Abstract
Adhesion and degranulation-promoting adapter protein (ADAP) is a multifunctional scaffold that regulates T cell receptor-mediated activation of integrins via association with the SKAP55 adapter and the NF-κB pathway through interactions with both the CARMA1 adapter and serine/threonine kinase transforming growth factor β-activated kinase 1 (TAK1). ADAP-deficient T cells exhibit impaired proliferation following T cell receptor stimulation, but the contribution of these distinct functions of ADAP to this defect is not known. We demonstrate that loss of ADAP results in a G1-S transition block in cell cycle progression following T cell activation due to impaired accumulation of cyclin-dependent kinase 2 (Cdk2) and cyclin E. The CARMA1-binding site in ADAP is critical for mitogen-activated protein (MAP) kinase kinase 7 (MKK7) phosphorylation and recruitment to the protein kinase C θ (PKCθ) signalosome and subsequent c-Jun kinase (JNK)-mediated Cdk2 induction. Cyclin E expression following T cell receptor stimulation of ADAP-deficient T cells is transient and associated with enhanced cyclin E ubiquitination. Both the CARMA1- and TAK1-binding sites in ADAP are critical for restraining cyclin E ubiquitination and turnover independently of ADAP-dependent JNK activation. T cell receptor-mediated proliferation was most dramatically impaired by the loss of ADAP interactions with CARMA1 or TAK1 rather than SKAP55. Thus, ADAP coordinates distinct CARMA1-dependent control of key cell cycle proteins in T cells.
INTRODUCTION
Tlymphocyte activation requires physical contact with an antigen-presenting cell and the propagation of signals from the antigen-specific T cell receptor (TCR) that result in proliferation and differentiation. Adapter proteins coordinate the assembly of signalosomes that are essential for optimal T cell activation (36). In T cells, adhesion and degranulation-promoting adapter protein (ADAP) positively regulates T cell receptor signaling by facilitating the activation of integrin receptors that enhances T cell contact with antigen-presenting cells and by promoting the activation of NF-κB (4, 5, 16, 24, 28, 38, 46). These two functions of ADAP are controlled by biochemically and functionally distinct pools of ADAP that are defined by SKAP55, another adapter that constitutively associates with a subset of the total ADAP expressed in a T cell (4, 5). The pool of ADAP associated with SKAP55 regulates integrin function, while the pool of ADAP not associated with SKAP55 regulates NF-κB via TCR-inducible association with the CARMA1 adapter and the serine/threonine kinase transforming growth factor β-activated kinase 1 (TAK1) (4, 5, 24, 38). These inducible interactions facilitate the formation of the CARMA1-Bcl10-Malt1 (CBM) complex and the assembly of the protein kinase C θ (PKCθ) signalosome that are required for optimal T cell receptor-mediated activation of NF-κB (42). Three discrete sites in ADAP mediate the association of ADAP with SKAP55, CARMA1, and TAK1 (24, 38). T cells lacking ADAP exhibit impaired TCR-mediated proliferation (16, 27, 28), but the contribution of these individual protein interactions with ADAP to this proliferative defect remains undefined.
The successful progression of T cells through the cell cycle following TCR stimulation involves the temporal induction and activation of cyclins and cyclin-dependent kinases (Cdk's) (47). D-type cyclins, Cdk4, and Cdk6 are induced during the G1 phase of the cell cycle, followed by the induction of cyclin E and the induction and activation of Cdk2 at the late G1 restriction point. Expression of cyclin E is controlled by transcriptional regulation of cyclin E as well as by ubiquitin-dependent degradation of cyclin E. Both the cullin-3 E3 ubiquitin ligase (6, 37) and the SCFFbw7 E3 ubiquitin ligase control cyclin E levels in a manner that is dependent on cyclin E phosphorylation and the association of cyclin E with Cdk2 (20, 25, 40, 48). The signaling pathways that control the induction of cell cycle regulatory proteins in T cells remain incompletely characterized. NF-κB has been implicated in the activation of cyclin D1 and cyclin A transcription, and IκB kinase (IKK) has been proposed to play a role in cell cycle regulation (1, 13, 18, 19, 21, 30). The c-Jun kinase (JNK) signaling pathway has also been reported to regulate cell cycle progression of multiple cell types. In fibroblasts, the JNK1 and JNK2 isoforms differentially regulate G1-S-phase transition and cell cycle progression via c-Jun, a downstream target of JNK (34). Similar differential functions for JNK1 and JNK2 have also been reported for T cell differentiation and various T cell functional responses that may be affected by the differentiation and activation state of the T cell (7, 11, 29, 41). However, T cells lacking either JNK1 or JNK2 display impaired proliferation in response to antibody stimulation of the TCR and the CD28 coreceptor, suggesting common functions for JNK1 and JNK2 in T cells (35).
The CARMA1 signalosome may be particularly critical for the induction and activation of cell cycle regulatory proteins following TCR stimulation, as T cells lacking either CARMA1, Bcl10, or Malt1 exhibit impaired activation of NF-κB and JNK (3, 12, 15, 17, 31–33, 44). In this study, we demonstrate that the impaired proliferative response of ADAP-deficient T cells is associated with impaired induction of Cdk2 and increased turnover of cyclin E that is mediated by discrete ADAP-dependent pathways. ADAP association with CARMA1, but not TAK1, is critical for JNK-dependent expression of Cdk2, while the stabilization of cyclin E is JNK independent and impaired by loss of ADAP association with either CARMA1 or TAK1.
MATERIALS AND METHODS
Mice.
ADAP−/− mice and DO11.10/ADAP−/− mice crossed to hCAR transgenic mice expressing the human coxsackie adenovirus receptor have been previously described (5, 24, 27). CARMA1−/− mice were provided by M. Farrar (University of Minnesota). Mice were housed in specific-pathogen-free facilities at the University of Minnesota and were used at between 8 and 12 weeks of age. All experimental protocols involving the use of mice were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Antibodies and reagents.
Anti-Thy1.1 allophycocyanin (APC), anti-CD4 Alexa Fluor 700, anti-CD8 peridinin chlorophyll protein (PerCP)-Cy5.5, anti-CD11c PerCP-Cy5.5, anti-B220 PerCP-Cy5.5, anti-D011.10 TCR biotin KJ1-26, and streptavidin phycoerythrin-Cy7 were purchased from eBioscience (San Diego, CA). Anti-extracellular signal-regulated kinase (anti-ERK; 9102), anti-phospho-ERK (9101), anti-Jnk (56G8), anti-CycD1 (2926), anti-mitogen-activated protein (anti-MAP) kinase (anti-MAPK) kinase 7 (anti-MKK7; 4172), anti-phospho-MKK7 (4171S), anti-MKK4 (9152), anti-phospho-MKK4 (9151S), anti-Cdk4 (2906), anti-Cdk2 (2546), anti-phospho-Cdk2 (2561S), and anti-phospho-Jnk (2924) were from Cell Signaling (Danvers, MA). Mouse anti-PKCθ (C-18), anti-Bcl10 (H-197), anti-cyclin E (H-145), anti-c-Jun, anti-phospho-c-Jun (KM-1), and antiubiquitin (P4D1) were from Santa Cruz Biotechnology (Santa Cruz, CA). Sheep anti-ADAP N terminus polyclonal antiserum was the kind gift of G. Koretzky, as previously described (5). Anti-rabbit or anti-mouse Alexa Fluor 680-conjugated antibodies (Invitrogen, Carlsbad, CA), donkey anti-sheep IR800-conjugated antibodies, and donkey anti-rabbit secondary IR680-conjugated antibodies (LI-COR Biosciences, Lincoln, NE) were used for Western blotting. 4′,6-Diamidino-2-phenylindole (DAPI), carboxyfluorescein succinimidyl ester (CFSE), and ovalbumin peptide from amino acids (aa) 323 to 339 (OVAp) were from Invitrogen. The JNK inhibitor SP600125 was purchased from Sigma.
