Abstract
Since 1957, it has been proposed that the dissemination of inhalational anthrax required spores to be transported from the lumena of the lungs into the lymphatic system. In 2002, this idea was expanded to state that alveolar macrophages act as a “Trojan horse” capable of transporting spores across the lung epithelium into draining mediastinal lymph nodes. Since then, the Trojan horse model of dissemination has become the most widely cited model of inhalational infection as well as the focus of the majority of studies aiming to understand events initiating inhalational anthrax infections. However, recent observations derived from animal models of Bacillus anthracis infection are inconsistent with aspects of the Trojan horse model and imply that bacterial dissemination patterns during inhalational infection may be more similar to the cutaneous and gastrointestinal forms than previously thought. In light of these studies, it is of significant importance to reassess the mechanisms of inhalational anthrax dissemination, since it is this form of anthrax that is most lethal and of greatest concern when B. anthracis is weaponized. Here we propose a new “jailbreak” model of B. anthracis dissemination which applies to the dissemination of all common manifestations of the disease anthrax. The proposed model impacts the field by deemphasizing the role of host cells as conduits for dissemination and increasing the role of phagocytes as central players in innate defenses, while moving the focus toward interactions between B. anthracis and lymphoid and epithelial tissues.
INTRODUCTION
Bacillus anthracis is a Gram-positive, spore-forming bacterium that is the causative agent of the disease anthrax (45). Most commonly associated with grazing livestock and game, anthrax is a zoonotic disease for humans who associate with them, such as herders and wool processors (76). Classically, anthrax can be contracted through several routes of exposure: by inhalation, cutaneously, and gastrointestinally (1, 41, 58). In addition, it should be noted that, recently, a new form of anthrax was recognized and deemed “injection anthrax,” because of its association with the injection of B. anthracis-contaminated illicit drugs, such as heroin (60). Cutaneous anthrax is the most common form of infection in humans, accounting for 95% of all cases (76). The cutaneous form has the lowest mortality rate, at 1% with antibiotic treatment and 20% without. Inhalational anthrax has the highest mortality rate, at 45% with antibiotic treatment and greater than 97% without (8). Gastrointestinal anthrax has roughly the same mortality rate as the inhalational form of infection but likely goes underreported due to difficulties in diagnosis and its prevalence in developing countries (5).
B. anthracis has multiple virulence factors. The most important of these are exotoxins and capsule, which are encoded on two different virulence plasmids, pXO1 and pXO2, respectively (42). The tripartite AB exotoxin consists of a receptor-binding translocase subunit, protective antigen (PA), and two enzymatic subunits, lethal factor (LF) and edema factor (EF). When PA is bound to LF or EF, the complex becomes lethal toxin (LT) or edema toxin (ET), respectively, but it should be noted that oligomerized PA has been modeled to bind multiple subunits at the same time, and thus a single exotoxin complex may contain PA, LF, and EF at the same time (44). LF is a metalloprotease that cleaves most mitogen-activated protein kinase kinases (MAPKKs), or MEKs, when delivered to the cytosol by PA (2, 20, 29, 57). As implied by its name, LT can cause the death of cells and experimental animals (57). EF is a calmodulin-activated adenylate cyclase that converts ATP to cyclic AMP (cAMP) and induces edema in host tissues when introduced into the cytosol by PA (4, 46). B. anthracis capsule's biosynthetic machinery is encoded on pXO2 and is responsible for producing a capsule that consists of a gamma-linked polyglutamic acid that is covalently bound to the cell wall (9, 61). Capsule serves to protect B. anthracis from phagocytosis and complement deposition (50, 51, 67).
While the vegetative form of the bacterium can cause infection in the laboratory (72), the spore is the infectious particle in nature, presumably because of a spore's ability to persist in the environment as a viable entity for decades (53). For this reason, the spore can itself be considered vital in causing infection. Spores are metabolically inactive and resistant to environmental stresses, such as extreme heat, cold, and radiation. These properties also make spores resistant to killing by the host immune system. Spores are generally 1 to 2 μm in length and are composed of a core, a thick peptidoglycan layer called the cortex, a proteinaceous coat, and an exosporium, which is a loose-fitting sac-like structure on the exterior of the spore (10, 27). The size of aerosol particles in which spores are carried plays a large role in how effectively an inhalational infection can be established. Particles greater than 12 μm containing spores are much less infective in guinea pigs and rhesus monkeys than spores less than 5 μm (19).
Most of what is known about initiation of anthrax has been derived from animal models of infection. While caveats are inherent when animal infections are compared with human infections, animals are the only ethical means of studying anthrax in a complete host system (31). While nonhuman primates are arguably the best model of anthrax infection, their use is often cost prohibitory and there are relatively few molecular, genetic, and immunological tools with which to study the infection. On the other hand, there is a greater diversity of genetic tools and reagents available for mice and, to a lesser degree, for rabbits and guinea pigs. These tools permit a more precise analysis of the dissemination of bacteria within the host, the host response, and the disease. Many, but not all, of the key characteristics of anthrax are recapitulated in mouse models of infection (31). Information gleaned from studies using genetic tools in mice improves the design and implementation of infection studies performed in nonhuman primates and other less experimentally tractable animals.
