Abstract
Human and bovine neutrophils release neutrophil extracellular traps (NETs), which are protein-studded DNA matrices capable of extracellular trapping and killing of pathogens. Recently, we reported that bovine neutrophils release NETs in response to the important respiratory pathogen Mannheimia haemolytica and its leukotoxin (LKT). Here, we demonstrate macrophage extracellular trap (MET) formation by bovine monocyte-derived macrophages exposed to M. haemolytica or its LKT. Both native fully active LKT and noncytolytic pro-LKT (produced by an lktC mutant of M. haemolytica) stimulated MET formation. Confocal and scanning electron microscopy revealed a network of DNA fibrils with colocalized histones in extracellular traps released from bovine macrophages. Formation of METs required NADPH oxidase activity, as previously demonstrated for NET formation. METs formed in response to LKT trapped and killed a portion of the M. haemolytica cells. Bovine alveolar macrophages, but not peripheral blood monocytes, also formed METs in response to M. haemolytica cells. MET formation was not restricted to bovine macrophages. We also observed MET formation by the mouse macrophage cell line RAW 264.7 and by human THP-1 cell-derived macrophages, in response to Escherichia coli hemolysin. The latter is a member of the repeats-in-toxin (RTX) toxin family related to the M. haemolytica leukotoxin. This study demonstrates that macrophages, like neutrophils, can form extracellular traps in response to bacterial pathogens and their exotoxins.
INTRODUCTION
Mannheimia haemolytica is the most important bacterial pathogen of the bovine respiratory disease complex. In its most severe form it causes a severe fibrinous pleuropneumonia characterized by intense leukocyte infiltration in alveoli, intra-alveolar hemorrhage, fibrin deposition, and consolidation of the lungs (38). The most important virulence factor for M. haemolytica is its leukotoxin (LKT), a 104-kDa exotoxin released during logarithmic-phase growth (16, 22). LKT is a member of the repeats-in-toxin (RTX) toxin family of exoproteins produced by a wide variety of Gram-negative bacteria, including Escherichia coli, Actinobacillus pleuropneumoniae, and Aggregatibacter actinomycetemcomitans (47). Activation of pro-LKT requires acylation by the transacylase encoded by lktC (39). The acylated LKT then binds amino acids 5 to 17 of the signal sequence of bovine CD18 on ruminant leukocytes (29), leading to cell death. This restricts cytotoxicity to ruminant leukocytes, because the signal sequence for CD18 is not present on mature leukocytes from other mammalian species (19, 37, 40). A similar RTX toxin, the hemolysin produced by uropathogenic E. coli, is neither species nor cell type restricted (37).
Activated neutrophils undergo a form of cell death, called NETosis, in which nuclear DNA is released into the extracellular environment with little concomitant release of lactate dehydrogenase (LDH) (10, 11, 21, 34, 35, 46). The resulting network of extracellular DNA and associated proteins (e.g., histones and granule constituents) are called neutrophil extracellular traps (NETs) (10). The extracellular DNA within NETs is studded with antimicrobial proteins. These include histones and primary, secondary, and tertiary granular components such as neutrophil elastase, myeloperoxidase, lactoferrin, and gelatinase (10). Activation of human neutrophils with interleukin-8, phorbol 12-myristate 13-acetate (PMA), or lipopolysaccharide (LPS) alone causes NET formation (34, 46). NET formation also occurs in response to prokaryotic and eukaryotic pathogens (10, 21, 46). Extracellular trap formation has also been demonstrated for other types of granulocytes, such as eosinophils and mast cells (44, 48).
NETs are capable of trapping and killing a variety of Gram-negative and Gram-positive bacteria, fungi, and protozoa (6, 7, 10, 11, 13, 18, 21, 23, 24, 34, 41, 46). Recently published data demonstrate that NETs are formed in response to M. haemolytica and its leukotoxin and that some of the M. haemolytica cells are killed during this process (4). In this report, we present evidence that bovine macrophages also form extracellular traps (i.e., macrophage extracellular traps [METs]) that are capable of snaring and killing M. haemolytica cells in vitro. Furthermore, we present evidence that MET formation is also a property of murine and human macrophage cell lines. These findings are consistent with recent reports that murine macrophages form METs in response to Staphylococcus aureus, resulting in death of a portion of the bacterial cells (17). Similarly, human monocytes formed METs in response to certain types of gold nanoparticles in vitro (5).
MATERIALS AND METHODS
Cell lines and primary cell preparation.
RAW 264.7 (mouse macrophage) and THP-1 (human monocyte) cell lines were grown in RPMI 1640 (Cellgro, Manassas, VA) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Cellgro). All cells were grown at 37°C with 5% CO2 in a humidified incubator. Differentiation of the THP-1 cells into macrophage-like cells was performed by incubation with 100 nM PMA in culture medium for 7 days at 37°C with 5% CO2 (36). Differentiated THP-1 cells were deemed acceptable when >95% of the THP-1 cells were adherent (36).
Whole blood was collected by venipuncture from healthy Holstein cows housed at the University of Wisconsin—Madison Dairy Cattle Center using 0.38% (vol/vol) sodium citrate as anticoagulant. Blood was centrifuged at 1,000 × g for 15 min, and the buffy coat was removed. The buffy coat, containing mononuclear cells, was suspended in Hanks' balanced salt solution (HBSS; Cellgro) with 4 mM EDTA (without calcium or magnesium), layered onto Histopaque-1083 (Sigma-Aldrich, St. Louis, MO), and centrifuged at 1,000 × g for 30 min at room temperature. Mononuclear cells were removed, and contaminating red blood cells (RBCs) were lysed in a 1:10 dilution of lysis buffer (150 mM ammonium chloride, 10 mM Tris [pH 7.5]) while rotating at 8 rpm for 10 min. Cells were pelleted at 1,000 × g and washed 3 times with HBSS with 4 mM EDTA. Mononuclear cells were resuspended in RPMI 1640 with 1% (vol/vol) FBS and incubated at 37°C with 5% CO2 for 2 h on 100-mm carboxyl-coated dishes (Becton, Dickinson and Company, Franklin Lakes, NJ). Nonadherent cells were removed by repeated washing. Adherent monocytes were allowed to differentiate into monocyte-derived macrophages by incubating them in RPMI 1640 with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin for 7 days at 37°C. The medium was exchanged twice during this period. Monolayers with greater than 99% viability, as determined by trypan blue staining and light microscopy, were deemed acceptable for further use.
Neutrophils were isolated by lysis of the red blood cell pellet using a 1:3 dilution in lysis buffer while rotating at 8 rpm for 10 min. Bovine neutrophils (bovine polymorphonuclear leukocytes [bPMNs]) were pelleted at 1,000 × g and washed 4 times with HBSS. Cells were resuspended in serum- and phenol red-free RPMI 1640 medium and examined by light microscopy. Cell suspensions found to be >98% bPMNs, as determined by cell morphology, and to have >99% viability, as determined by trypan blue staining, were deemed acceptable for use in experiments.
Bovine alveolar macrophages were isolated from bronchoalveolar lavage (BAL) fluid obtained from donor cows at the University of Wisconsin—Madison Veterinary Medicine Teaching Hospital. Lavage fluid was filtered through a 0.2-μm-pore-size nylon filter, washed 3 times, and resuspended in RPMI 1640 at a concentration of 106 macrophages/ml. Bovine alveolar macrophages were allowed to adhere for 1 h, washed 2 times with phosphate-buffered saline (PBS), and used immediately in RPMI 1640. Cultures with greater than 90% viability, as determined by trypan blue staining and light microscopy, were used in experiments.