Adenovirus production and transduction.
Adenovirus expression plasmids and recombinant adenovirus were generated as previously described (5, 24). The ADAPΔCAR (deletion of aa 426 to 541 in mouse ADAP), ADAPΔTAK (deletion of aa 691 to 708 in mouse ADAP), and ADAPΔSKAP (deletion of aa 338 to 358 in mouse ADAP) mutants were generated as previously described (5, 24, 38). Freshly isolated resting hCAR-positive (hCAR+) control and ADAP−/− lymph node T cells were transduced with control virus or ADAP virus and incubated at 37°C for 3 days in complete T cell medium containing 10 ng/ml mouse interleukin-7 (R&D Systems) as previously described (4, 5, 24, 38). All recombinant adenoviruses used in this study express a Thy1.1 expression marker, used to assess the efficiency of viral infection of resting T cells (Fig. 1A and B).
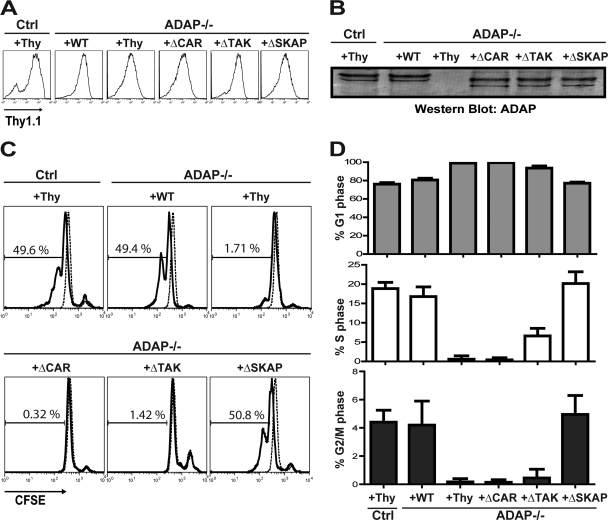
Fig 1.
Impaired proliferation and block in G1-S transition in ADAP−/− T cells upon CD3/CD28 stimulation. Purified naïve hCAR control (Ctrl) or hCAR/ADAP−/− T cells were transduced with control adenovirus expressing Thy1.1 (Thy) or adenovirus expressing Thy1.1 and wild-type ADAP (WT), ADAPΔCAR mutant (ΔCAR), ADAPΔTAK mutant (ΔTAK), or ADAPΔSKAP mutant (ΔSKAP). (A) Cells were stained with anti-Thy1.1-APC and analyzed by flow cytometry. (B) Cell lysates were prepared, and Western blotting was performed with anti-ADAP antibody. (C) Cells were labeled with CFSE and stimulated with anti-CD3 and anti-CD28 antibodies for 42 h prior to flow cytometry analysis. (D) Cell cycle analysis was performed on the transduced T cells using DAPI staining. Results for both the proliferation and cell cycle analysis are representative of at least five independent experiments.
Western blotting.
Western blotting was performed as previously described (5, 38). Briefly, freshly harvested lymphocytes were transduced as described above and then stimulated with 1 μg anti-CD3 (clone 2C11) and 0.1 μg anti-CD28 per 3 × 106 cells in a 50-μl volume. Samples were lysed at the indicated time point by the addition of 50 μl of 2% NP-40 lysis buffer (5, 38). For extended time course assays and inhibitor studies, T cells were assessed for viability by trypan blue exclusion, and an equivalent number of viable cells was lysed. The lysates were spun to pellet insoluble materials, and the supernatant was added to 4× NuPAGE sample buffer (Invitrogen) and separated by SDS-PAGE. For whole-cell lysates, 1 million cell equivalents were loaded per lane. After Western transfer, a polyvinylidene difluoride membrane was blocked with 5% bovine serum albumin (BSA) for 1 h, and primary antibody was incubated for 2 h at room temperature or overnight at 4°C in phosphate-buffered saline (PBS)–2.5% BSA–0.2% Tween 20, washed with PBS containing 0.2% Tween 20, incubated for 1 h in secondary antibody in PBS–2.5% BSA–0.2% Tween 20, and finally washed with PBS–0.2% Tween 20 and stored in PBS. The membrane was imaged with on Odyssey infrared imager (LI-COR Biosciences, Lincoln, NE).
Immunoprecipitation.
Cells were stimulated with anti-CD3/CD28 and lysed as described above at a density of 10 × 106 cells/400 μl. For immunoprecipitation, anti-PKCθ, anti-Bcl10, or anti-cyclin E antibody was cross-linked to GammaBind Plus Sepharose beads (GE Healthcare, Piscataway, NJ) at a ratio of 10 μg to 20 μl settled beads as previously described (5, 38). Beads were added to cleared cell lysates, rotated overnight at 4°C, and washed with 1% NP-40 lysis buffer, and samples were prepared for SDS-PAGE by resuspending the samples into 30 μl of 1× lysis buffer and bringing the samples to 1× SDS loading buffer and 5% β-mercaptoethanol.
Cell cycle analysis.
Cells were activated as described above and resuspended at 20 × 106 cells/ml in PBS with 5% calf serum. CFSE (5 mM in PBS) was added at a 1:1 volume to the cell suspension, and the mixture was vortexed immediately. Cells were incubated for 5 min at room temperature in the dark and then washed twice with PBS containing 5% calf serum. For DAPI staining, samples were initially stained with an antibody cocktail for surface markers and then incubated with Foxp3 fix-perm buffer (eBioscience) according to product instructions. Samples were then resuspended in 200 μl of 0.1 μg/ml DAPI in Foxp3 perm buffer for 20 min at room temperature. Cells of interest were analyzed by fluorescence-activated cell sorting (FACS) for CFSE dilution using a FACSCalibur apparatus. Cells in the undivided CFSE peak were used for cell cycle analysis by DAPI staining using a Watson (Pragmatic) model in FlowJo software (Tree Star, Ashland, OR).
In vitro activation and proliferation assays.
Naïve CD4 T cells were purified using negative selective on MACS LS columns (Miltenyi Biotec, Auburn, CA). For kinetic analysis of activation, purified T cells were cultured at a density of 2 × 106/ml in 2 ml on plates precoated overnight with anti-CD3 and anti-CD28 antibodies (both at 2 μg/ml). Cells were harvested by washing the plates with PBS at the indicated times, and samples were processed for quantitative PCR (qPCR) or Western blotting as described above. To monitor proliferation, purified T cells were transduced with different ADAP mutant viruses or control virus and then labeled with CFSE as described above. Cells were cultured at a density of 1 × 106 cells/ml for different time points (24, 36, and 48 h) on plates coated with anti-CD3 (2 μg/ml) and anti-CD28 (2 μg/ml) antibodies prior to harvesting and analysis by flow cytometry.
In vivo proliferation assay.
T cells from DO11.10 hCAR+ control or ADAP−/− mice were transduced with the different ADAP viruses and control virus as described above. Transduced cells were labeled with CFSE, and 1 × 106 cells were transferred into BALB/c mice. Two hours after T cell transfer, host mice were challenged with an intravenous injection of OVAp (5 μg). Spleens were harvested 48 h after OVAp injection. Cells were stained for surface markers CD4, CD8, Thy1.1, D011.10 TCR, B220, and CD11c, incubated with Foxp3 fix-perm buffer (eBioscience), and stained with DAPI. Cells of interest (KJ1-26+ Thy1.1+) were analyzed by FACS for CFSE dilution. Cells in the undivided CFSE peak were used for cell cycle analysis as described above.
qRT-PCR.