The manner by which B. anthracis initiates infection has been a focus of research by a number of laboratories. In 2002, Guidi-Rontani coined the name “Trojan horse” for her proposed model of dissemination based on long-accumulating data (34), with particular emphasis on the data of Joan Ross (62). This model is described in greater detail below. The Trojan horse model has become the most widely cited model for inhalation anthrax. Of late, however, data derived from animal models of infection have necessitated a reassessment of the Trojan horse concept. In this review, we will describe the observations that gave birth to the Trojan horse model, review recent research which modifies the Trojan horse model as proposed in 2002, and review research which questions the necessity for a Trojan horse to cause infection. Lastly, a new model will be outlined that describes the establishment and dissemination of B. anthracis infections.
DISSEMINATION FACTORS
B. anthracis is nonmotile; thus, dissemination beyond the initial site of contact necessitates the involvement of the host by one means or another (71, 76). The means by which virulence factors produced by B. anthracis influence host function are essential for understanding dissemination. Exotoxins play a significant role in dissemination and have been shown to influence many aspects of host function. Unencapsulated pXO2-deficient B. anthracis with the genes for LT and ET knocked out are incapable of progressing beyond the draining lymph node during inhalational and subcutaneous infection in A/J mice (49). This may reflect the exotoxins' ability to break down epithelial and endothelial cell barriers and cause hemorrhaging in animal models (22, 57, 74). The ability to affect endothelial barrier function can be seen even with low exotoxin concentrations (100 ng ET and 1 μg LT) in vitro, where ET and LT alter the transendothelial electrical resistance of endothelial cell monolayers (69, 74). Likewise, capsule plays an important role in dissemination. It has been shown that encapsulated B. anthracis is able to disseminate from initial sites of infection to blood more quickly than its unencapsulated counterparts, although the mechanism behind this phenomenon is currently ill defined (1, 20, 30).
B. anthracis produces, in addition to the major virulence factors, at least four separate proteases that promote host tissue degradation to break down barriers to bacterial dissemination (59). These proteases break down gelatin, casein, fibronectin, laminin, and collagen. Supernatants of B. anthracis containing these proteases administered to mice led to hemorrhaging or death within 2 to 3 days when introduced intratracheally (11, 59). Thus, known and unidentified factors beyond the classical major virulence determinants should not be overlooked when considering how B. anthracis mediates its growth and dissemination through a host.
DISSEMINATION
While the mortality rates of the four types of B. anthracis infection differ, the majority of reported lethal anthrax cases in animals and humans exhibit bacteremia and toxemia at the time of death independently of the route of spore entry. Autopsies reveal B. anthracis lesions containing vegetative bacteria associated with edema and hemorrhaging throughout the body (1, 24, 26, 30). While studying how B. anthracis travels through the host, Lincoln et al. reported that B. anthracis arrived in the mediastinal lymph nodes after aerosol challenge in Rhesus monkeys and also arrived in the thoracic duct after intradermal infection (47). This was the first direct evidence to suggest that B. anthracis first spreads via the lymphatics before entering the bloodstream in a manner that is not dependent on the original route of exposure, although previous experiments had implied this to be the case. These observations were later supported with bioluminescent imaging (BLI) studies utilizing light-producing bacteria to infect BALB/c and A/J mice. These studies demonstrated that after infection initiated, bacteria were next detected in the regional draining lymph nodes before their appearance in the bloodstream in subjects with subcutaneous, gastrointestinal, and inhalational anthrax (21, 28, 30, 49). While it is generally accepted that before infection can spread hematogenously, B. anthracis must escape the lymphatic system, disagreement remains regarding whether the lymphatic system is where spores germinate to initiate vegetative outgrowth, as elaborated below.
BIRTH OF THE TROJAN HORSE MODEL
The Trojan horse model of infection posits that during inhalational anthrax, spores do not germinate in the lungs and must be transported by alveolar macrophages past the lung epithelial barrier to lymph nodes in order to germinate and initiate the production of virulence factors (34). This idea has its roots in very early B. anthracis research. Initial research on inhalational anthrax involved infecting small and large animals; however, in all cases, very few vegetative bacteria were found in the lumena of the lungs at any stage of infection (62, 78). These early observations have been supported by many others since (1, 8, 12, 16, 25, 39). This observation suggested that the lumen of a lung itself is, for the most part, refractory to germination of spores. The concept that spores need to traverse from the lumena of the lungs into deeper tissue in order to germinate and cause disseminated disease resulted from these observations. A mechanism for the movement of spores out of the lumena of the lung was proposed in 1957 by Joan Ross, who observed a macrophage containing a phagocytosed spore in transit to the draining tracheal bronchial lymph node (62). Lincoln et al.'s 1965 study built upon Ross's observations by determining that B. anthracis infection in Rhesus monkeys progressed from the lungs lymphatically and not hematogenously (47). Later research determined that phagocytosed spores were capable of germinating and escaping from phagocytes (18, 35, 65). A germination operon (gerX) was found to be linked with the germination of B. anthracis within macrophages (36). These observations led to Guidi-Rontani's 2002 opinion article focusing on B. anthracis dissemination, in which alveolar macrophages were named the Trojan horse cells that gave rise to disseminated disease by transporting spores from the lumena of the lungs to the mediastinal lymph nodes (34). This model also proposed that differences in lethality between animal species, and perhaps different types of infection, could be explained by the presence or absence of germinants within macrophages from different species.
Another important aspect of the Trojan horse model revolves around the endotoxins' effects on macrophages and whether exotoxins prevent the destruction of B. anthracis. Exotoxins have been reported to modulate the oxidative burst in both macrophages and neutrophils (17, 37). The idea that exotoxins target the innate immune system is supported by research using transgenic mice whose CMG2 cellular toxin receptor was selectively eliminated from cells of myeloid lineage, which led to the complete protection of the mice from subcutaneous and intravenous infection (48). Likewise, exotoxins have been shown to modify multiple behaviors of innate immune cells, including chemotaxis, cytokine secretion, and activation (2, 52, 70).