Bacteria.
Mannheimia haemolytica A1 (obtained from a bovine pneumonic lung), M. haemolytica ΔlktC (SH1562; a gift from S. Highlander), and M. haemolytica ΔlktA (a gift from R. Briggs) were grown in brain heart infusion (BHI) broth without shaking at 37°C for 10 h. Bacterial cells were pelleted at 3,750 × g, washed 3 times in PBS, and resuspended in RPMI to an optical density of 0.7, which corresponds to 5 × 109 CFU/ml. The number of CFU in each broth culture was extrapolated from growth curves performed in our laboratory and confirmed by dilution plating on tryptic soy agar (TSA) with 5% sheep red blood cells (Becton, Dickinson and Company) to enumerate the CFU.
Production and purification of toxins.
LKT, pro-LKT, and ΔlktA LKT were prepared from Mannheimia haemolytica A1, M. haemolytica ΔlktC strain SH1562 (20), and the M. haemolytica ΔlktA strain, respectively (39). Hemolysin (HLY) was prepared from Escherichia coli strain WAM1824 (kindly provided by R. A. Welch, Madison, WI) (33) grown to logarithmic growth phase in RPMI 1640 medium supplemented with 10 μg/ml chloramphenicol. Toxins were produced and purified as described previously (2). Briefly, bacteria were pelleted from 12-h broth cultures by centrifugation at 3,750 × g for 10 min. The pelleted cells were resuspended in RPMI 1640 medium and incubated for 6 h at 37°C until they reached logarithmic growth phase. Bacterial cells were centrifuged at 7,500 × g for 30 min at 4°C. The supernatant was removed, filtered (pore size, 0.2 μm), and concentrated using an Amicon filtration system with a 100-kDa-molecular-mass-cutoff membrane (Millipore, Billerica, MA). The LKT or HLY was stored at −70°C with 20% (vol/vol) glycerol until used in an experiment. One unit of LKT or HLY is defined as the greatest dilution of toxin that killed 50% of target cells (106 bovine lymphoblastoid [BL-3] cells and human kidney epithelial [A498] cells, respectively) in 2 h at 37°C, as determined by the CellTiter 96 AQueous One Solution cell proliferation assay (Promega, Madison, WI). lktC and lktA deletion mutant LKT preparations did not exhibit any cytotoxicity in this assay. Protein concentrations were determined using a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL).
Quantification of extracellular DNA.
Macrophage extracellular DNA was quantified using a modified technique described by Fuchs et al. (21). Briefly, macrophages were incubated for the indicated times with various stimuli and then pelleted at 500 × g for 3 min. The supernatant was removed, micrococcal nuclease buffer with 0.1 U/μl micrococcal nuclease was added (New England BioLabs, Ipswich, MA), and the mixture was incubated for 15 min at 37°C (as described by the manufacturer). A 1:200 dilution of PicoGreen reagent (Invitrogen, Carlsbad, CA) in 10 mM Tris base buffered with 1 mM EDTA was added to an equal volume of the nuclease-treated macrophage mixture. Fluorescence was determined at an excitation wavelength of 488 nm and an emission wavelength of 520 nm using an automated plate reader (DTX 800 multimode detector; Beckman Coulter, Brea, CA). Extracellular trap production was quantified as the fold increase in the number of fluorescence units for LKT-treated macrophages divided by the number of fluorescence units for untreated control macrophages.
Reagents.
DNase I (source, bovine pancreas), PMA, and cytochalasin D (Cyto D) were purchased from Sigma-Aldrich. The NADPH oxidase inhibitor diphenyleneiodonium chloride (DPI) was purchased from Calbiochem/EMD (Darmstadt, Germany). The pancaspase inhibitor fluoromethylketone (Z-VAD-FMK) was purchased from Alexis Biochemicals (Plymouth Meeting, PA). The anti-CD18 antibody (BAQ20A) was purchased from VMRD (Pullman, WA). M. haemolytica A1 LPS was kindly provided by D. McClenahan (Cedar Falls, IA) (32). The anti-LKT antibody (MM601) was kindly provided by S. Srikumaran (Pullman, WA). The antihistone antibody was purchased from AbCam (Cambridge, MA), and the Alexa Fluor 488 anti-rabbit IgG antibody and Alexa Fluor 488 anti-mouse IgG antibody were purchased from Invitrogen. LDH release was determined using the CytoTox 96 nonradioactive cytotoxicity assay as described by the manufacturer (Promega).
Immunofluorescence.
To perform immunofluorescence microscopy, macrophages (2.5 × 105) were grown on 12-mm glass coverslips for 16 h at 37°C (Fisher Scientific, Hanover Park, IL). Slides were washed, resuspended in RPMI 1640, and incubated for 30 min at 37°C with 1 U LKT, 5 × 107 fluorescein-labeled M. haemolytica, 250 nM LPS, 100 μm PMA, or 1 U LKT and 180 U DNase. Slides were washed 3 times with PBS and fixed for 10 min with 4% paraformaldehyde. The slides were then washed, permeabilized with cold acetone for 5 min, washed again, and blocked with 1% bovine serum albumin (BSA; Pierce) in PBS for 20 min at room temperature. Slides were incubated for 1 h with 1 μM TOPRO stain. In some experiments, an antihistone monoclonal antibody (AbCam, Cambridge, MA) was added, and slides were washed and then incubated for 1 h at room temperature with an Alexa Fluor 488-conjugated anti-mouse IgG antibody (Molecular Probes, Eugene, OR) in 1% BSA (in PBS). Slides were washed and examined by confocal microscopy (Eclipse TE2000-U microscope; Nikon Corporation, Tokyo, Japan).
SEM.
Bovine macrophages (5 × 106) were incubated on glass slides overnight at 37°C, washed twice with PBS, and then incubated with 5 × 107 M. haemolytica cells for 5 min. Slides were washed and fixed using 4% paraformaldehyde in PBS, postfixed with 2.5% glutaraldehyde, and prepared as previously described (9). Images were taken at Winona State University using a Feico Phenom scanning electron microscope (SEM; FEI Company, Hillsboro, OR).
Bacterial trapping and killing.
M. haemolytica cells were grown to log phase as described earlier, washed 3 times in PBS, and resuspended for 15 min on ice in 0.5 mg/ml fluorescein isothiocyanate (FITC; Sigma-Aldrich) in 50 mM sodium carbonate buffer. The M. haemolytica cells were then washed 3 times with PBS and resuspended at a concentration of 5 × 109 CFU/ml in serum-free RPMI 1640. FITC-labeled M. haemolytica cells were serially diluted and plated on TSA agar with 5% sheep RBCs, and colonies were enumerated to confirm viability.
Macrophages (105) were incubated with FITC-labeled or unlabeled M. haemolytica cells (107) for various times and analyzed for trapping and killing of bacterial cells. As a control, 180 U DNase I was added to some cultures to cleave extracellular DNA and free the bacteria. In a similar experiment, 0.5 U LKT was incubated with macrophages (105) for 30 min at 37°C prior to the addition of M. haemolytica. DNase-treated and LKT-treated macrophages were washed and then incubated with FITC-labeled or unlabeled M. haemolytica cells (107) in RPMI 1640 for various times at 37°C. Some macrophages were incubated with 180 U DNase I and M. haemolytica cells for the entire length of the experiment.