Quantitative real-time PCR (qRT-PCR) was performed as previously described (10). Briefly, 2 million T cells from the indicated treatment were lysed with Qiashredder columns (Qiagen, Valencia, CA) and frozen at −80°C until samples were collected at all time points. A Qiagen RNeasy Plus miniprep kit was used to isolate total mRNA, and first-strand cDNA synthesis starting with 200 ng of mRNA was performed using an Applied Biosystems cDNA kit (Applied Biosystems Life Technologies, Carlsbad, CA). qRT-PCRs were run and analyzed on an ABI 7300 thermal cycler, and the dissociation melting point and agarose gel electrophoresis were used to confirm the size and uniformity of all products. β-Actin was used to normalize samples, and the comparative threshold cycle (CT) method was used to quantify relative mRNA expression as previously described (10). The primer sets used were used β-actin (forward [Fwd], 5′-CAGCTTCTTTGCAGCTCCTT; reverse [Rev], 5′GACCAGCGCAGCGATA); CD69 (Fwd, 5′-TGGTCCTCATCACGTCCTTAATAA; Rev, 5′-TCCAACTTCTCGTACAAGCCTG); Cyclin E1 (Fwd, 5′-GACCTTTCAGTCCGCTCCA; Rev, 5′-CAATCTTGGCAATTTCTTCATC); and Cdk2 (Fwd, 5′-TGCACCAGGACCTCAAGAAA; Rev, 5′-ACGGTGAGAATGGCAGAAAG).
RESULTS
The CARMA1- and TAK1-binding sites in ADAP are critical for T cell proliferation and cell cycle progression.
We used an in vitro stimulation system to examine the requirements for ADAP in promoting proliferation and cell cycle progression following TCR stimulation. Purified wild-type or ADAP-deficient naïve T cells expressing the hCAR transgene were infected with adenovirus constructs expressing a Thy1.1 marker gene and wild-type ADAP or mutant ADAP constructs with selective mutation of motifs that regulate binding of ADAP to either SKAP55 (ADAPΔSKAP), CARMA1 (ADAPΔCAR), or TAK1 (ADAPΔTAK) prior to labeling with CFSE and stimulation with immobilized anti-CD3 and anti-CD28 antibodies (5, 24, 38). Consistent with previous results (38), each ADAP construct was expressed at comparable levels in ADAP−/− DO11.10 T cells, as shown by flow cytometry for Thy1.1 expression (Fig. 1A) and Western blotting for ADAP (Fig. 1B). ADAP−/− T cells infected with a control adenovirus exhibited impaired proliferation compared to control T cells infected with the same control adenovirus (Fig. 1C). Wild-type ADAP restored proliferation to levels similar to those for control T cells (Fig. 1C). In contrast, expression of either the ADAPΔCAR or ADAPΔTAK mutant of ADAP failed to restore proliferation. These mutants are not globally nonfunctional, as we previously demonstrated that they restore defects in integrin-dependent conjugate formation when expressed in ADAP−/− T cells (5, 24). TCR stimulation with immobilized antibody in this in vitro system obviates the usual requirement for integrin-dependent T cell adhesion to an antigen-presenting cell. Thus, we observed that the ADAPΔSKAP mutant, which does not restore the conjugate defect in ADAP−/− T cells (4, 5), is nevertheless able to restore proliferation to levels comparable to those for wild-type ADAP (Fig. 1C).
In order to examine a more physiological response that requires integrin-dependent adhesive interactions with an antigen-presenting cell, we transferred CFSE-labeled DO11.10 T cells into recipient mice and then challenged them 1 day later with antigen (ovalbumin peptide aa 323 to 339). Similar to the results observed in vitro, DO11.10 ADAP−/− T cells exhibited dramatically impaired proliferation in response to antigen challenge in vivo that was restored by expression of wild-type ADAP but not the ADAPΔCAR or ADAPΔTAK mutant (Fig. 2A). However, in this in vivo system, the ADAPΔSKAP mutant did not restore proliferation to the levels observed with either control T cells or ADAP−/− T cells expressing wild-type ADAP (Fig. 2A). We conclude that the CARMA1- and TAK1-binding sites in ADAP are critical for TCR-mediated proliferation both in vitro and in vivo.
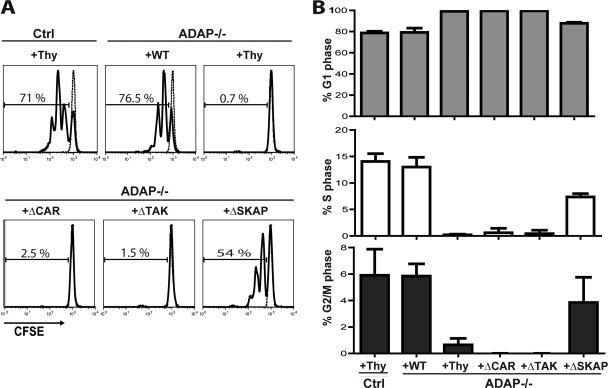
Fig 2.
Impaired proliferation and block in G1-S transition in ADAP−/− T cells upon antigen challenge in vivo. (A) Naïve DO11.10 hCAR control (Ctrl) or DO11.10 hCAR/ADAP−/− T cells were transduced with control adenovirus expressing Thy1.1 (Thy) or adenovirus expressing Thy1.1 and wild-type ADAP (WT), ADAPΔCAR mutant (ΔCAR), ADAPΔTAK mutant (ΔTAK), or ADAPΔSKAP mutant (ΔSKAP), stained with CFSE, and then transferred into BALB/c mice. Two hours after T cell transfer, host mice were injected with OVAp and spleens were harvested 42 h after OVAp injection. T cells expressing Thy1.1 and the DO11.10 T cell receptor were identified by flow cytometry and analyzed for CFSE dye dilution. (B) CD4 T cells transduced and transferred as described for panel A were analyzed for cell cycle transition using DAPI staining. Results for both the proliferation and cell cycle analysis are representative of at least three independent experiments.
The impaired proliferative response of ADAP−/− T cells and ADAP−/− T cells expressing the ADAPΔCAR or ADAPΔTAK mutant both in vitro and in vivo was associated with a dramatic increase in the percentage of T cells in the G1 phase of the cell cycle, with very few cells in the S or G2/M phases of the cell cycle (Fig. 1D and 2B). This result suggests that ADAP−/− T cells have a block in G1-S-phase transition following TCR stimulation that is dependent on the CARMA1- and TAK1-binding sites in ADAP. Compared to control T cells or ADAP−/− T cells expressing wild-type ADAP, ADAP−/− T cells expressing the ADAPΔSKAP mutant that were activated in vivo but not in vitro exhibited an increase in the percentage of cells in the G1 phase and a decrease in the percentage of cells in the S phase (Fig. 1D and 2B). This is consistent with the partial restoration of proliferation observed in vivo with the ADAPΔSKAP mutant (Fig. 2A).
Differential regulation of cyclin E and Cdk2 by the CARMA1- and TAK1-binding sites in ADAP.
Since activated ADAP−/− T cells exhibit a G1-S transition block, we examined the induction of G1-phase cell cycle regulatory mRNA and proteins during CD3/CD28 stimulation of control and ADAP−/− T cells. We first assessed mRNA levels at various time points after CD3/CD28 stimulation. At all of the time points examined, there was no impairment in mRNA levels for the early-G1-phase D-type cyclins or Cdk4 (data not shown) or the late-G1-phase cell cycle proteins cyclin E and its partner, Cdk2 (Fig. 3A), in ADAP−/− T cells. For cyclin E, we consistently observed elevated levels of cyclin E mRNA after 24, 32, and 48 h of CD3/CD28 stimulation of ADAP−/− T cells. Cyclin E protein was clearly detectable at 24 h after CD3/CD28 stimulation of wild-type control T cells and remained elevated at 32 and 48 h (Fig. 3B). In contrast, cyclin E protein was only transiently expressed in ADAP−/− T cells following CD3/CD28 stimulation. Cyclin E was detected at 17 and 24 h after CD3/CD28 stimulation of ADAP−/− T cells but was not observed at the 32- and 48-h time points. While increased expression of Cdk2 was observed following CD3/CD28 stimulation of wild-type T cells, we failed to observe increased Cdk2 protein in CD3/CD28-stimulated ADAP−/− T cells at any of the time points examined (Fig. 3B).