Because the Trojan horse model had been hinted at since the 1950s and is currently the only well-defined model of inhalational B. anthracis dissemination, since 2002 the Trojan horse model has been the most widely cited model of inhalational B. anthracis infection. Over time, this model has been incrementally modified after it was demonstrated in mice that lung dendritic cells are capable of phagocytosing spores and transporting them into the lymphatic system more effectively than alveolar macrophages (7, 12).
REDEFINING THE TROJAN HORSE
Because the Trojan horse model is the best-defined model of dissemination, a significant body of research has focused on refining its mechanistic details. These studies have brought to light data which suggest that a reassessment of a number of the Trojan horse model's central tenets, as they were originally defined, is needed. It was shown in vitro that epithelial cells and fibroblasts can engulf and transcytose spores and vegetative bacilli through cell monolayers without disrupting barrier integrity (63, 64, 72). These data raised questions as to whether phagocytes are the sole cell type that can act as Trojan horses and thus whether phagocytes are an absolute necessity for spores to transit from the lumena of the lungs. However, transport through nonprofessional phagocytes occurs less frequently than spore uptake by phagocytes, and the ability of spores to cross epithelial barriers has been difficult to demonstrate definitively with in vivo models.
Two studies performed by Cote et al. demonstrated that rates of the inhalational lethality of B. anthracis infections increase with the depletion of alveolar macrophages, and thus spores do not require alveolar macrophages to bypass epithelial barriers (15, 16). However, it must be noted that in these studies chlodronate liposome was used as the means to deplete macrophages, which may either incompletely eliminate the targeted resident macrophages or deplete other cell types (68). Relatedly, Wu et al. found that human alveolar macrophages are more resistant to intoxication than murine macrophages and do not express significant amounts of anthrax toxin receptors (77). The facts that human alveolar macrophages are less susceptible to B. anthracis exotoxins than those in mice and that their presence increases survival in inhalational infections suggest that alveolar macrophages may be better than other phagocytes at clearing B. anthracis infection from the lungs (77). These results imply that it would be advantageous for spores to avoid phagocytosis, because interactions with alveolar macrophages would often lead to the death of the bacteria (40, 43). This may explain why, at autopsy of both humans and animals, few spores and vegetative bacteria are found within the alveolar spaces (3, 12, 16, 33). Depletion of other innate immune cells implicated to be Trojan horses has demonstrated that monocytes, macrophages, and neutrophils on the whole protect against B. anthracis rather than act to promote infection, in contrast to what might have been predicted by the Trojan horse model (16, 48).
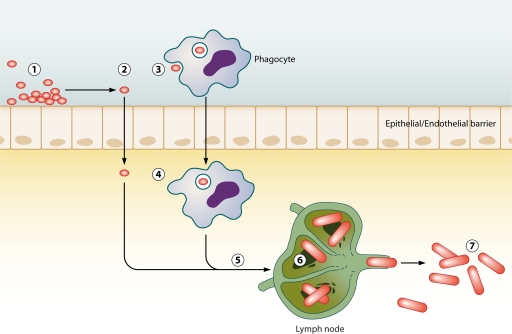
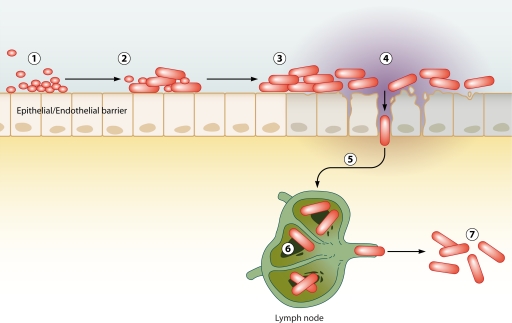
Together, the above observations reveal that spores are not solely dependent on phagocytes for transport from the lumena of the lungs beyond the epithelial barrier. However, these observations also do not necessarily detract from the aspect of the Trojan horse that implies that spores that have trafficked to the draining lymph nodes are the founding population of disseminated disease. The idea that B. anthracis may use multiple mechanisms to transverse epithelial barriers to arrive at a draining lymph node should not be discounted. Many other pathogens, including pathogenic bacteria such as salmonellae, shigellae, and Listeria monocytogenes, utilize multiple distinct methods for crossing epithelial barriers (13). It should also be noted that even if a majority of spores or vegetative bacteria are cleared at the initial site of infection, the trafficking of a subset of spores to the lymph nodes may cause infection according to the Trojan horse model. In light of these observations, the Trojan horse model should be expanded to take into account all possible mechanisms of spore trafficking to draining lymph nodes. In summary, the Trojan horse model can be simplified to state that spores trafficking to the regional draining lymph node through an intermediate intracellular step leads to disseminated disease, as demonstrated in Fig. 1.
Fig 1.
Trojan horse model of infection. Spores enter the lumena of the airways, gastrointestinal tract, or skin at exposure (1). Spores either invade the epithelium directly (2) or are phagocytosed by a host phagocyte (3). In the original model, this phagocyte was predicted to be an alveolar macrophage but can likely also be a dendritic cell. Spores then cross the epithelium by transcytosis through the epithelial cell or within the phagocyte (4). Spores then traffic to a mediastinal lymph node (5). Once in the lymph node, spores germinate into vegetative bacteria, grow, and produce exotoxins and capsule, eventually resulting in the loss of the structural integrity of the lymph node (6). Bacteria escape the lymph node and enter the bloodstream, leading to bacteremia and host death (7).