To quantify the bacterial cells trapped within the METs, samples were removed and washed 3 times with PBS and fluorescence was determined using an automated plate reader at 488 nm. To determine bacterial killing by METs, macrophages incubated with M. haemolytica suspensions were serially diluted in PBS and plated on TSA with 5% sheep RBCs. At each time point, serial dilutions of M. haemolytica cells that had been incubated in RPMI (without macrophages) were plated to quantify the total numbers of CFU. The percent bactericidal activity of METs was determined as described previously (4).
Statistical analysis.
Group means were compared by analysis of variance, followed by the Tukey-Kramer pairwise comparison test, as performed by the Instat statistical package (GraphPad, San Diego, CA). The level of significance was set at a P value of <0.05.
RESULTS
LKT and pro-LKT cause MET formation.
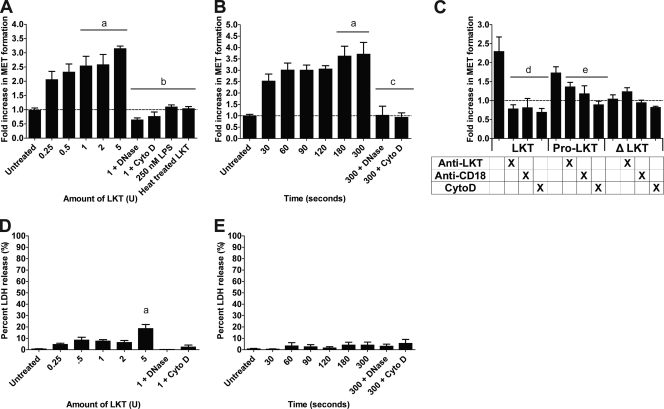
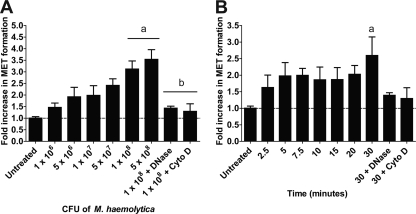
Previous research on extracellular trap formation has focused on human neutrophils (10). We previously showed that bovine neutrophils produce NETs in response to M. haemolytica and its LKT (4). Here, we show that bovine monocyte-derived macrophages produced METs (up to a 3-fold increase in extracellular DNA) in a dose- and time-dependent manner in response to LKT (Fig. 1A and B). In a separate experiment, we established that approximately 7% of total macrophage DNA (i.e., cells lysed with 0.5% SDS) was released during a 20-min incubation with 2 U LKT (data not shown). We did not observe release of extracellular DNA by peripheral blood monocytes incubated with LKT under the same conditions (data not shown). MET formation was reduced when DNase I or Cyto D was added to cleave extracellular DNA or inhibit intracellular transport of LKT, respectively (Fig. 1A). Neither purified M. haemolytica LPS nor heat-inactivated LKT (100°C for 1 h) induced MET formation (Fig. 1A). Taken together these observations indicate that contaminating LPS alone does not cause MET formation. We quantified LDH release as an indicator of necrosis (21, 35) and observed no significant increase in LDH except when larger amounts (e.g., 5 U) of LKT were incubated with bovine macrophages (Fig. 1D and E).
Fig 1.
LKT and pro-LKT cause MET formation by bovine macrophages. (A and B) Bovine macrophages (105) were incubated with various amounts of LKT for 20 min (A) or were incubated with 0.5 U of LKT for various times (B). As a control, 105 bovine macrophages were preincubated with 180 U of DNase I or 10 μg/ml Cyto D, to degrade extracellular DNA or inhibit LKT internalization, respectively. In some experiments, 105 bovine macrophages were incubated with 250 nM M. haemolytica LPS and 1 U heat-inactivated LKT (100°C for 30 min). Untreated cells were incubated with RPMI 1640 as a negative control. (C) Bovine macrophages (105) were incubated with 1 U LKT, pro-LKT, or ΔLKT for 20 min. Some bovine macrophages were incubated with an anti-CD18 antibody or 10 μg/ml cytochalasin D, or the toxins were incubated with an anti-LKT antibody for 30 min prior to addition to macrophages. For panels A to C, extracellular DNA was quantified by the addition of a 1:200 dilution of PicoGreen and fluorescence was quantified using an automated plate reader. Results are expressed as the fold increase compared to untreated cells. (D and E) Bovine macrophages (105) were incubated with various amounts of LKT for 20 min (D) or were incubated with 0.5 U of LKT for various times (E). LDH was quantified using the CytoTox 96 nonradioactive cytotoxicity assay as described by the manufacturer. Data represent the mean ± SEM of 5 independent experiments. a, P < 0.05 compared to untreated macrophages; b, P < 0.05 compared to macrophages treated with 1 U LKT; c, P < 0.05 compared to macrophages treated with LKT for 5 min; d, P < 0.05 compared to macrophages incubated with LKT alone; e, P < 0.05 compared to macrophages incubated with pro-LKT alone.
MET formation in response to LKT was further examined using two LKT variants. The first is a pro-LKT purified from a ΔlktC strain of M. haemolytica (a kind gift from S. K. Highlander, Houston, TX), and the second is a truncated leukotoxin protein (ΔLKT) purified from a ΔlktA M. haemolytica strain (kind gift from R. E. Briggs, Ames, IA). The ΔlktC strain of M. haemolytica does not produce an active LKTC protein; therefore, acylation of the LKTA protein does not occur and the resulting pro-LKT is noncytolytic in conventional assays, despite being capable of binding to CD18 (4). The ΔlktA strain produces a ΔLKT that lacks amino acids 34 to 378, rendering it incapable of binding CD18 or causing cytotoxicity (39). Incubation of bovine macrophages with pro-LKT caused MET formation, although it was somewhat reduced compared to that achieved with native LKT (Fig. 1C). MET formation in response to LKT and pro-LKT was reduced when bovine macrophages were preincubated with an anti-CD18 receptor antibody or cytochalasin D or when the toxins were incubated with a neutralizing anti-LKT antibody (MM601) before being added to the macrophages (Fig. 1C). As expected, the truncated ΔLKT did not cause MET formation (Fig. 1C). These data confirm that MET formation requires LKT binding to its receptor, CD18.
Bovine macrophages form METs in response to M. haemolytica cells that produce LKT or pro-LKT.
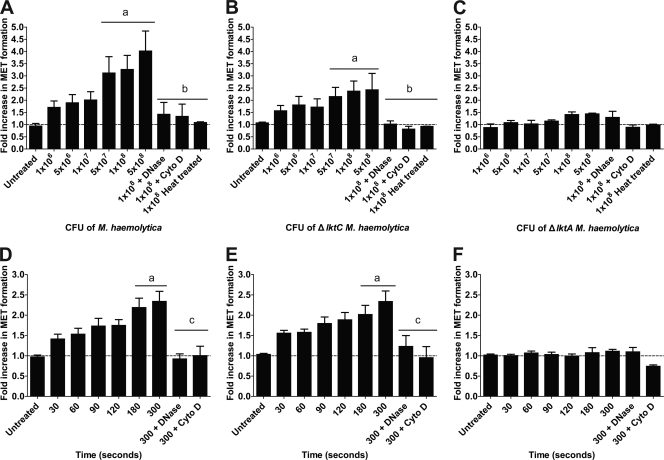
We next tested if M. haemolytica cells stimulated MET formation. Bovine macrophages produced METs in a time- and dose-dependent manner when incubated with wild-type M. haemolytica cells that produce active LKT or ΔlktC M. haemolytica cells that produce a nonacylated pro-LKT (Fig. 2). MET formation did not occur in response to ΔlktA M. haemolytica cells that produce a truncated noncytolytic LKT, nor were METs observed when DNase I or cytochalasin D was added to macrophages incubated with M. haemolytica cells that produce LKT (Fig. 2). MET formation was not due to macrophage lysis. Little LDH release was observed in medium from macrophages incubated with wild-type or ΔlktC M. haemolytica cells (data not shown), suggesting that DNA release did not simply reflect necrosis.