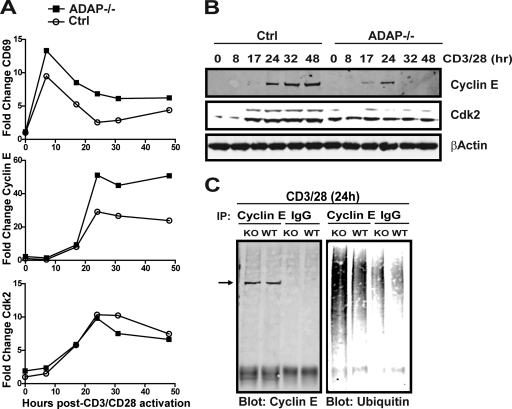
Fig 3.
ADAP is critical for efficient cyclin E and Cdk2 accumulation. (A and B) Purified naïve control (Ctrl) or ADAP−/− CD4 T cells were activated with anti-CD3 and anti-CD28 antibodies for the indicated times, and cell extracts were prepared for qRT-PCR or Western blotting. (A) qRT-PCR showing fold change relative to β-actin for the early activation marker CD69, cyclin E, and Cdk2. (B) Total cell lysates from the indicated time course performed in parallel with the experiments whose results are shown in panel A were subjected to Western blotting with antibodies to cyclin E, Cdk2, and β-actin. (C) Wild-type or ADAP−/− T cells were activated with anti-CD3 and anti-CD28 antibodies for 24 h. Cyclin E immunoprecipitates or control IgG were prepared, and Western blots were probed for cyclin E and ubiquitin. IP, immunoprecipitation; KO, knockout. Results are representative of two independent experiments performed.
Since cyclin E levels are regulated by ubiquitin-dependent proteasomal degradation (6, 20, 25, 37, 40), we next sought to assess changes in cyclin E ubiquitination in the absence of ADAP. We immunoprecipitated cyclin E from wild-type and ADAP−/− T cells stimulated for 24 h, a time point at which similar levels of cyclin E are present in both cell types (Fig. 3B). Western blotting with an anti-ubiquitin antibody showed that compared to wild-type T cells, ubiquitination was markedly elevated in cyclin E immunoprecipitates from ADAP−/− T cells (Fig. 3C). These results indicate that the transient expression of cyclin E in ADAP−/− T cells following CD3/CD28 stimulation is associated with enhanced ubiquitination of cyclin E.
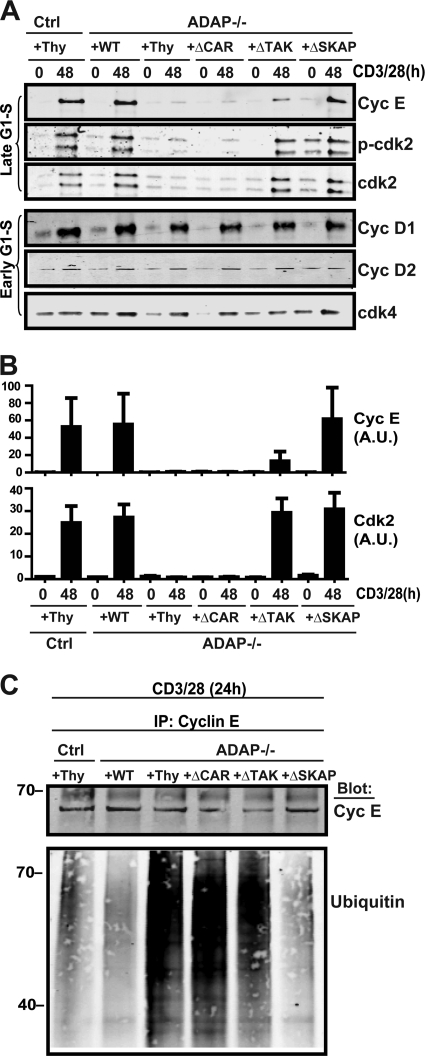
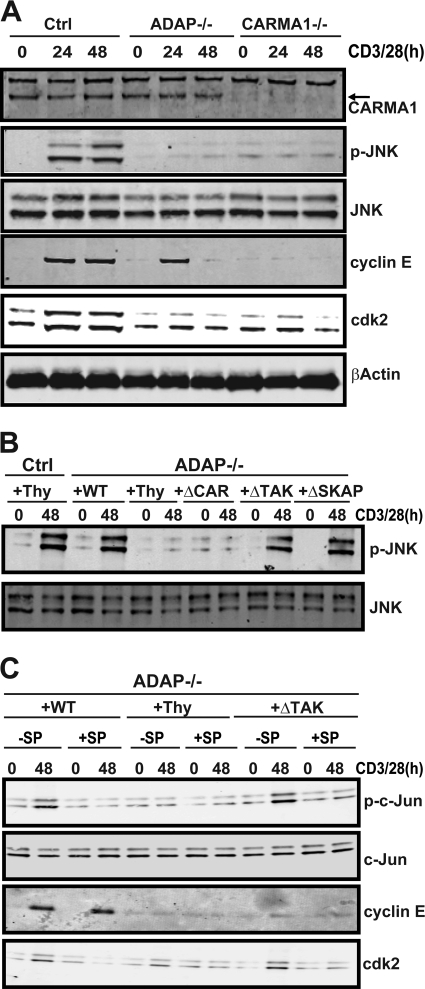
We next used the panel of ADAP mutants to determine the mechanism of ADAP-dependent control of cyclin E stability. CD3/CD28 stimulation resulted in comparable induction of D-type cyclins and Cdk4, which are induced in the early G1 phase of the cell cycle, in control T cells, ADAP−/− T cells, and ADAP−/− T cells expressing wild-type or mutant ADAP constructs (Fig. 4A). In contrast, CD3/CD28 stimulation of ADAP−/− T cells failed to result in the induction of cyclin E and Cdk2 (Fig. 4A). Expression of the ADAPΔCAR mutant in ADAP−/− T cells did not restore induction of either cyclin E or Cdk2 (Fig. 4A). CD3/CD28 stimulation of ADAP−/− T cells expressing the ADAPΔTAK mutant resulted in only a minimal induction of cyclin E expression but did lead to Cdk2 induction that was similar to that observed in control T cells and ADAP−/− T cells expressing wild-type ADAP. The ADAPΔSKAP mutant fully restored induction of both cyclin E and Cdk2 (Fig. 4A and densitometry in Fig. 4B). We also stimulated ADAP−/− T cells expressing the panel of ADAP mutants for 24 h and assessed cyclin E ubiquitination (Fig. 4C). Consistent with Fig. 3C, hyperubiquitination of immunoprecipitated cyclin E was observed in ADAP−/− T cells expressing the control virus (Fig. 4C). Expression of either wild-type ADAP or the ADAPΔSKAP mutant resulted in a reduction in cyclin E ubiquitination down to levels observed with stimulated control T cells. In contrast, the ubiquitination of cyclin E remained elevated in activated ADAP−/− T cells expressing the ADAPΔCAR or ADAPΔTAK mutants (Fig. 4C). These results suggest that the CARMA1- and TAK1-binding sites in ADAP are both critical for cyclin E induction, while the CARMA1-binding site is singularly critical for Cdk2 induction.
Fig 4.