REEVALUATING THE NECESSITY OF A TROJAN HORSE CELL
While study of the Trojan horse mechanism of dissemination has led to a much greater understanding of how spores and the immune system interact in the lungs, there has been no direct evidence demonstrating whether or not spores in the draining mediastinal lymph nodes are the population of bacteria that give rise to disseminated anthrax. Establishing the location of the founding population of bacteria was particularly technologically challenging until the recent development of BLI technologies, which have led to significant advances in understanding where infections initiate and how dissemination of infection progresses temporally and spatially. These technologies rely on light-producing bacteria that can be tracked within an intact host using highly sensitive cameras. The major advantages of these methods are that subject animals do not need to be euthanized for every time point and that data can be collected noninvasively in a dynamic fashion. Studies using a B. anthracis construct that expresses luminescence under a germination promoter demonstrated that spores can germinate in the lungs of mice (28, 66). These results were at odds with the original Trojan horse model, which states that spores do not germinate in the lungs. However, germination in the lungs was observed only in animals that received a large bolus of spores intranasally, and therefore, this may not represent the typical inhalational infection. Furthermore, multiple studies utilizing B. anthracis bacteria that produce light only during vegetative growth indicated that throughout early infection, when bacteria are growing in the upper airways, all bacteria in the mediastinal lymph nodes remain dormant spores (30, 49).
The relationship between spore germination and pathogenesis is complex (14). Spore germination alone does not necessarily mean the establishment of infection and can leave B. anthracis susceptible to destruction, as demonstrated by Hu et al. (40). Additionally, BLI studies raised concern about traditional experimental infection techniques. Glomski et al. in 2007 suggested that mucosal epithelial breaches caused by experimental techniques are prone to infection (30). The propensity for traumatized epithelial barriers to be the initiation site of infection is consistent with studies on cutaneous infection, in which breaks in the skin are more readily infected in mouse models of cutaneous infection (6). It is also important to note that Ross observed spores within a macrophage in transit to a regional lymph node after intratracheal intubation, which she noted to frequently cause breaks in the airway mucosa, and she did not observe spore transport to the lymph after noninvasive aerosol challenge (62).
In addition, BLI technologies have revealed that during intranasal and aerosol spore challenges, the predominant site of initiation of infection in mice is in the nasally associated lymphoid tissue (NALT) and not in the mediastinal lymph nodes (28, 30, 49). However, spore-containing aerosol size many influence this observation, as it has been demonstrated that large particle sizes tend to accumulate in the upper respiratory tract in primates (38). As such, this scenario likely reflects what would be predicted to occur with most natural infections and with poorly executed bioweapon delivery because of the propensity of spores to clump. At a time when luminescence signals from an unencapsulated B. anthracis Sterne strain or a nontoxigenic capsulated strain with a luciferase cassette under the control of the protective antigen promoter indicated that vegetative bacteria multiply and produce exotoxin in the NALT, only ungerminated spores were found both in the lungs and in the mediastinal lymph nodes of mice (28, 30, 49). These data were later supported by research using a bioluminescent, fully virulent clinical isolate (21). This suggests that in mouse infections, spores in the lungs and mediastinal lymph nodes may be ancillary to the more robust infection in the NALT at early time points. These mouse studies are supported by Lincoln et al.'s monkey data (47), which demonstrated that after inoculation, infection next spread into the lymphatic system and then progressed into the bloodstream, leading to death. However, a key difference between these two models was that in the mice, bacterial growth initiated in the NALT and then progressed to the cervical lymph node rather than the mediastinal lymph node, as was suggested by the Trojan horse model. This does not rule out the possibility that Trojan horse cells deliver spores from the NALT to the cervical lymph node but instead implies that bacteria are delivered into an unexpected anatomical location—the cervical lymph node rather than the mediastinal lymph node.
REFORMING THE TROJAN HORSE MODEL IN LIGHT OF NEW DATA
In both the gastrointestinal and subcutaneous forms of murine anthrax, vegetative bacilli grow at the initial sites of spore entry either in the intestines (Peyer's patches) or nasally associated lymphoid tissues/oropharynx and then spread to the lymphatic system before escaping into the blood (5). In the cutaneous form of anthrax, spores have been found to rapidly germinate at the site of initial spore contact in the dermis of the host (6, 30). The question becomes, if B. anthracis does not require macrophages to act as Trojan horses for dissemination in the case of gastrointestinal, cutaneous, or subcutaneous infections, why would a Trojan horse be required solely for the inhalational form?