Fig 2.
Wild-type M. haemolytica and ΔlktC M. haemolytica cells cause MET formation by bovine macrophages. Monocyte-derived macrophages (105) were incubated for 20 min with various cell numbers of M. haemolytica (A), ΔlktC M. haemolytica (B), or ΔlktA M. haemolytica (C). In some experiments, bacteria were inactivated at 100°C for 30 min prior to addition to macrophages. (D to F) Macrophages (105) were incubated for various times with 1 × 107 cells of wild-type M. haemolytica (D), ΔlktC M. haemolytica (E), or ΔlktA M. haemolytica (F). In some experiments, 180 U of DNase I or 10 μg/ml cytochalasin D was incubated with the macrophages for 30 min at 37°C prior to addition of the bacterial cells. Extracellular DNA was quantified using PicoGreen as described in Materials and Methods. Data represent the mean ± SEM of 5 independent experiments. a, P < 0.05 compared to untreated macrophages; b, P < 0.05 compared to macrophages incubated with 1 × 108 bacteria; c, P < 0.05 compared to macrophages incubated for 300 s with bacterial cells alone.
MET production is dependent on NADPH oxidase activity.
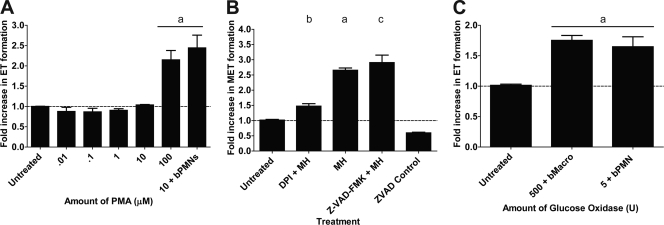
NET formation by human neutrophils is dependent on the generation of reactive oxygen species (ROS) by NADPH oxidase (21). Neutrophils from chronic granulomatous disease patients or from patients who have a mutation in the NADPH oxidase gene, which renders them susceptible to recurrent infections, do not form NETs (7, 21, 28). We tested if incubation with PMA, which activates the assembly and production of NADPH oxidase in phagocytes (21), stimulates MET production. We found that bovine macrophages formed METs in response to PMA, although this required concentrations higher than those needed to stimulate NET formation by bovine neutrophils (100 μM versus 10 μM, respectively) (Fig. 3A). Addition of the NADPH oxidase inhibitor DPI significantly decreased M. haemolytica-induced MET formation (Fig. 3B), as described previously for neutrophils. METs also formed when bovine macrophages were incubated with glucose oxidase (GO), which produces hydrogen peroxide and activates ROS production within macrophages (Fig. 3C). It should be noted that the concentrations of PMA, GO, and DPI required to exert their effects were greater than those reported for trap formation by neutrophils (21). MET formation in response to LKT was not inhibited by the pancaspase inhibitor Z-VAD-FMK, indicating that MET formation is not the result of apoptosis (Fig. 3B).
Fig 3.
MET formation requires NADPH oxidase activity. (A) Bovine macrophages (105) were incubated with various concentrations of PMA. As a positive control, 105 bovine neutrophils were incubated with 10 μM PMA. (B) Bovine macrophages (105) were preincubated with 50 μM DPI for 30 min at 37°C. DPI-treated and untreated macrophages (105) were then incubated with 5 × 107 M. haemolytica cells (MH). As a control, some macrophages were incubated with 200 μM the pancaspase inhibitor Z-VAD-FMK prior to addition of 5 × 107 M. haemolytica cells. (C) Bovine macrophages or neutrophils (105) were incubated with 500 U or 5 U glucose oxidase, respectively. Extracellular DNA was quantified using PicoGreen as described in Materials and Methods. Data represent the mean ± SEM of 5 independent experiments. a, P < 0.05 compared to untreated macrophages; b, P < 0.05 compared to macrophages incubated with 5 × 107 M. haemolytica; c, P < 0.05 compared to Z-VAD-FMK control macrophages.
METs trap and kill M. haemolytica cells.
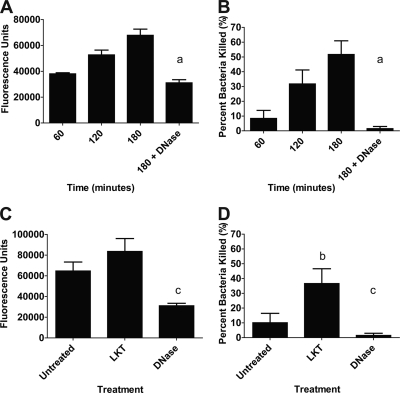
Previous investigations have shown that NETs and METs are capable of trapping and killing extracellular bacteria (17, 21). Using fluorescein-labeled M. haemolytica cells, we found that bacterial cells were trapped, in a time-dependent manner, by METs formed in response to M. haemolytica cells (Fig. 4A). Furthermore, approximately 50% of the M. haemolytica cells were killed by METs during a 180-min incubation (Fig. 4B). To confirm that the bacteria were trapped in METs, we added DNase I to cleave extracellular DNA and free trapped bacterial cells. DNase I treatment significantly reduced the number of M. haemolytica cells trapped and killed by bovine macrophages (Fig. 4A and B). We infer that the remaining small proportion of bacterial cells that were killed by DNase I-treated macrophages (<2%) represents bacterial cells that were phagocytosed and killed intracellularly.
Fig 4.
Preincubating bovine macrophages with LKT increases the trapping and killing of M. haemolytica by METs. Bovine macrophages (105) were incubated with 107 fluorescein-labeled M. haemolytica cells (A) or unlabeled M. haemolytica cells (B) for 60, 120, or 180 min. Similarly, LKT-treated (0.5 U LKT for 30 min) or untreated macrophages were incubated for 60 min with 107 fluorescein-labeled M. haemolytica cells (C) or 107 unlabeled M. haemolytica cells (D). Untreated macrophages were used as controls. Additional controls included macrophages incubated with M. haemolytica and 180 U DNase I. Bacterial trapping was estimated by fluorescence using an automated plate reader. Bacterial survival was estimated by plating serial dilutions of lysates on TSA–5% sheep RBC plates. Data represent the mean ± SEM of 5 independent experiments. a, P < 0.05 compared to macrophages incubated with M. haemolytica for 180 min; b, P < 0.05 compared to untreated macrophages; c, P < 0.05 compared to macrophages treated with LKT alone.