ADAP interaction with CARMA1 and TAK1 is critical for expression and maintenance of Cdk2 and cyclin E. (A) Naïve hCAR control type (Ctrl) or hCAR ADAP−/− T cells (ADAP−/−) were transduced with control adenovirus expressing Thy1.1 (Thy) or adenovirus expressing Thy1.1 and wild-type ADAP (WT), ADAPΔCAR mutant (ΔCAR), ADAPΔTAK mutant (ΔTAK), or ADAPΔSKAP mutant (ΔSKAP) and then stimulated with anti-CD3 and anti-CD28 antibodies for 48 h. Lysates were probed for cyclin E (Cyc E), phospho-Cdk2 (p-cdk2), Cdk2, cyclin D1 (Cyc D1), cyclin D2 (Cyc D2), and Cdk4. (B) Densitometry values for cyclin E and Cdk2 from three independent experiments performed as described for panel A were obtained using Odyssey software. Values were normalized to the control wild-type unstimulated level and are expressed as arbitrary units (A.U.). (C) Cells transduced and activated as described for panel A were harvested at 24 h and immunoprecipitated for cyclin E, and Western blots were performed for cyclin E and ubiquitin. Similar results were obtained in two independent experiments.
We also examined cyclin E and Cdk2 levels following CD3/CD28 stimulation of CARMA1−/− T cells. Similar to our results with ADAP−/− T cells, activation of CARMA1−/− T cells did not result in induction of cyclin E or increases in Cdk2 protein (Fig. 5A). However, while cyclin E was clearly detectable 24 h after CD3/CD28 stimulation of ADAP−/− T cells, we failed to detect cyclin E at this same time point in CARMA1−/− T cells (Fig. 5A).
Fig 5.
The CARMA1-binding site in ADAP is critical for TCR-dependent JNK activation and induction of Cdk2. (A) Control (Ctrl), CARMA−/−, and ADAP−/− T cells were stimulated with anti-CD3 and anti-CD28 antibodies for 48 h, lysed, and analyzed by Western blotting with antibodies specific for JNK, phospho-JNK, cyclin E, Cdk2, and β-actin. (B) Naïve hCAR control or hCAR/ADAP−/− T cells were transduced with control adenovirus expressing Thy1.1 (Thy) or adenovirus expressing Thy1.1 and wild-type ADAP (WT), ADAPΔCAR mutant (ΔCAR), ADAPΔTAK mutant (ΔTAK), or ADAPΔSKAP mutant (ΔSKAP) and then stimulated with anti-CD3 and anti-CD28 antibodies. Lysates were probed by Western blotting with anti-phospho-JNK (p-JNK) and anti-JNK antibodies. (C) Naïve hCAR control or hCAR/ADAP−/− T cells were transduced with the indicated adenoviruses as described for panel B and stimulated with anti-CD3 and anti-CD28 antibodies for 48 h in the presence (+SP) or absence (−SP) of the JNK inhibitor SP6000125 (30 μM). Cells were harvested, lysed, and probed by Western blotting with anti-phospho-c-Jun, anti-c-Jun, anti-cyclin E, and anti-Cdk2 antibodies. Similar results were observed in at least three independent experiments.
The CARMA1-binding site in ADAP regulates JNK-mediated induction of Cdk2.
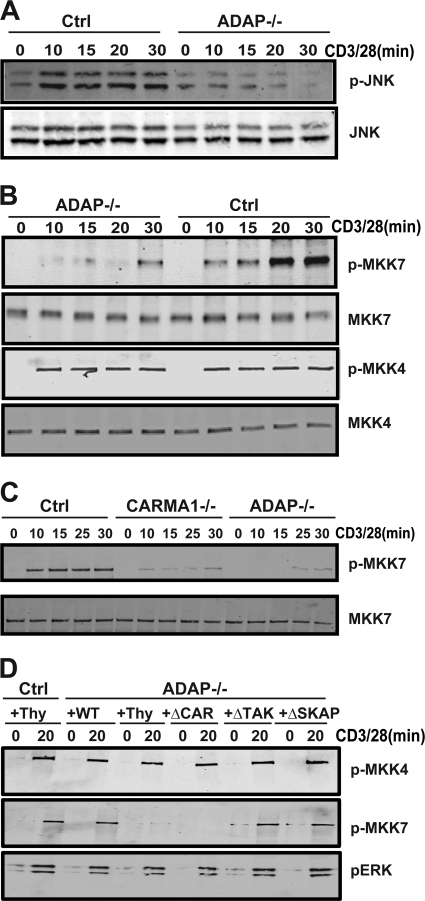
As we previously demonstrated that the CARMA1- and TAK1-binding sites in ADAP were both critical for TCR-mediated activation of NF-κB (24, 38), the impairment of Cdk2 induction in ADAP−/− T cells expressing the ADAPΔCAR but not the ADAPΔTAK mutant suggests that Cdk2 induction is not dependent on NF-κB. CARMA1-deficient T cells have been reported to have a defect in JNK phosphorylation following CD3/CD28 stimulation (3, 17). We directly compared JNK phosphorylation, cyclin E levels, and Cdk2 levels in CARMA−/− T cells and ADAP−/− T cells following CD3/CD28 stimulation (Fig. 5A). Consistent with previous studies, we observed impaired JNK phosphorylation following CD3/CD28 stimulation of CARMA1−/− T cells (Fig. 5A). When we examined ADAP−/− T cells, we also observed that CD3/CD28 stimulation resulted in impaired JNK phosphorylation (Fig. 5A and B). While expression of the ADAPΔTAK mutant or the ADAPΔSKAP mutant in ADAP−/− T cells restored JNK phosphorylation, expression of the ADAPΔCAR mutant failed to restore JNK phosphorylation (Fig. 5B). Thus, the CARMA1-binding site in ADAP is critical for Cdk2 induction and JNK phosphorylation.
To further define a role for JNK signaling in the induction of Cdk2 in activated T cells, we demonstrated that treatment of ADAP−/− T cells expressing wild-type ADAP with the JNK inhibitor SP600125 (2) blocked c-Jun phosphorylation and Cdk2 induction following CD3/CD28 stimulation (Fig. 5C). Similar results were obtained with ADAP−/− T cells expressing the ADAPΔTAK mutant (Fig. 5C), which is also consistent with their normal levels of JNK activation (Fig. 5B) and Cdk2 induction (Fig. 4A). Treatment of ADAP−/− T cells expressing wild-type ADAP with the JNK inhibitor did not affect cyclin E induction (Fig. 5C). The concentrations of the JNK inhibitor used in these experiments did not affect the viability of the T cells (data not shown). These results are consistent with a JNK-independent mechanism by which TCR stimulation results in induction of cyclin E.
The CARMA1-binding site in ADAP is critical for phosphorylation and recruitment of MKK7.
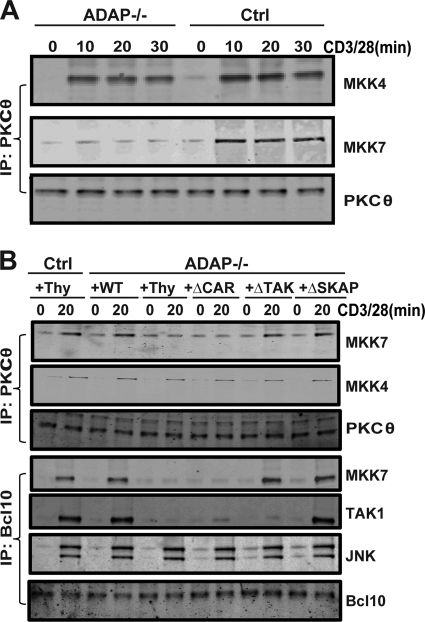
To establish a mechanism by which ADAP regulates JNK activation via CARMA1, we examined the phosphorylation status of JNK and of MKK7 and MKK4, which have both been reported to serve as mitogen-activated protein (MAP) kinase kinases for JNK (9). CD3/CD28 stimulation of control T cells resulted in rapid phosphorylation of JNK (Fig. 6A) and MKK4 and MKK7 (Fig. 6B). While MKK4 phosphorylation was unaffected by the loss of ADAP, MKK7 phosphorylation and JNK phosphorylation were impaired at early time points after CD3/CD28 stimulation of ADAP−/− T cells. Defects in MKK7 phosphorylation in ADAP−/− T cells were most evident at 10 and 20 min after stimulation (Fig. 6B). CARMA1-deficient T cells also exhibited a defect in MKK7 phosphorylation following CD3/CD28 stimulation (Fig. 6C). We also examined the activation-dependent recruitment of both MKK7 and MKK4 to PKCθ in control and ADAP−/− T cells. While there was minimal coimmunoprecipitation of either MKK7 or MKK4 with PKCθ in unstimulated T cells, CD3/CD28 stimulation resulted in coimmunoprecipitation of both MKKs within 10 min and was sustained out to 30 min (Fig. 7A). In ADAP−/− T cells, there was a dramatic impairment in the coimmunoprecipitation of MKK7, but not MKK4, with PKCθ following CD3/CD28 stimulation (Fig. 7A).