New animal models of inhalational anthrax seem to disagree with some aspects of the earlier observations of disease dissemination; however, it is possible to reinterpret previous findings in a new context. Since early inhalational anthrax exhibits some clinical manifestations similar to those of pneumonia, the disease had been referred to as “anthrax pneumonia” as early as 1882 (32). However, there is typically little evidence of significant bacterial growth within the alveolar space, as is characteristic of classical pneumonia. Today it remains difficult to diagnose inhalational anthrax; many of the 2001 victims of the mailing of anthrax spores in the United States were initially diagnosed with bronchitis or pneumonia (55). Thus, it is not surprising that early research focused on the lungs and trachea. Once patients display symptoms of inhalational anthrax, unfortunately, the infection has generally progressed to a point where even effective bactericidal antibiotic treatment can save only 55% of the infected individuals (8). This “point of no return” may reflect the fact that infection has progressed so far as to have disseminated from the original site of infection and spread hematogenously into the lungs and mediastinum to cause the classical diagnostic mediastinal widening associated with advanced inhalational anthrax, a point where it has been suggested that enough total exotoxin has been released to kill the host even in the absence of viable bacteria (73). This idea has some historical precedent, having first been proposed in 1924 by Fraenkel, who proposed that the initiating site of infection was in the tracheo-bronchial mucosa and that pneumonia developed after infection had been established (23). In light of data acquired in experiments using luminescent bacteria that demonstrate bacterial growth within the NALT, it is possible that past studies of inhalational anthrax mistakenly focused on the lungs, rather than an alternate site, as the origin of disseminated bacteria, possibly one analogous to the NALT in mice.
The B. anthracis infection cycle relies on killing grazing animals through contraction of what is presumably gastrointestinal anthrax in order to increase spore concentrations in existing grazing areas or to introduce spores into spore-free grazing areas (58). Therefore, it is likely that B. anthracis has evolved to exploit the gut-associated lymphoid tissue (GALT), as observed in mouse models of infection (30), in order to kill grazing animals. When considering the similarities in structure of the NALT and GALT (30), it is conceivable that the deadly nature of inhalational anthrax is a by-product of B. anthracis exploiting a niche similar to those that would be targeted in a gastrointestinal infection. Following this line of reasoning, the difference in lethality between mucosal and cutaneous infections may result from differences in the immune responses between a mucosal and a nonmucosal infection. This has been alluded to in the literature on mouse studies, where the role of neutrophils and monocytes/macrophages in controlling subcutaneous versus inhalational infections has been assessed (15, 16, 48, 56). These studies have found that in subcutaneous infections, neutrophils are vital in clearing infection (56). However, in aerosol and intranasal infections, macrophages are the most important cell type mediating the clearance of the infection, with neutrophils providing minimal protection (16, 56). Likewise, research examining cutaneous anthrax infections with nude mice, which lack most adaptive immunity but retain innate immune system function, showed that an influx of neutrophils was associated with clearance of bacteria (75). These data can be combined with findings by Liu et al., who reported that when CMG2 is selectively eliminated in myeloid lineage cells, to eliminate their sensitivity to B. anthracis exotoxins, complete protection against subcutaneous B. anthracis infections is acquired (48). This suggests that while macrophages and neutrophils are protective against B. anthracis infection, exotoxins are capable of disarming the innate immune system to allow bacterial outgrowth and dissemination. Differences in cellular subtypes that respond to mucosal versus dermal infections may explain why B. anthracis has a higher mortality rate in inhalational than in cutaneous anthrax. Macrophages, which are protective in inhalational infection (15), rely heavily on oxidative killing, which exotoxins have been shown to affect (37). Neutrophils, shown to be protective in cutaneous infection, do not rely solely on their oxidative burst to kill pathogens but have granules that contain many different antimicrobial effectors (54). Indeed, human neutrophils kill B. anthracis independently of an oxidative burst with alpha defensins, which are key components of primary granules (54). The addition of nonoxidative killing in neutrophils perhaps suggests why neutrophil-controlled cutaneous infections are less lethal than macrophage-controlled inhalational infections. It is important to point out that while cell depletion experiments suggest that differential immune responses to a lung versus a subcutaneous infection occur, it has not been conclusively demonstrated experimentally. Overall, small-animal models suggest that, rather than inhalational anthrax being unique among the forms of anthrax, requiring a host immune cell to escape the lumena of the lungs, it may progress like gastrointestinal anthrax, where infection is established in a mucosa-associated lymphoid tissue.
JAILBREAK MODEL OF DISSEMINATION
While it has been demonstrated that Trojan horse-mediated movement of bacteria to the lymph nodes can occur at low levels (12) and that spores are found in the mediastinal lymph nodes at early stages of infection (30), it has not been shown experimentally that spores in draining lymph nodes are the founders of disseminated disease. In light of recent findings using animal models, we propose that B. anthracis germinates at favorable sites in the host, such as the NALT, GALT, and damaged cutaneous soft tissues, where it begins producing its virulence factors (Fig. 2, steps 1 and 2). Exotoxins and proteases produced by B. anthracis act to dampen the immune response and permeabilize tissues, effectively allowing vegetative bacilli to drain into the regional lymph node (Fig. 2, steps 3 to 5). Once the draining lymph node has been overwhelmed by exotoxins and bacteria draining from the initial site of infection, vegetative bacilli gain access to the bloodstream, causing bacteremia and eventually leading to host death (Fig. 2, steps 6 and 7). In summary, this model of dissemination proposes that differences in mortality between routes of infection are defined by the initial sites of spore contact with mucosally associated lymphoid tissue or cutaneous lesions. In addition, this model does not require intracellular transport of spores to the draining lymph nodes.
Fig 2.
Jailbreak model of infection. Spores enter the lumena of the airways, gastrointestinal tract, or skin at exposure (1). Spores germinate and become exotoxin-producing encapsulated vegetative bacteria at the site of initial entry into the host tissues (2). Continued vegetative bacterial growth increases exotoxin and protease concentrations and leads to the breakdown of the endothelial/epithelial barrier function (3). Vegetative bacteria pass through the damaged barrier (4). Vegetative bacteria traffic to a regional draining lymph node by bulk lymphatic flow without the necessity of phagocytic transport (5). Bacteria in the lymph node continue to produce virulence factors and replicate, eventually causing the loss of lymph node integrity (6). Bacteria escape the lymph node and enter the bloodstream, leading to bacteremia and host death (7).