During pulmonary infection with M. haemolytica, bovine leukocytes are likely exposed to LKT before they encounter the bacterial cells. We therefore examined whether prior exposure to LKT affects the ability of METs to trap and kill M. haemolytica cells. To do so, we incubated bovine macrophages with a small amount of LKT (0.5 U) for 30 min before M. haemolytica cells (10 bacterial cells per macrophage) were added. Prior incubation with LKT had little effect on the numbers of M. haemolytica cells trapped within METs (Fig. 4C) but significantly increased the percentage of bacterial cells killed (Fig. 4D). DNase I treatment reduced the numbers of bacteria that were trapped and killed (Fig. 4C and D), implicating DNA-containing METs in bacterial killing. We also used a Syto 9 and propidium iodide staining kit (Live/Dead BacLight bacterial viability kit; Invitrogen) to confirm that a proportion of the M. haemolytica cells trapped within METs were killed and to exclude the possibility that the reduced numbers of CFU simply reflect clumped bacteria (data not shown).
Scanning electron and confocal microscopy examination of METs.
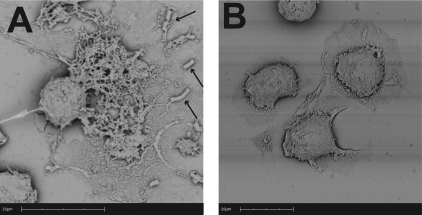
Using SEM, we observed web-like structures in which M. haemolytica cells were trapped, protruding from some macrophages (Fig. 5A). The extracellular network appeared to exceed 10 μm in length (Fig. 5A). These structures were not seen when bovine macrophages were incubated with medium that did not contain M. haemolytica cells (Fig. 5B).
Fig 5.
Scanning electron photomicrographs of METs formed by bovine macrophages in response to M. haemolytica cells. Bovine macrophages (2.5 × 105) were incubated with 5 × 107 M. haemolytica cells or RPMI 1640 (negative control) for 5 min at 37°C. Cells were washed, fixed, and processed for SEM as described in Materials and Methods. (A) A matrix of extracellular DNA strands released in response to M. haemolytica cells (arrows indicate trapped bacterial cells); (B) control bovine macrophages incubated with RPMI that do not exhibit extracellular DNA fibrils. Photomicrographs are of representative cells from 3 independent experiments.
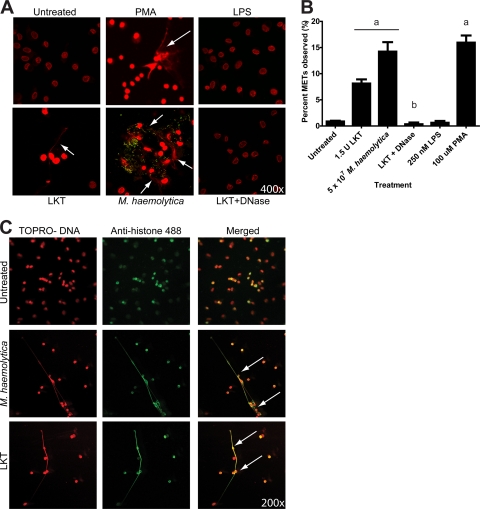
To further examine the structure of the METs, we incubated bovine macrophages with LKT or fluorescein-labeled M. haemolytica cells and then fluorescently stained nucleic acids with TOPRO (red). Confocal microscopy analysis confirmed the presence of large extracellular strands and clumps of DNA released from macrophages incubated with LKT, M. haemolytica cells (green), or 100 μM PMA (Fig. 6A). Extracellular DNA was not observed for untreated bovine macrophages, macrophages incubated with M. haemolytica LPS, or macrophages concomitantly incubated with LKT and DNase I, even when the intensity of the micrographs was increased (Fig. 6A). To quantify the proportion of cells undergoing MET formation, we counted 500 macrophages and found that approximately 8% and 15% of macrophages appeared to form METs in response to LKT and M. haemolytica cells, respectively (Fig. 6B). These numbers were significantly decreased when DNase I was added along with LKT and macrophages (Fig. 6B). As a positive control, incubation with 100 μM PMA stimulated MET formation by a percentage of macrophages similar to that seen with M. haemolytica cells (Fig. 6B).
Fig 6.
Confocal microscopy of METs formed by bovine macrophages in response to LKT and M. haemolytica. (A) Macrophages (2.5 × 105) were incubated for 30 min at 37°C with 1 U LKT, 1 U LKT with 180 U DNase I, 107 fluorescein-labeled M. haemolytica cells, 100 mM PMA, 250 nM M. haemolytica LPS, or RPMI 1640 (negative control). Cells were fixed, permeabilized, stained for DNA using TOPRO, and examined by confocal microscopy. Arrows indicate representative METs emanating from macrophages. (B) Percentage of macrophages that formed METs, based on scoring of 500 macrophages in multiple micrographs. a, P < 0.05 compared to untreated macrophages; b, P < 0.05 compared to macrophages incubated with 1.5 U LKT. Photomicrographs are of representative cells from 3 independent experiments. (C) To assess colocalization of DNA and histones in METs, 2.5 × 105 macrophages were incubated with 1 U LKT or 107 M. haemolytica cells for 30 min. Cells were fixed, permeabilized, stained for DNA using TOPRO (red), and probed for histones using an antihistone antibody followed by an antimouse antibody labeled with Alexa Fluor 488 (green). Cells were examined by confocal microscopy. Arrows indicate areas of colocalization of signals for extracellular DNA and histones. Photomicrographs are of representative cells from 3 independent experiments.
To examine whether the DNA in METs was derived from the nucleus or mitochondria, we incubated macrophages with LKT or M. haemolytica cells and then fluorescently stained for DNA (red) and histones (green). Confocal microscopy revealed that DNA and histones colocalized within the nucleus in untreated control bovine macrophages (Fig. 6C). Similarly, DNA and histones colocalized to both the extracellular DNA and nuclei of macrophages treated with LKT or M. haemolytica cells. These observations are consistent with the extracellular DNA being of nuclear origin.
Bovine alveolar macrophages produce METs.
We next examined whether other types of bovine macrophages produce extracellular traps. We found that bovine alveolar macrophages formed METs in a dose- and time-dependent manner in response to M. haemolytica cells (Fig. 7). Preincubation of alveolar macrophages with DNase I or cytochalasin D reduced MET formation, as previously observed for monocyte-derived macrophages (Fig. 1) and bovine neutrophils (4). In contrast to these results, we did not observe extracellular traps when peripheral blood monocytes were incubated with LKT (data not shown).
Fig 7.
Bovine alveolar macrophages produce METs in response to M. haemolytica. Bovine alveolar macrophages (105) were incubated with various numbers of M. haemolytica cells for 30 min (A) or were incubated with 107 M. haemolytica cells for various times (B). As a control, 105 bovine alveolar macrophages were preincubated with 180 U of DNase I or 10 μg/ml Cyto D. Extracellular DNA was quantified using PicoGreen as described in Materials and Methods. Data represent the mean ± SEM of 5 independent experiments. a, P < 0.05 compared to untreated macrophages; b, P < 0.05 compared to macrophages incubated with 1 × 108 M. haemolytica cells.
Production of extracellular traps by murine and human macrophage cell lines.