Fig 6.
The CARMA1-binding site in ADAP regulates TCR-mediated activation of MKK7. (A and B) Naïve control (Ctrl) and ADAP−/− T cells were stimulated for the indicated time points (min) with anti-CD3 and anti-CD28 antibodies. Cell lysates were analyzed by Western blotting with antibodies specific for JNK and phospho-JNK (A) or MKK7, MKK4, phospho-MKK7 (p-MKK7), and phospho-MKK4 (B). (C) Naïve control, CARMA1−/−, and ADAP−/− T cells were stimulated as described for panels A and B. Cell lysates were analyzed by Western blotting with antibodies specific for MKK7 and phospho-MKK7. (D) Naïve hCAR control or hCAR/ADAP−/− T cells were transduced with control adenovirus expressing Thy1.1 (Thy) or adenovirus expressing Thy1.1 and wild-type ADAP (WT), ADAPΔCAR mutant (ΔCAR), ADAPΔTAK mutant (ΔTAK), or ADAPΔSKAP mutant (ΔSKAP) and then stimulated with anti-CD3 and anti-CD28 antibodies for 20 min. Cell lysates were analyzed by Western blotting with antibodies specific for phospho-MKK7, phospho-MKK4, and phospho-ERK (p-ERK). Similar results were obtained in at least three independent experiments.
Fig 7.
TCR-inducible MKK7 recruitment is dependent on the CARMA1-binding site in ADAP. (A) Naïve control (Ctrl) and ADAP−/− T cells were stimulated for the indicated time points (min) with anti-CD3 and anti-CD28 antibodies. PKCθ immunoprecipitates were analyzed by Western blotting with antibodies specific for MKK4, MKK7, and PKCθ. (B) Naïve hCAR control or hCAR/ADAP−/− T cells were transduced with control adenovirus expressing Thy1.1 (Thy) or adenovirus expressing Thy1.1 and wild-type ADAP (WT), ADAPΔCAR mutant (ΔCAR), ADAPΔTAK mutant (ΔTAK), or ADAPΔSKAP mutant (ΔSKAP) and then stimulated with anti-CD3 and anti-CD28 antibodies for 20 min. Cell lysates were subjected to immunoprecipitation with either an anti-PKCθ antibody or an anti-Bcl10 antibody and then analyzed by Western blotting with antibodies specific for MKK7, MKK4, PKCθ, TAK1, JNK, or Bcl10. Results are representative of three independent experiments performed.
Expression of wild-type ADAP in ADAP−/− T cells restored CD3/CD28-mediated MKK7 phosphorylation (Fig. 6D) and association with PKCθ (Fig. 7B). The ADAPΔTAK and ADAPΔSKAP mutants were also able to restore MKK7 phosphorylation (Fig. 6D) and PKCθ association to levels observed with wild-type ADAP (Fig. 7B). In contrast, the ADAPΔCAR mutant was unable to rescue this defect in MKK7 phosphorylation and PKCθ association (Fig. 6D and 7B). Since Bcl10 has been proposed to be a scaffold that nucleates CARMA1-dependent recruitment of TAK1, MKK7, and JNK following TCR stimulation (3), we also examined the function of ADAP in this recruitment to Bcl10. Consistent with previous studies in Jurkat T cells (3), CD3/CD28 stimulation resulted in inducible association of Bcl10 with MKK7, TAK1, and JNK (Fig. 7C). In ADAP−/− T cells, the association of Bcl10 with MKK7 and TAK1 but not with JNK was impaired (Fig. 7C). Expression of wild-type ADAP or the ADAPΔSKAP mutant in ADAP−/− T cells restored the Bcl10 association with TAK1 and MKK7. The ADAPΔCAR mutant was unable to restore the Bcl10 association with either MKK7 or TAK1, while the ADAPΔTAK mutant restored the Bcl10 association with MKK7 but not TAK1 (Fig. 7C). Overall, these results suggest that the CARMA1-binding site in ADAP plays a critical role in JNK signaling by regulating MKK7 phosphorylation and recruitment with TAK1 to the PKCθ signalosome via Bcl10.
DISCUSSION
T cells lacking expression of the multifunctional scaffold protein ADAP exhibit impaired proliferation in response to stimulation of the T cell receptor (16, 27, 28). In this study, we have demonstrated that the inability of ADAP-deficient T cells to respond optimally to this mitogenic signal is associated specifically with a G1-S-phase block in cell cycle progression and the impaired induction of the late G1-S-phase transition proteins Cdk2 and cyclin E. Using ADAP mutants that selectively disrupt the constitutive association of ADAP with SKAP55 or the inducible association of ADAP with CARMA1 or TAK1, we demonstrate a critical requirement for the CARMA1-binding site in ADAP for the TCR-dependent induction of both cyclin E and Cdk2. As CARMA1−/− T cells also exhibit a defect in TCR-mediated T cell proliferation (12) and induction of cyclin E and Cdk2 (Fig. 5A) and the CARMA1-binding site in ADAP is critical for the TCR-mediated formation of the CBM signalosome (24), our results suggest that the induction of both cyclin E and Cdk2 is dependent on the efficient formation of this signalosome. We recently identified a distinct site in the C-terminal end of ADAP that is critical for the TCR-inducible association of ADAP with the TAK1 kinase (38). TCR stimulation of T cells expressing an ADAP mutant lacking the TAK1-binding site results in efficient formation of the CBM signalosome but impaired IKKα/β phosphorylation. Consequently, NF-κB activation remains impaired in T cells expressing the ADAPΔTAK mutant (38). Our finding that the TCR-dependent induction of cyclin E is not restored in ADAP−/− T cells expressing either the ADAPΔCAR or ADAPΔTAK mutant is consistent with a model where cyclin E induction is regulated by ADAP-dependent activation of NF-κB via CBM signalosome assembly. Although other studies have demonstrated a role for NF-κB in regulating cyclin E induction (14, 23), we did not find that a loss of ADAP impaired the transcriptional activation of cyclin E following T cell stimulation. Instead, we observed accelerated loss of cyclin E protein in ADAP−/− T cells following CD3/CD28 stimulation. In our kinetic analysis of cyclin E expression, we could detect cyclin E only transiently in ADAP−/− T cells at 24 h after stimulation. Furthermore, there was dramatically increased detection of ubiquitin in cyclin E immunoprecipitates from ADAP−/− T cells activated for 24 h. Our results suggest that ADAP restrains cyclin E ubiquitination via a mechanism that is dependent on ADAP interactions with CARMA1 and TAK1, as expression of either the ADAPΔCAR or ADAPΔTAK mutant did not reduce cyclin E ubiquitination at 24 h or restore cyclin E expression at the later 48-h time point.