CONCLUSIONS
Often, B. anthracis is still lethal after treatment with antibiotics initiated when distinct symptoms of anthrax manifest (such as mediastinal widening); thus, a better understanding of where infection initiates and how B. anthracis disseminates is necessary in order to begin to design treatment options that interrupt crucial early events in infection. The proposed jailbreak model presents an alternative to the Trojan horse model of dissemination, with the key difference being that, instead of spores requiring an intracellular step to establish infection in lymph nodes, the jailbreak model requires spore germination and exotoxin production at the initial site of spore exposure to cause damage to cellular barriers in order to gain access to the lymph and then the circulatory system. The proposed model of infection has several important ramifications for future research. Most importantly, this proposed model suggests a shift away from the current focus on potential Trojan horses and the lumena of the lungs and instead refocuses on the mucosa-associated lymphoid tissues in the upper respiratory tract in inhalational anthrax and the gastrointestinal tract for gastrointestinal anthrax. Additionally, the jailbreak model suggests that future research should have greater focus on differences in immune responses to mucosal versus nonmucosal surfaces and mechanisms by which B. anthracis circumvents these responses. However, there are several caveats with the jailbreak model of infection. The most notable is that evidence for the jailbreak model has been derived primarily from mice, and results similar to those acquired with BLI have not been substantiated in other animal model systems. As a result, the universality of the jailbreak model of infection will need to be assessed using nonmurine models of infection.
ACKNOWLEDGMENT
We have no conflicting financial interests in the data presented in this report.
Biographies

Zachary P. Weiner is from Greensboro, NC. He graduated with B.S. degrees in microbiology, food science, and science technology and society at North Carolina State University in 2008. While an undergraduate, Zach worked at the National Institute of Environmental Health Sciences studying DNA repair mechanisms and at NCSU developing detection protocols for Campylobacter jejuni. He is currently a doctoral candidate in the University of Virginia Department of Microbiology, Immunology, and Cancer Biology, where he became interested in studying the interactions between pathogens and hosts during infection.

Ian J. Glomski received his B.S. in biology as well as environmental science from Tufts University and his Ph.D. in molecular and cell biology from the University of California, Berkeley, in the lab of Daniel A. Portnoy and was a Judith P. Sulzberger Postdoctoral Fellow at the Institute Pasteur in Paris, France, in the laboratory of Michele Mock. Currently, Dr. Glomski is an Assistant Professor in the University of Virginia Department of Microbiology, Immunology, and Cancer Biology. His past, current, and future research focus is on Gram-positive bacterium-host interactions, with emphasis on Bacillus anthracis. In particular, his interests lie in the examination of the immunomodulation of antimicrobial host responses by bacteria and the development of novel tools to study this phenomenon in vivo in real time, with an ultimate goal that the data will be derived from minimally perturbed maximally “real” models of infection.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH. 1993. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. U. S. A. 90:2291–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agrawal A, et al. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424:329–334 [DOI] [PubMed] [Google Scholar]
- 3. Albrink WS, Goodlow RJ. 1959. Experimental inhalation anthrax in the chimpanzee. Am. J. Pathol. 35:1055–1065 [PMC free article] [PubMed] [Google Scholar]
- 4. Barth H, Aktories K, Popoff MR, Stiles BG. 2004. Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol. Mol. Biol. Rev. 68:373–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beatty ME, Ashford DA, Griffin PM, Tauxe RV, Sobel J. 2003. Gastrointestinal anthrax: review of the literature. Arch. Intern. Med. 163:2527–2531 [DOI] [PubMed] [Google Scholar]
- 6. Bischof TS, Hahn BL, Sohnle PG. 2007. Experimental cutaneous Bacillus anthracis infections in hairless HRS/J. mice. Int. J. Exp. Pathol. 88:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brittingham KC, et al. 2005. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J. Immunol. 174:5545–5552 [DOI] [PubMed] [Google Scholar]
- 8. Brookmeyer R, Blades N. 2002. Prevention of inhalational anthrax in the U.S. outbreak. Science 295:1861. [DOI] [PubMed] [Google Scholar]
- 9. Candela T, Fouet A. 2005. Bacillus anthracis CapD, belonging to the gamma-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol. Microbiol. 57:717–726 [DOI] [PubMed] [Google Scholar]
- 10. Carrera M, Zandomeni RO, Fitzgibbon J, Sagripanti JL. 2007. Difference between the spore sizes of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 102:303–312 [DOI] [PubMed] [Google Scholar]
- 11. Chung MC, et al. 2006. Secreted neutral metalloproteases of Bacillus anthracis as candidate pathogenic factors. J. Biol. Chem. 281:31408–31418 [DOI] [PubMed] [Google Scholar]
- 12. Cleret A, et al. 2007. Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J. Immunol. 178:7994–8001 [DOI] [PubMed] [Google Scholar]
- 13. Cossart P, Sansonetti PJ. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242–248 [DOI] [PubMed] [Google Scholar]
