198 [DOI] [PubMed] [Google Scholar]
- 3. Balagopal P, et al. 2007. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J. Clin. Endocrinol. Metab. 92:1971–1974 [DOI] [PubMed] [Google Scholar]
- 4. Berry DC, Jin H, Majumdar A, Noy N. 2011. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc. Natl. Acad. Sci. U. S. A. 108:4340–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blaner WS. 2007. STRA6, a cell-surface receptor for retinol-binding protein: the plot thickens. Cell Metab. 5:164–166 [DOI] [PubMed] [Google Scholar]
- 6. Boesgaard TW, et al. 2008. The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients—the EUGENE2 study. Diabetologia 51:816–820 [DOI] [PubMed] [Google Scholar]
- 7. Cai D, et al. 2005. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 11:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chavez AO, et al. 2009. Retinol-binding protein 4 is associated with impaired glucose tolerance but not with whole body or hepatic insulin resistance in Mexican Americans. Am. J. Physiol. Endocrinol. Metab. 296:E758–E764 [DOI] [PubMed] [Google Scholar]
- 9. Cho YM, et al. 2006. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 29:2457–2461 [DOI] [PubMed] [Google Scholar]
- 10. Das M, et al. 2009. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell 136:249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng ZB, et al. 2009. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 58:2498–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erikstrup C, et al. 2009. RBP-to-retinol ratio, but not total RBP, is elevated in patients with type 2 diabetes. Diabetes Obes. Metab. 11:204–212 [DOI] [PubMed] [Google Scholar]
- 13. Frey SK, et al. 2008. Isoforms of retinol binding protein 4 (RBP4) are increased in chronic diseases of the kidney but not of the liver. Lipids Health Dis. 7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujisaka S, et al. 2009. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58:2574–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galic S, Oakhill JS, Steinberg GR. 2010. Adipose tissue as an endocrine organ. Mol. Cell Endocrinol. 316:129–139 [DOI] [PubMed] [Google Scholar]
- 16. Gavi S, et al. 2007. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J. Clin. Endocrinol. Metab. 92:1886–1890 [DOI] [PubMed] [Google Scholar]
- 17. Gomez-Ambrosi J, et al. 2008. Serum retinol-binding protein 4 is not increased in obesity or obesity-associated type 2 diabetes mellitus, but is reduced after relevant reductions in body fat following gastric bypass. Clin. Endocrinol. (Oxf.) 69:208–215 [DOI] [PubMed] [Google Scholar]
- 18. Graham TE, Wason CJ, Bluher M, Kahn BB. 2007. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia 50:814–823 [DOI] [PubMed] [Google Scholar]
- 19. Graham TE, et al. 2006. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 354:2552–2563 [DOI] [PubMed] [Google Scholar]
- 20. Gudas LJ. 2012. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim. Biophys. Acta 1821:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guha M, Mackman N. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 13:85–94 [DOI] [PubMed] [Google Scholar]
- 22. Haider DG, et al. 2007. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 92:1168–1171 [DOI] [PubMed] [Google Scholar]
- 23. Harmon GS, Lam MT, Glass CK. 2011. PPARs and lipid ligands in inflammation and metabolism. Chem. Rev. 111:6321–6340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirosumi J, et al. 2002. A central role for JNK in obesity and insulin resistance. Nature 420:333–336 [DOI] [PubMed] [Google Scholar]
- 25. Hosooka T, et al. 2008. Dok1 mediates high-fat diet-induced adipocyte hypertrophy and obesity through modulation of PPAR-gamma phosphorylation. Nat. Med. 14:188–193 [DOI] [PubMed] [Google Scholar]
- 26. Ingelsson E, et al. 2009. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis 206:239–244 [DOI] [PubMed] [Google Scholar]
- 27. Jia W, et al. 2007. Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J. Clin. Endocrinol. Metab. 92:3224–3229 [DOI] [PubMed] [Google Scholar]
- 28. Kane MA, Folias AE, Napoli JL. 2008. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal. Biochem. 378:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kershaw EE, Flier JS. 2004. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89:2548–2556 [DOI] [PubMed] [Google Scholar]
- 30. Kiernan UA, Phillips DA, Trenchevska O, Nedelkov D. 2011. Quantitative mass spectrometry evaluation of human retinol binding protein 4 and related variants. PLoS One 6:e17282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klöting N, et al. 2007. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 6:79–87 [DOI] [PubMed] [Google Scholar]
- 32. Kotnik P, Fischer-Posovszky P, Wabitsch M. 2011. RBP4—a controversial adipokine. Eur. J. Endocrinol. 165:703–711 [DOI] [PubMed] [Google Scholar]
- 33. Lago R, Gomez R, Lago F, Gomez-Reino J, Gualillo O. 2008. Leptin beyond body weight regulation—current concepts concerning its role in immune function and inflammation. Cell. Immunol. 252:139–145 [DOI] [PubMed] [Google Scholar]
- 34. Lebrun P, Van Obberghen E. 2008. SOCS proteins causing trouble in insulin action. Acta Physiol. (Oxf.) 192:29–36 [DOI] [PubMed] [Google Scholar]
- 35. Lee DC, Lee JW, Im JA. 2007. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism 56:327–331 [DOI] [PubMed] [Google Scholar]
- 36. Lee JW, et al. 2007. Visceral adiposity is associated with serum retinol binding protein-4 levels in healthy women. Obesity (Silver Spring). 15:2225–2232 [DOI] [PubMed] [Google Scholar]