Our results document a novel function for ADAP in controlling the ubiquitin-mediated degradation of cyclin E. Two distinct E3 ligases, cullin-3 and SCFFbw7, facilitate cyclin E ubiquitination. Cyclin E that is not complexed with Cdk2 is targeted by cullin-3 (6, 37), while cyclin E phosphorylation following association of cyclin E with Cdk2 results in the recognition of cyclin E by SCFFbw7 (20, 25, 40, 48). The role of ADAP in regulating the function of these two ligases is under investigation. It is interesting to note that in T cells expressing the ADAPΔTAK mutant, cyclin E ubiquitination remains elevated, although the levels of Cdk2 are comparable to those found in wild-type T cells. This suggests the possibility that ADAP might control distinct steps in the posttranslational modification of cyclin E that lead to its degradation. It is also notable that at 24 h postactivation, we can detect cyclin E in ADAP−/− T cells but not in CARMA1−/− T cells (Fig. 3B). The reason for this difference is currently not known, but it is possible that the complete absence of CARMA1 results in such rapid cyclin E degradation that it cannot be detected in our activated T cells. This is consistent with our previous work showing that T cells lacking ADAP do not have a complete block in the formation of the CBM signalosome (24).
While the induction of cyclin E following TCR stimulation is dependent on both the CARMA1- and TAK1-binding sites in ADAP, the induction of Cdk2 following TCR stimulation is dependent only on the CARMA1-binding site. This suggests that Cdk2 is regulated by a CARMA1-dependent signaling pathway that is distinct from the NF-κB pathway. We focused on the JNK pathway, as T cells lacking either CARMA1 or Malt1 have defects in TCR-mediated activation of JNK (3, 15, 17, 33). The proteolytic activity of Malt1 also regulates JNK signaling in T cells (39), although there are conflicting reports on the integrity of the JNK signaling pathway in Malt1−/− T cells following T cell stimulation (31, 33). We confirmed the impaired activation of JNK in CARMA1−/− T cells and also demonstrated that ADAP−/− T cells exhibit a defect in JNK activation. Analysis of ADAP−/− T cells expressing the panel of ADAP mutants clearly defined a role for the CARMA1-binding site and not the TAK1-binding site in ADAP for JNK signaling. Together with our finding that a JNK inhibitor blocks the induction of Cdk2 both in wild-type T cells and in ADAP−/− T cells expressing the ADAPΔTAK mutant, our results suggest that CARMA1-dependent regulation of JNK activation is critical for downstream induction of Cdk2 following T cell activation. As Cdk2 mRNA levels following CD3/CD28 stimulation are not affected by the loss of ADAP, our results suggest that the Cdk2 gene is not a direct downstream target of JNK.
Like other members of the MAPK family of kinases, JNK is phosphorylated and activated by MAP kinase kinases (MKKs), which are also phosphorylated and activated by an upstream MAP kinase kinase kinase (MKKK) (8). Both MKK4 and MKK7 have been proposed to be MKKs for JNK. Our studies suggest that MKK7 is particularly critical for JNK activation in T cells, as the loss of JNK signaling in ADAP−/− T cells and ADAP−/− T cells expressing the ADAPΔCAR mutant is associated with a loss of MKK7 phosphorylation and recruitment to the PKCθ signalosome following TCR stimulation. In contrast, MKK4 phosphorylation and recruitment to the PKCθ signalosome is not affected by the loss of ADAP. Our results are consistent with the known role of MKK7 as an MKK for JNK and with previous work indicating that MKK7 inducibly associates with Bcl10 in Jurkat T cells (3). TAK1 is a critical MKKK for JNK, as there is a loss of TCR-mediated activation of JNK in TAK1−/− thymocytes and effector T cells (22, 43). Thus, it is somewhat surprising that the TAK1-binding site in ADAP is not required for efficient activation of JNK and JNK-dependent activation of Cdk2. Our results suggest that the interaction of TAK1 with ADAP is required for TAK1-dependent phosphorylation of IKKα/β (38) but not for TAK1-dependent activation of JNK. Alternatively, it is possible that an MKKK distinct from TAK1 may be involved in ADAP-dependent activation of JNK.
Bcl10 has been proposed to serve as a scaffold that brings together TAK1, MKK7, and JNK2 following stimulation of Jurkat T cells with phorbol myristate acetate and ionomycin (3). We extended these findings by showing that CD3/CD28 stimulation of naïve T cells results in the association of Bcl10 with TAK1, MKK7, and JNK. Analysis of ADAP−/− T cells and ADAP−/− T cells expressing ADAP mutants revealed differential roles for ADAP and the CARMA1- and TAK1-binding sites in ADAP in the regulation of Bcl10 interactions with these molecules. The association of Bcl10 with both MKK7 and TAK1 but not JNK is affected by the loss of ADAP. The recruitment of TAK1 to Bcl10 is dependent on both the CARMA1- and TAK1-binding sites in ADAP, while the association of Bcl10 with MKK7 is dependent on the CARMA1-binding site in ADAP. As JNK activation is also dependent on the CARMA1-binding site but not the TAK1-binding site in ADAP, these results suggest a critical function for MKK7 recruitment to the CBM complex in ADAP-dependent regulation of JNK signaling. Previous studies using Jurkat T cells also suggest a selective role for CARMA1 in regulating activation of JNK2 but not JNK1 (3). Our results, obtained with an antibody that recognizes both JNK1 and JNK2, suggest a defect in total JNK activation in primary T cells and are consistent with other published work (15, 17, 26).
In addition to regulating NF-κB and JNK signaling, ADAP also promotes the efficient interaction of T cells with antigen-presenting cells by regulating the functional activity of integrin receptors. These distinct functions of ADAP are controlled by two biochemically distinct pools in T cells defined by the constitutive association of one pool of ADAP with SKAP55 (4). The fraction of ADAP that is associated with SKAP55 regulates integrin function and does not interact with the CBM signalosome following TCR stimulation (4). Analysis of the ADAP mutant lacking the SKAP55-binding site (ADAPΔSKAP) revealed that this mutant is able to restore Cdk2 and cyclin E induction and JNK signaling, when expressed in ADAP−/− T cells. This is consistent with our previous work showing that this mutant of ADAP can efficiently restore NF-κB signaling when expressed in ADAP−/− T cells (5). The ADAPΔSKAP mutant is also able to fully restore T cell proliferation in response to CD3/CD28 stimulation in vitro, even though this mutant does not restore integrin function in ADAP−/− T cells (5). This is likely due to the specific in vitro conditions that we used in this study, where T cell activation mediated by immobilized antibodies bypasses any contribution of integrins to facilitating signals provided to the TCR. We also examined the response of ADAP−/− T cells expressing the ADAPΔSKAP mutant to antigen challenge in vivo, where T cell stimulation involves the integrin-dependent interaction of T cells with an antigen-presenting cell. Under these conditions, we did observe that the ADAPΔSKAP mutant was unable to restore T cell proliferation to the levels observed with wild-type T cells or ADAP−/− T cells expressing wild-type ADAP. This is consistent with a requirement for the ADAP/SKAP55 complex in promoting efficient T cell interactions with antigen-presenting cells (4, 45). This pool of ADAP may set a critical threshold that must be reached in order to trigger optimal T cell stimulation.
In summary, we propose that ADAP regulates TCR-mediated proliferation and cell cycle progression by coordinately regulating the expression levels of cyclin E and Cdk2. The CARMA1- and TAK1-binding sites in ADAP are both critical for regulating the ubiquitination status and, thus, the stability of cyclin E following TCR stimulation. The CARMA1-binding site in ADAP also plays a distinct function in regulating the activation and recruitment of MKK7 to the PKCθ signalosome by facilitating formation of the CBM complex, resulting in JNK-mediated induction of Cdk2. Together, these ADAP-dependent pathways promote the induction of these key G1-S transition proteins required for optimal T cell proliferation and cell cycle progression. These findings provide novel insights into the mechanistic basis for impaired proliferative responses of ADAP−/− T cells and the biochemical pathways that control the expression of cell cycle regulatory proteins.
ACKNOWLEDGMENTS
We thank E. Peterson for helpful discussions, M. Farrar for reagents, and T. Lee for mouse genotyping and colony maintenance.