- 14. Cote CK, Bozue J, Twenhafel N, Welkos SL. 2009. Effects of altering the germination potential of Bacillus anthracis spores by exogenous means in a mouse model. J. Med. Microbiol. 58:816–825 [DOI] [PubMed] [Google Scholar]
- 15. Cote CK, Rea KM, Norris SL, van Rooijen N, Welkos SL. 2004. The use of a model of in vivo macrophage depletion to study the role of macrophages during infection with Bacillus anthracis spores. Microb. Pathog. 37:169–175 [DOI] [PubMed] [Google Scholar]
- 16. Cote CK, Van Rooijen N, Welkos SL. 2006. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect. Immun. 74:469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crawford MA, Aylott CV, Bourdeau RW, Bokoch GM. 2006. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J. Immunol. 176:7557–7565 [DOI] [PubMed] [Google Scholar]
- 18. Dixon TC, Fadl AA, Koehler TM, Swanson JA, Hanna PC. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell. Microbiol. 2:453–463 [DOI] [PubMed] [Google Scholar]
- 19. Druett HA, Henderson DW, Packman L, Peacock S. 1953. Studies on respiratory infection. I. The influence of particle size on respiratory infection with anthrax spores. J. Hyg. (Lond.) 51:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duesbery NS, et al. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734–737 [DOI] [PubMed] [Google Scholar]
- 21. Dumetz F, et al. 2011. Noninvasive imaging technologies reveal edema toxin as a key virulence factor in anthrax. Am. J. Pathol. 178:2523–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Firoved AM, et al. 2005. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 167:1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fraenkel E. 1924. Uber Inhalationsmilzbrand. Virchows Arch. Pathol. Anat. Physiol. 254:363–378 [Google Scholar]
- 24. Friedlander AM. 1997. Anthrax, p 467–478 In Zajtchuk R. (ed), Medical aspects of chemical and biological warfare. Office of the Surgeon General, Borden Institute, Walter Reed Army Medical Center, Washington, DC [Google Scholar]
- 25. Friedlander AM, et al. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239–1243 [DOI] [PubMed] [Google Scholar]
- 26. Fritz DL, et al. 1995. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab. Invest. 73:691–702 [PubMed] [Google Scholar]
- 27. Gerhardt P. 1967. Cytology of Bacillus anthracis. Fed. Proc. 26:1504–1517 [PubMed] [Google Scholar]
- 28. Glomski IJ, et al. 2008. Inhaled non-capsulated Bacillus anthracis in A/J mice: nasopharynx and alveolar space as dual portals of entry, delayed dissemination, and specific organ targeting. Microbes Infect. 10:1398–1404 [DOI] [PubMed] [Google Scholar]
- 29. Glomski IJ, et al. 2007. Murine splenocytes produce inflammatory cytokines in a MyD88-dependent response to Bacillus anthracis spores. Cell. Microbiol. 9:502–513 [DOI] [PubMed] [Google Scholar]
- 30. Glomski IJ, Piris-Gimenez A, Huerre M, Mock M, Goossens PL. 2007. Primary involvement of pharynx and Peyer's patch in inhalational and intestinal anthrax. PLoS Pathog. 3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goossens PL. 2009. Animal models of human anthrax: the quest for the Holy Grail. Mol. Aspects Med. 30:467–480 [DOI] [PubMed] [Google Scholar]
- 32. Greenfield WS. 1882. Supplementary report on woolsorters disease in the Bradford district. Local Government Board, London, United Kingdom [Google Scholar]
- 33. Grinberg LM, Abramova FA, Yampolskaya OV, Walker DH, Smith JH. 2001. Quantitative pathology of inhalational anthrax I: quantitative microscopic findings. Mod. Pathol. 14:482–495 [DOI] [PubMed] [Google Scholar]
- 34. Guidi-Rontani C. 2002. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol. 10:405–409 [DOI] [PubMed] [Google Scholar]
- 35. Guidi-Rontani C, Levy M, Ohayon H, Mock M. 2001. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol. Microbiol. 42:931–938 [DOI] [PubMed] [Google Scholar]
- 36. Guidi-Rontani C, Weber-Levy M, Labruyere E, Mock M. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9–17 [DOI] [PubMed] [Google Scholar]
- 37. Hanna PC, Kruskal BA, Ezekowitz RA, Bloom BR, Collier RJ. 1994. Role of macrophage oxidative burst in the action of anthrax lethal toxin. Mol. Med. 1:7–18 [PMC free article] [PubMed] [Google Scholar]
- 38. Harper GJ, Morton JD. 1953. The respiratory retention of bacterial aerosols: experiments with radioactive spores. J. Hyg. (Lond.) 51:372–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Henderson DW, Peacock S, Belton FC. 1956. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. (Lond.) 54:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu H, Sa Q, Koehler TM, Aronson AI, Zhou D. 2006. Inactivation of Bacillus anthracis spores in murine primary macrophages. Cell. Microbiol. 8:1634–1642 [DOI] [PubMed] [Google Scholar]
- 41. Inglesby TV, et al. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252 [DOI] [PubMed] [Google Scholar]
- 42. Ivanova N, et al. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87–91 [DOI] [PubMed] [Google Scholar]
- 43. Kang TJ, et al. 2005. Murine macrophages kill the vegetative form of Bacillus anthracis. Infect. Immun. 73:7495–7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kintzer AF, et al. 2009. The protective antigen component of anthrax toxin forms functional octameric complexes. J. Mol. Biol. 392:614–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koch R. 1878. Die Aetiologie der Milzbrand-Krankheit bergundet auf die Entwicklungsgeschichte des Bacillus anthracis. Beitr. Biol. Pflazen 2:277–311 [Google Scholar]
- 46. Leppla SH. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 79:3162–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lincoln RE, et al. 1965. Role of the lymphatics in the pathogenesis of anthrax. J. Infect. Dis. 115:481–494 [DOI] [PubMed] [Google Scholar]