- 37. McGrane MM. 2007. Vitamin A regulation of gene expression: molecular mechanism of a prototype gene. J. Nutr. Biochem. 18:497–508 [DOI] [PubMed] [Google Scholar]
- 38. Meisinger C, et al. 2011. Retinol-binding protein 4 is associated with prediabetes in adults from the general population: the Cooperative Health Research in the Region of Augsburg (KORA) F4 Study. Diabetes Care 34:1648–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mills JP, Furr HC, Tanumihardjo SA. 2008. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp. Biol. Med. (Maywood) 233:1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munkhtulga L, et al. 2010. Regulatory SNP in the RBP4 gene modified the expression in adipocytes and associated with BMI. Obesity (Silver Spring). 18:1006–1014 [DOI] [PubMed] [Google Scholar]
- 41. Ng TW, Watts GF, Barrett PH, Rye KA, Chan DC. 2007. Effect of weight loss on LDL and HDL kinetics in the metabolic syndrome: associations with changes in plasma retinol-binding protein-4 and adiponectin levels. Diabetes Care 30:2945–2950 [DOI] [PubMed] [Google Scholar]
- 42. Olefsky JM, Glass CK. 2010. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72:219–246 [DOI] [PubMed] [Google Scholar]
- 43. Ost A, et al. 2007. Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. FASEB J. 21:3696–3704 [DOI] [PubMed] [Google Scholar]
- 44. Pino-Lagos K, Guo Y, Noelle RJ. 2010. Retinoic acid: a key player in immunity. Biofactors 36:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Preitner F, Mody N, Graham TE, Peroni OD, Kahn BB. 2009. Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297:E1420–E1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Promintzer M, et al. 2007. Insulin resistance is unrelated to circulating retinol binding protein and protein C inhibitor. J. Clin. Endocrinol. Metab. 92:4306–4312 [DOI] [PubMed] [Google Scholar]
- 47. Qi Q, et al. 2007. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J. Clin. Endocrinol. Metab. 92:4827–4834 [DOI] [PubMed] [Google Scholar]
- 48. Redlich CA, et al. 1996. Characterization of carotenoid, vitamin A, and alpha-tocopheral levels in human lung tissue and pulmonary macrophages. Am. J. Respir. Crit. Care Med. 154:1436–1443 [DOI] [PubMed] [Google Scholar]
- 49. Rocha VZ, Folco EJ. 2011. Inflammatory concepts of obesity. Int. J. Inflamm. 2011:529061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saltiel AR, Kahn CR. 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806 [DOI] [PubMed] [Google Scholar]
- 51. Samuel VT, Petersen KF, Shulman GI. 2010. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375:2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shi H, Cave B, Inouye K, Bjorbaek C, Flier JS. 2006. Overexpression of suppressor of cytokine signaling 3 in adipose tissue causes local but not systemic insulin resistance. Diabetes 55:699–707 [DOI] [PubMed] [Google Scholar]
- 53. Silswal N, et al. 2005. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem. Biophys. Res. Commun. 334:1092–1101 [DOI] [PubMed] [Google Scholar]
- 54. Solini A, Santini E, Madec S, Rossi C, Muscelli E. 2009. Retinol-binding protein-4 in women with untreated essential hypertension. Am. J. Hypertens. 22:1001–1006 [DOI] [PubMed] [Google Scholar]
- 55. Suganami T, Nishida J, Ogawa Y. 2005. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 25:2062–2068 [DOI] [PubMed] [Google Scholar]
- 56. Tschoner A, et al. 2008. Retinol-binding protein 4, visceral fat, and the metabolic syndrome: effects of weight loss. Obesity (Silver Spring) 16:2439–2444 [DOI] [PubMed] [Google Scholar]
- 57. Vallabhapurapu S, Karin M. 2009. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27:693–733 [DOI] [PubMed] [Google Scholar]
- 58. van Hoek M, et al. 2008. An RBP4 promoter polymorphism increases risk of type 2 diabetes. Diabetologia 51:1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ventura JJ, et al. 2006. Chemical genetic analysis of the time course of signal transduction by JNK. Mol. Cell 21:701–710 [DOI] [PubMed] [Google Scholar]
- 60. Villamor E, Fawzi WW. 2005. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 18:446–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weisberg SP, et al. 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wellen KE, Hotamisligil GS. 2005. Inflammation, stress, and diabetes. J. Clin. Invest. 115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu H, et al. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang Q, et al. 2012. Quantitative measurement of full-length and C-terminal proteolyzed RBP4 in serum of normal and insulin-resistant humans using a novel mass spectrometry immunoassay. Endocrinology 153:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang Q, et al. 2005. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436:356–362 [DOI] [PubMed] [Google Scholar]
- 66. Yao-Borengasser A, et al. 2007. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J. Clin. Endocrinol. Metab. 92:2590–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yoshida A, Matsutani Y, Fukuchi Y, Saito K, Naito M. 2006. Analysis of the factors contributing to serum retinol binding protein and transthyretin levels in Japanese adults. J. Atheroscler. Thromb. 13:209–215 [DOI] [PubMed] [Google Scholar]
- 68. Yu XX, Watts LM, Manchem P, Monia BP, Bhanot S. 2008. Antisense reduction of retinol-binding protein 4 expression in liver and adipose tissues causes robust improvements in insulin sensitivity in diabetic and obese mice. Diabetes 57(Suppl 1A):LB21 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.