This work was supported by Public Health Service grant R01-AI038474 from the National Institutes of Health. Y.S. is also supported in part by the Harry Kay Chair in Biomedical Research at the University of Minnesota.
Footnotes
Published ahead of print 12 March 2012
REFERENCES
- 1. Albanese C, et al. 2003. IKKα regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol. Biol. Cell 14:585–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett BL, et al. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. U. S. A. 98:13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blonska M, et al. 2007. The CARMA1-Bcl10 signaling complex selectively regulates JNK2 kinase in the T cell receptor-signaling pathway. Immunity 26:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burbach BJ, Srivastava R, Ingram MA, Mitchell JS, Shimizu Y. 2011. The pleckstrin homology domain in the SKAP55 adapter protein defines the ability of the adapter protein ADAP to regulate integrin function and NF-κB activation. J. Immunol. 186:6227–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burbach BJ, et al. 2008. Distinct regulation of integrin-dependent T cell conjugate formation and NF-κB activation by the adapter protein ADAP. J. Immunol. 181:4840–4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clurman BE, Sheaff RJ, Thress K, Groudine M, Roberts JM. 1996. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 10:1979–1990 [DOI] [PubMed] [Google Scholar]
- 7. Conze D, et al. 2002. c-Jun NH2-terminal kinase (JNK)1 and JNK2 have distinct roles in CD8+ T cell activation. J. Exp. Med. 195:811–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuevas BD, Abell AN, Johnson GL. 2007. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene 26:3159–3171 [DOI] [PubMed] [Google Scholar]
- 9. Davis RJ. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239–252 [DOI] [PubMed] [Google Scholar]
- 10. DeNucci C, Pagan AJ, Mitchell JS, Shimizu Y. 2010. Control of α4β7 integrin expression and CD4 T cell homing by the β1 integrin subunit. J. Immunol. 184:2458–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dong C, et al. 1998. Defective T cell differentiation in the absence of Jnk1. Science 282:2092–2095 [DOI] [PubMed] [Google Scholar]
- 12. Egawa T, et al. 2003. Requirement for CARMA1 in antigen receptor-induced NF-κB activation and lymphocyte proliferation. Curr. Biol. 13:1252–1258 [DOI] [PubMed] [Google Scholar]
- 13. Eto I. 2000. Molecular cloning and sequence analysis of the promoter region of mouse cyclin D1 gene: implication in phorbol ester-induced tumour promotion. Cell Prolif. 33:167–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng B, et al. 2004. NF-κB inducible genes BCL-X and cyclin E promote immature B-cell proliferation and survival. Cell. Immunol. 232:9–20 [DOI] [PubMed] [Google Scholar]
- 15. Gaide O, et al. 2002. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nat. Immunol. 3:836–843 [DOI] [PubMed] [Google Scholar]
- 16. Griffiths EK, et al. 2001. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science 293:2260–2263 [DOI] [PubMed] [Google Scholar]
- 17. Hara H, et al. 2003. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity 18:763–775 [DOI] [PubMed] [Google Scholar]
- 18. Joyce D, et al. 2001. NF-κB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 12:73–90 [DOI] [PubMed] [Google Scholar]
- 19. Joyce D, et al. 1999. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-κB-dependent pathway. J. Biol. Chem. 274:25245–25249 [DOI] [PubMed] [Google Scholar]
- 20. Koepp DM, et al. 2001. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294:173–177 [DOI] [PubMed] [Google Scholar]
- 21. Li ZW, et al. 1999. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J. Exp. Med. 189:1839–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu HH, Xie M, Schneider MD, Chen ZJ. 2006. Essential role of TAK1 in thymocyte development and activation. Proc. Natl. Acad. Sci. U. S. A. 103:11677–11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lupino E, et al. 2010. In CD28-costimulated human naive CD4+ T cells, I-κB kinase controls the expression of cell cycle regulatory proteins via interleukin-2-independent mechanisms. Immunology 131:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medeiros RB, et al. 2007. Regulation of NF-κB activation in T cells via association of the adapter proteins ADAP and CARMA1. Science 316:754–758 [DOI] [PubMed] [Google Scholar]
- 25. Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. 2001. Archipelago regulates cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 413:311–316 [DOI] [PubMed] [Google Scholar]
- 26. Moreno-Garcia ME, et al. 2009. Serine 649 phosphorylation within the protein kinase C-regulated domain down-regulates CARMA1 activity in lymphocytes. J. Immunol. 183:7362–7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mueller KL, Thomas MS, Burbach BJ, Peterson EJ, Shimizu Y. 2007. Adhesion and degranulation promoting adapter protein (ADAP) positively regulates T cell sensitivity to antigen and T cell survival. J. Immunol. 179:3559–3569 [DOI] [PubMed] [Google Scholar]
- 28. Peterson EJ, et al. 2001. Coupling of the TCR to integrin activation by SLAP-130/Fyb. Science 293:2263–2265 [DOI] [PubMed] [Google Scholar]
- 29. Rincon M, Pedraza-Alva G. 2003. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol. Rev. 192:131–142 [DOI] [PubMed] [Google Scholar]
- 30. Rudolph D, et al. 2000. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 14:854–862 [PMC free article] [PubMed] [Google Scholar]
- 31. Ruefli-Brasse AA, French DM, Dixit VM. 2003. Regulation of NF-κB-dependent lymphocyte activation and development by paracaspase. Science 302:1581–1584 [DOI] [PubMed] [Google Scholar]
- 32. Ruland J, et al. 2001. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104:33–42 [DOI] [PubMed] [Google Scholar]
- 33. Ruland J, Duncan GS, Wakeham A, Mak TW. 2003. Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity 19:749–758 [DOI] [PubMed] [Google Scholar]
- 34. Sabapathy K, et al. 2004. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell 15:713–725 [DOI] [PubMed] [Google Scholar]
- 35. Sabapathy K, et al. 2001. c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J. Exp. Med. 193:317–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Samelson LE. 2002. Signal transduction mediated the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 20:371–394 [DOI] [PubMed] [Google Scholar]
- 37. Singer JD, Gurian-West M, Clurman B, Roberts JM. 1999. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev. 13:2375–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srivastava R, Burbach BJ, Shimizu Y. 2010. NF-κB activation in T cells requires discrete control of IκB kinase α/β (IKKα/β) phosphorylation and IKKγ ubiquitination by the ADAP adapter protein. J. Biol. Chem. 285:11100–11105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Staal J, et al. 2011. T-cell receptor-induced JNK activation requires proteolytic inactivation of CYLD by MALT1. EMBO J. 30:1742–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strohmaier H, et al. 2001. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413:316–322 [DOI] [PubMed] [Google Scholar]
- 41. Tao J, et al. 2007. JNK2 negatively regulates CD8+ T cell effector function and anti-tumor immune response. Eur. J. Immunol. 37:818–829 [DOI] [PubMed] [Google Scholar]
- 42. Thome M. 2004. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat. Rev. Immunol. 4:348–359 [DOI] [PubMed] [Google Scholar]
- 43. Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. 2006. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat. Immunol. 7:851–858 [DOI] [PubMed] [Google Scholar]
- 44. Wang D, et al. 2002. A requirement for CARMA1 in TCR-induced NF-κB activation. Nat. Immunol. 3:830–835 [DOI] [PubMed] [Google Scholar]
- 45. Wang H, Rudd CE. 2008. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 18:486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang HY, et al. 2004. ADAP-SLP-76 binding differentially regulates supramolecular activation cluster (SMAC) formation relative to T cell-APC conjugation. J. Exp. Med. 200:1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weinberg RA. 1995. The retinoblastoma protein and cell cycle control. Cell 81:323–330 [DOI] [PubMed] [Google Scholar]
- 48. Welcker M, et al. 2003. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol. Cell 12:381–392 [DOI] [PubMed] [Google Scholar]