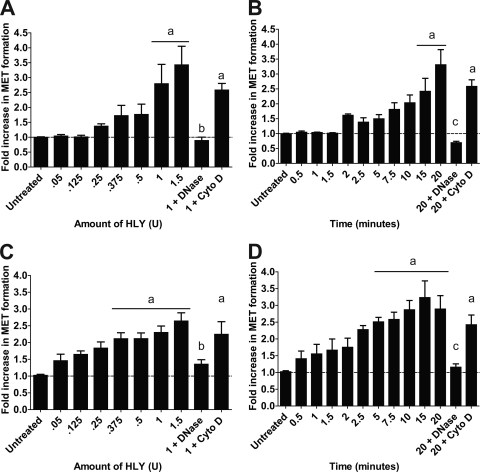
Finally, we examined whether MET formation was restricted to bovine macrophages or was representative of a broader response by macrophages from other mammalian species. To trigger MET formation by nonruminant macrophages, we used the E. coli HLY, which is the prototypical RTX toxin that is active against leukocytes from multiple species (47). When we incubated HLY with the mouse macrophage cell line RAW 264.7, we observed MET formation in a dose- and time-dependent manner (Fig. 8A and B). MET formation was reduced when DNase I was present but not when cytochalasin D was added (Fig. 8A and B). Similarly, we examined extracellular trap formation by the human THP-1 monocyte cell line after those cells were treated with PMA to differentiate them into macrophage-like cells. THP-1 cells formed METs in a dose- and time-dependent manner in response to the E. coli HLY (Fig. 8C and D). As for RAW 264.7 cells, MET formation was reduced by DNase I but was not inhibited by cytochalasin D. We did not observe MET formation by either cell line in response to LPS (data not shown).
Fig 8.
RAW 264.7 murine and THP-1-derived human macrophages produce METs in response to E. coli HLY. (A and B) RAW 264.7 macrophages (105) were incubated with various amounts of E. coli HLY for 20 min (A) or were incubated with 0.5 U HLY for various times (B). (C and D) THP-1-derived macrophages (105) were incubated with various amounts of HLY for 20 min (C) or were incubated with 0.5 U HLY for various times (D). As additional controls, 105 macrophages were preincubated with 180 U of DNase I or 10 μg/ml Cyto D. Extracellular DNA was quantified using PicoGreen as described in Materials and Methods. Data represent the mean ± SEM of 5 independent experiments. a, P < 0.05 compared to untreated macrophages; b, P < 0.05 compared to macrophages incubated with 1 U of HLY; c, P < 0.05 compared to macrophages treated for 20 min with HLY.
DISCUSSION
We present here a detailed characterization of macrophage extracellular trap formation. Bovine macrophages produce extracellular traps in a dose- and time-dependent manner in response to M. haemolytica and its LKT (Fig. 1 and 2). MET formation peaked at a 3-fold increase, which is similar to results for NET formation published by other investigators in response to various pathogens (35). We used several methods to confirm that LKT, and not contaminating LPS, was responsible for MET formation. These included the use of (i) cytochalasin D, to inhibit LKT-mediated internalization (2, 3); (ii) antibodies to LKT and its CD18 receptor; (iii) heat-inactivated LKT, heat-inactivated M. haemolytica cells, or heat-inactivated ΔlktC M. haemolytica cells (100°C for 1 h); (iv) a truncated ΔLKT; and (v) purified M. haemolytica LPS alone (Fig. 1). Taken together, these data indicate that LKT causes MET formation via binding to its CD18 receptor and that M. haemolytica LPS (25 nM to 2.5 μM) by itself does not cause MET formation by bovine macrophages (data not shown). Similar results were reported using bovine neutrophils (4). The lack of LPS-mediated extracellular trap formation is similar to the results of Clark et al. (18), who reported that LPS alone did not stimulate NET production by human neutrophils, unless platelets were also present. However, we cannot completely exclude a role for LPS as a cofactor in the MET response to M. haemolytica cells or to LKT.
Greater concentrations of RTX toxins can disrupt the cytoplasmic membrane and cause lysis of host cells (47). To examine what was responsible for the release of DNA, we quantified LDH release, which is a marker for necrosis (21, 35). LDH release remained low at LKT concentrations (0.25 to 2 U) that trigger MET formation, indicating that necrosis was not responsible for extracellular DNA. However, LDH release did increase when macrophages were incubated with a higher dose (5 U) of LKT (Fig. 1D and E). These data are similar to those presented by Pilsczek et al. (35). These investigators observed little LDH release during NET formation, which they interpret to be evidence that NET formation does not lead to neutrophil lysis. Rather, these investigators observed that NETs are found in budding vesicles that burst upon leaving the neutrophil (35). We infer from these results that low concentrations of LKT (<5 U) cause extracellular DNA release and MET formation by a process that is largely independent of necrosis.
Interestingly, extracellular traps were produced by macrophages in response to either native fully active LKT or nonacylated pro-LKT (Fig. 1C). This observation is similar to that reported for bovine neutrophils (4). These data suggest that binding of pro-LKT to CD18 is sufficient to trigger extracellular trap formation by bovine leukocytes in the absence of an overt cytotoxic effect, as detected by standard cytotoxicity assays (4). MET formation was inhibited by preincubation of LKT or pro-LKT with a neutralizing anti-LKT antibody (Fig. 1C). MET formation in response to LKT or pro-LKT was reduced when macrophages were preincubated with an anti-CD18 antibody or cytochalasin D, the latter of which we have previously shown inhibits intracellular trafficking of LKT (2) (Fig. 1C). Overall, these data confirm that the interaction between LKT or pro-LKT and the CD18 receptor and the subsequent reorganization of intracellular actin are required for MET formation in response to LKT or pro-LKT.
We used monocyte-derived macrophages (cultured in vitro for 7 days) for most of our experiments. Likewise, bovine alveolar macrophages also formed METs in a dose- and time-dependent manner in response to M. haemolytica (Fig. 7). These data are consistent with earlier reports that alveolar macrophages exhibit streaming nuclei during M. haemolytica infection of cattle (1). It is interesting that maximal MET formation by alveolar macrophages occurred somewhat later (30 min) than that observed with monocyte-derived macrophages (Fig. 3). However, it should be noted that a limited number of alveolar macrophage samples were tested (n = 3). In contrast to the findings described above, freshly adherent monocytes did not form extracellular traps when incubated with LKT (data not shown). These findings suggest that the maturation state of bovine mononuclear phagocytes influences their release of nuclear DNA in response to LKT.
Investigators have reported that NET formation occurs within 10 min to 4 h (35), whereas eosinophils produce extracellular traps within seconds (45). We found that MET formation occurred faster than NET formation, with significant accumulation of extracellular DNA occurring within 2 min after the addition of stimuli to macrophages (Fig. 2D and E) (4). This appears to be similar to findings of a recent study that demonstrated NET formation at as early as 5 min in response to S. aureus (35). The mechanisms by which extracellular traps are formed by various types of leukocytes have not been firmly established. However, the temporal differences in DNA release suggest that the intracellular signaling that regulates MET and NET formation may differ.
We used confocal and scanning electron microscopy to analyze the structure of METs. Confocal analysis confirmed that extracellular DNA fibrils were released in response to LKT or M. haemolytica cells, and these fibrils were disrupted by the addition of DNase I (Fig. 6A). SEM confirmed the presence of a large network (>10 μm2) of DNA fibrils that, upon higher magnification, clearly contained M. haemolytica cells trapped within them (Fig. 6). The origin of DNA in extracellular traps is generally thought to be nuclear, although it is reported that eosinophils release mitochondrial DNA (48). We addressed this issue by examining the colocalization of TOPRO, which stains DNA, with the signal from a fluorescently conjugated antibody that stains nuclear histones. The rationale for this approach is based on histones being associated with nuclear but not mitochondrial DNA (14). Colocalization of the signal for TOPRO (red) and the antihistone antibodies (green) suggests that the DNA in METs that bovine macrophages produced in response to M. haemolytica and its LKT is of nuclear origin (Fig. 6C). Incubation with DNase I confirmed that extracellular staining of TOPRO was specific for DNA.