- 48. Liu S, et al. 2010. Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe 8:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loving CL, et al. 2009. Role of anthrax toxins in dissemination, disease progression, and induction of protective adaptive immunity in the mouse aerosol challenge model. Infect. Immun. 77:255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Makino S, Uchida I, Terakado N, Sasakawa C, Yoshikawa M. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol. 171:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Makino S, Watarai M, Cheun HI, Shirahata T, Uchida I. 2002. Effect of the lower molecular capsule released from the cell surface of Bacillus anthracis on the pathogenesis of anthrax. J. Infect. Dis. 186:227–233 [DOI] [PubMed] [Google Scholar]
- 52. Maldonado-Arocho FJ, Bradley KA. 2009. Anthrax edema toxin induces maturation of dendritic cells and enhances chemotaxis towards macrophage inflammatory protein 3beta. Infect. Immun. 77:2036–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Manchee RJ, Broster MG, Stagg AJ, Hibbs SE. 1994. Formaldehyde solution effectively inactivates spores of Bacillus anthracis on the Scottish island of Gruinard. Appl. Environ. Microbiol. 60:4167–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mayer-Scholl A, et al. 2005. Human neutrophils kill Bacillus anthracis. PLoS Pathog. 1:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mayer TA, et al. 2003. Inhalational anthrax due to bioterrorism: would current Centers for Disease Control and Prevention guidelines have identified the 11 patients with inhalational anthrax from October through November 2001? Clin. Infect. Dis. 36:1275–1283 [DOI] [PubMed] [Google Scholar]
- 56. Moayeri M, et al. 2010. Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 6:e1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moayeri M, Haines D, Young HA, Leppla SH. 2003. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J. Clin. Invest. 112:670–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mock M, Fouet A. 2001. Anthrax. Annu. Rev. Microbiol. 55:647–671 [DOI] [PubMed] [Google Scholar]
- 59. Popov SG, et al. 2005. Effective antiprotease-antibiotic treatment of experimental anthrax. BMC Infect. Dis. 5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramsay CN, et al. An outbreak of infection with Bacillus anthracis in injecting drug users in Scotland. Euro Surveill. 15(2):pii=19465 [DOI] [PubMed] [Google Scholar]
- 61. Richter S, et al. 2009. Capsule anchoring in Bacillus anthracis occurs by a transpeptidation reaction that is inhibited by capsidin. Mol. Microbiol. 71:404–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ross JM. 1957. The pathogenesis of anthrax following the administration of spores by the respiratory route J. Pathol. Bacteriol. 73:9 [Google Scholar]
- 63. Russell BH, et al. 2008. In vivo demonstration and quantification of intracellular Bacillus anthracis in lung epithelial cells. Infect. Immun. 76:3975–3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Russell BH, Vasan R, Keene DR, Xu Y. 2007. Bacillus anthracis internalization by human fibroblasts and epithelial cells. Cell. Microbiol. 9:1262–1274 [DOI] [PubMed] [Google Scholar]
- 65. Ruthel G, Ribot WJ, Bavari S, Hoover TA. 2004. Time-lapse confocal imaging of development of Bacillus anthracis in macrophages. J. Infect. Dis. 189:1313–1316 [DOI] [PubMed] [Google Scholar]
- 66. Sanz P, et al. 2008. Detection of Bacillus anthracis spore germination in vivo by bioluminescence imaging. Infect. Immun. 76:1036–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schneerson R, et al. 2003. Poly(gamma-d-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc. Natl. Acad. Sci. U. S. A. 100:8945–8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tacke F, Randolph GJ. 2006. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 211:609–618 [DOI] [PubMed] [Google Scholar]
- 69. Tessier J, et al. 2007. Contributions of histamine, prostanoids, and neurokinins to edema elicited by edema toxin from Bacillus anthracis. Infect. Immun. 75:1895–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tournier JN, et al. 2005. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J. Immunol. 174:4934–4941 [DOI] [PubMed] [Google Scholar]
- 71. Turnbull PC. 1999. Definitive identification of Bacillus anthracis—a review. J. Appl. Microbiol. 87:237–240 [DOI] [PubMed] [Google Scholar]
- 72. van Sorge NM, et al. 2008. Anthrax toxins inhibit neutrophil signaling pathways in brain endothelium and contribute to the pathogenesis of meningitis. PLoS One 3:e2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Walsh JJ, et al. 2007. A case of naturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin. Infect. Dis. 44:968–971 [DOI] [PubMed] [Google Scholar]
- 74. Warfel JM, Steele AD, D'Agnillo F. 2005. Anthrax lethal toxin induces endothelial barrier dysfunction. Am. J. Pathol. 166:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Watts CJ, Hahn BL, Sohnle PG. 2009. Resistance of athymic nude mice to experimental cutaneous Bacillus anthracis infection. J. Infect. Dis. 199:673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. WHO 2008. Anthrax in humans and animals. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 77. Wu W, et al. 2009. Resistance of human alveolar macrophages to Bacillus anthracis lethal toxin. J. Immunol. 183:5799–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Young GA, Jr, Zelle MR, Lincoln RE. 1946. Respiratory pathogenicity of Bacillus anthracis spores; methods of study and observations on pathogenesis. J. Infect. Dis. 79:233–246 [DOI] [PubMed] [Google Scholar]