NET formation by human neutrophils is dependent on NADPH oxidase activity (10, 21). Neutrophils from chronic granulomatous disease patients, whose leukocytes do not produce NADPH oxidase, do not form NETs in response to PMA or S. aureus (21). Here, we provide evidence that bovine MET formation is also dependent on NADPH oxidase activity. We tested two chemicals (PMA and glucose oxidase) reported to stimulate NET formation (21) and observed MET formation in response to each (Fig. 3). PMA stimulates production of NADPH oxidase through activation of protein kinase C, while glucose oxidase causes NET production without activation of NADPH oxidase (21). Conversely, preincubation of macrophages with DPI, an NADPH oxidase inhibitor, reduced MET formation in response to M. haemolytica cells (Fig. 3B). Interestingly, bovine macrophages required higher concentrations of PMA and GO to stimulate MET formation and higher concentrations of DPI to inhibit M. haemolytica-induced MET formation than those previously reported for human NET formation (Fig. 4) (21).
We found that METs trap and kill M. haemolytica cells in a time-dependent manner. Coincubation of M. haemolytica-stimulated macrophages with DNase I reduced the numbers of M. haemolytica cells trapped and killed (Fig. 4A and B), indicating that cleavage of extracellular DNA released bacterial cells snared within the DNA fibrils and hence reduced MET-mediated killing. The mechanism by which extracellular traps kill bacteria is not clear. Because trap formation relies on glucose oxides or NADPH oxidase activity, local release of reactive oxygen intermediates may contribute to bacterial death. We also speculate that antimicrobial proteins (histones and others) enmeshed in the DNA fibrils might contribute to bacterial killing. Because LKT is secreted by M. haemolytica cells, we hypothesized that macrophages might be exposed to LKT prior to contact with M. haemolytica cells during natural infection. We explored this possibility and found that preincubation with LKT increased the numbers of M. haemolytica cells that were trapped and killed by METs (Fig. 4C and D). We speculate that release of extracellular traps by bovine macrophages and neutrophils in response to LKT is an effort by the host to localize and impede invasion by the pathogen. However, one might envision that M. haemolytica cells which are trapped, but not killed, by METs could release LKT and LPS to stimulate local inflammation. Whether MET formation enhances the trapping and killing of bacterial cells in vivo or provides a niche in which surviving M. haemolytica cells release LKT and LPS to augment inflammation in the lung remains to be answered.
Other researchers found that only a minority (∼10 to 40%) of neutrophils form NETs when incubated with a stimulus (21, 35). Using confocal microscopy to quantify cells forming traps, we observed similar percentages of macrophages undergoing MET formation in response to LKT, M. haemolytica cells, or PMA (Fig. 6B). It should be noted that a single cluster or strand of DNA was scored as one MET, although it is possible that more than one macrophage could have contributed to that DNA staining.
Earlier histopathology studies revealed streaming leukocytes resembling extracellular traps within the alveoli of cattle with M. haemolytica pneumonia (8, 38). Extensive extracellular DNA was found in M. haemolytica-infected lung tissue (4), although the origin of the DNA was not determined. We have observed colocalization of extracellular DNA and the macrophage marker MIP-1α in histological sections from M. haemolytica-infected lung tissue (data not shown). These observations do not provide direct evidence for MET formation in vivo but are consistent with prior reports that some streaming leukocytes might be leukocytes (including macrophages) that have formed extracellular traps (12, 15).
The relevance of this study is broadened by evidence that extracellular trap formation is not restricted to bovine macrophages. Using the mouse macrophage cell line RAW 264.7 and the human monocyte cell line THP-1 (Fig. 8), we observed MET formation in response to a related RTX toxin, the HLY from a uropathogenic strain of E. coli (33). LKT did not induce MET formation in these cell lines (data not shown). Unlike LKT, whose activity is specific for ruminant leukocytes, HLY is active against leukocytes from many species (33). Cytochalasin D did not reduce MET formation by RAW 264.7 or THP-1 cells in response to HLY (Fig. 8). This result is consistent with our previous finding that HLY intoxication of bovine lymphoblastoid (BL-3) cells is unaffected by cytochalasin D and, hence, independent of host cell actin rearrangement (unpublished observations). Overall, these data suggest that MET formation in response to RTX toxins is not restricted to bovine macrophages and is representative of a broader response exhibited by mouse and human macrophages.
While preparing the manuscript, Chow et al. reported MET formation by RAW 264.7 cells and thioglycolate-elicited murine peritoneal macrophage cells in response to S. aureus cells. A portion of the S. aureus cells was killed in this process (17). Treatment of RAW 264.7 cells or murine peritoneal macrophages with statins enhanced MET-mediated S. aureus killing (17). Formation of METs by human monocytes and macrophages in response to gold nanoparticles was also recently reported (5). Whether extracellular traps are beneficial or detrimental to host defense is unclear at this time. Although the potential beneficial effect of trapping and killing extracellular bacterial cells is obvious, the presence of NETs has recently been linked to several human disease states, including systemic lupus erythematosus (27), peritonitis (42, 43), vasculitis (30), septic arthritis (31), and pre-eclampsia (25, 26). What role macrophage extracellular traps play in host defense and the pathophysiology of infectious diseases will be the subject of future investigations.
ACKNOWLEDGMENTS
We thank Andrea Kutny for assisting with some experiments. We also thank Lawrence Rueter, Chris Scott, Blaine Rathmann, and Jacob Larrison for their help with the SEM preparation. Also, we thank Keith Poulsen and Sheila McGuirk for the bronchoalveolar lavage fluid.
This work was supported by the USDA National Research Initiative (2004-14841, 2006-17522), a USDA National Institute of Food and Agriculture grant (2010-67015-30171), the Walter and Marth Renk Endowed Laboratory for Food Safety, and the University of Wisconsin—Madison Graduate School.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Ackermann MR, et al. 1994. Distribution of anti-CD68 (EBM11) immunoreactivity in formalin-fixed, paraffin-embedded bovine tissues. Vet. Pathol. 31:340–348 [DOI] [PubMed] [Google Scholar]
- 2. Atapattu DN, Albrecht RM, McClenahan DJ, Czuprynski CJ. 2008. Dynamin-2-dependent targeting of Mannheimia haemolytica leukotoxin to mitochondrial cyclophilin D in bovine lymphoblastoid cells. Infect. Immun. 76:5357–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aulik N, Hellenbrand K, Kisiela D, Czuprynski C. 2011. Mannheimia haemolytica leukotoxin binds cyclophilin D on bovine neutrophil mitochondria. Microb. Pathog. 50:168–178 [DOI] [PubMed] [Google Scholar]
- 4. Aulik N, Hellenbrand K, Klos H, Czuprynski C. 2010. Mannheimia haemolytica and its leukotoxin causes neutrophil extracellular trap (NET) formation by bovine neutrophils. Infect. Immun. 78:4454–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartneck M, Keul H, Zwadlo-Klarwasser G, Groll J. 2010. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett. 10:59–63 [DOI] [PubMed] [Google Scholar]
- 6. Beiter K, et al. 2006. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr. Biol. 16:401–407 [DOI] [PubMed] [Google Scholar]
- 7. Bianchi M, et al. 2009. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114:2619–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Breider MA, Walker RD, Hopkins FM, Schultz TW, Bowersock TL. 1988. Pulmonary lesions induced by Pasteurella haemolytica in neutrophil sufficient and neutrophil deficient calves. Can. J. Vet. Res. 52:205–209 [PMC free article] [PubMed] [Google Scholar]
- 9. Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. 2010. Neutrophil extracellular traps: how to generate and visualize them. J. Vis. Exp. 36:pii=1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brinkmann V, et al. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 11. Brinkmann V, Zychlinsky A. 2007. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5:577–582 [DOI] [PubMed] [Google Scholar]
- 12. Brogden KA, DeBey B, Audibert F, Lehmkuhl H, Chedid L. 1995. Protection of ruminants by Pasteurella haemolytica A1 capsular polysaccharide vaccines containing muramyl dipeptide analogs. Vaccine 13:1677–1684 [DOI] [PubMed] [Google Scholar]
- 13. Buchanan JT, et al. 2006. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16:396–400 [DOI] [PubMed] [Google Scholar]
- 14. Caron F, Jacq C, Rouviere-Yaniv J. 1979. Characterization of a histones-like protein extracted from yeast mitochondria. Proc. Natl. Acad. Sci. U. S. A. 76:4265–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caswell JL, Middleton DM, Sorden SD, Gordon JR. 1998. Expression of the neutrophil chemoattractant interleukin-8 in the lesions of bovine pneumonic pasteurellosis. Vet. Pathol. 35:124–131 [DOI] [PubMed] [Google Scholar]
- 16. Chang YF, Young R, Post D, Struck DK. 1987. Identification and characterization of the Pasteurella haemolytica leukotoxin. Infect. Immun. 55:2348–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow O, et al. 2010. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 8:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark SR, et al. 2007. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13:463–469 [DOI] [PubMed] [Google Scholar]
- 19. Deshpande MS, Ambagala TC, Ambagala AP, Kehrli ME, Jr, Srikumaran S. 2002. Bovine CD18 is necessary and sufficient to mediate Mannheimia (Pasteurella) haemolytica leukotoxin-induced cytolysis. Infect. Immun. 70:5058–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fedorova ND, Highlander SK. 1997. Generation of targeted nonpolar gene insertions and operon fusions in Pasteurella haemolytica and creation of a strain that produces and secretes inactive leukotoxin. Infect. Immun. 65:2593–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuchs TA, et al. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gentry MJ, Srikumaran S. 1991. Neutralizing monoclonal antibodies to Pasteurella haemolytica leukotoxin affinity-purify the toxin from crude culture supernatants. Microb. Pathog. 10:411–417 [DOI] [PubMed] [Google Scholar]
- 23. Grinberg N, Elazar S, Rosenshine I, Shpigel NY. 2008. Beta-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia coli. Infect. Immun. 76:2802–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guimaraes-Costa AB, et al. 2009. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U. S. A. 106:6748–6753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta A, Hasler P, Gebhardt S, Holzgreve W, Hahn S. 2006. Occurrence of neutrophil extracellular DNA traps (NETs) in pre-eclampsia: a link with elevated levels of cell-free DNA? Ann. N. Y. Acad. Sci. 1075:118–122 [DOI] [PubMed] [Google Scholar]
- 26. Gupta AK, Hasler P, Holzgreve W, Hahn S. 2007. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin. Immunopathol. 29:163–167 [DOI] [PubMed] [Google Scholar]
- 27. Hakkim A, et al. 2010. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. U. S. A. 107:9813–9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heyworth PG, Cross AR, Curnutte JT. 2003. Chronic granulomatous disease. Curr. Opin. Immunol. 15:578–584 [DOI] [PubMed] [Google Scholar]
- 29. Highlander SK, Engler MJ, Weinstock GM. 1990. Secretion and expression of the Pasteurella haemolytica leukotoxin. J. Bacteriol. 172:2343–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kessenbrock K, et al. 2009. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15:623–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Logters T, et al. 2009. Diagnostic accuracy of neutrophil-derived circulating free DNA (cf-DNA/NETs) for septic arthritis. J. Orthop. Res. 27:1401–1407 [DOI] [PubMed] [Google Scholar]
- 32. McClenahan D, et al. 2008. Effects of lipopolysaccharide and Mannheimia haemolytica leukotoxin on bovine lung microvascular endothelial cells and alveolar epithelial cells. Clin. Vaccine Immunol. 15:338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moayeri M, Welch RA. 1997. Prelytic and lytic conformations of erythrocyte-associated Escherichia coli hemolysin. Infect. Immun. 65:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papayannopoulos V, Zychlinsky A. 2009. NETs: a new strategy for using old weapons. Trends Immunol. 30:513–521 [DOI] [PubMed] [Google Scholar]
- 35. Pilsczek FH, et al. 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185:7413–7425 [DOI] [PubMed] [Google Scholar]
- 36. Schwende H, Fitzke E, Ambs P, Dieter P. 1996. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 59:555–561 [PubMed] [Google Scholar]
- 37. Shanthalingam S, Srikumaran S. 2009. Intact signal peptide of CD18, the beta-subunit of beta2-integrins, renders ruminants susceptible to Mannheimia haemolytica leukotoxin. Proc. Natl. Acad. Sci. U. S. A. 106:15448–15453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slocombe RF, Malark J, Ingersoll R, Derksen FJ, Robinson NE. 1985. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am. J. Vet. Res. 46:2253–2258 [PubMed] [Google Scholar]
- 39. Thumbikat P, Briggs RE, Kannan MS, Maheswaran SK. 2003. Biological effects of two genetically defined leukotoxin mutants of Mannheimia haemolytica. Microb. Pathog. 34:217–226 [DOI] [PubMed] [Google Scholar]
- 40. Thumbikat P, Dileepan T, Kannan MS, Maheswaran SK. 2005. Characterization of Mannheimia (Pasteurella) haemolytica leukotoxin interaction with bovine alveolar macrophage beta2 integrins. Vet. Res. 36:771–786 [DOI] [PubMed] [Google Scholar]
- 41. Urban CF, Reichard U, Brinkmann V, Zychlinsky A. 2006. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 8:668–676 [DOI] [PubMed] [Google Scholar]
- 42. Vitkov L, Klappacher M, Hannig M, Krautgartner WD. 2009. Extracellular neutrophil traps in periodontitis. J. Periodontal Res. 44:664–672 [DOI] [PubMed] [Google Scholar]
- 43. Vitkov L, Klappacher M, Hannig M, Krautgartner WD. 2010. Neutrophil fate in gingival crevicular fluid. Ultrastruct. Pathol. 34:25–30 [DOI] [PubMed] [Google Scholar]
- 44. von Kockritz-Blickwede M, et al. 2008. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 111:3070–3080 [DOI] [PubMed] [Google Scholar]
- 45. von Kockritz-Blickwede M, Nizet V. 2009. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 87:775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wartha F, Beiter K, Normark S, Henriques-Normark B. 2007. Neutrophil extracellular traps: casting the NET over pathogenesis. Curr. Opin. Microbiol. 10:52–56 [DOI] [PubMed] [Google Scholar]
- 47. Welch RA. 2001. RTX toxin structure and function: a story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 257:85–111 [DOI] [PubMed] [Google Scholar]
- 48. Yousefi S, et al. 2008. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14:949–953 [DOI] [PubMed] [Google Scholar]