Abstract
Recent studies suggest that extracellular DNA promotes biofilm formation in Staphylococcus aureus and, conversely, that extracellular nucleases limit the ability to form a biofilm. S. aureus produces at least two extracellular nucleases, and in the study described in this report, we examined the impact of each of these nucleases on biofilm formation under both in vitro and in vivo conditions. Our results demonstrate that both nucleases impact biofilm formation in the clinical isolate UAMS-1. Under certain in vitro conditions, this impact is negative, with mutation of either or both of the nuclease genes (nuc1 and nuc2) resulting in an enhanced capacity to form a biofilm. However, this effect was not apparent in vivo in a murine model of catheter-associated biofilm formation. Rather, mutation of either or both nuclease genes appeared to limit biofilm formation to a degree that could be correlated with increased susceptibility to daptomycin.
INTRODUCTION
Adefining characteristic of many Staphylococcus aureus infections is formation of a biofilm. Because this compromises the efficacy of antimicrobial therapy, it is important to understand the mechanistic basis for biofilm formation. One factor recently shown to be relevant in this regard is extracellular DNA (eDNA). Under in vitro conditions, the two possible sources of eDNA are the growth medium and the bacteria themselves. In fact, recent data suggest that it is the latter that play a primary role (13). Current models also suggest that the S. aureus lytSR two-component regulatory system and CidR collectively control the release of eDNA by influencing expression of the cid and lrg operons, with the latter two operons serving opposing roles with respect to each other in modulating the production of murein hydrolases and, consequently, cell lysis (18). Specifically, mutation of cidA results in reduced production of murein hydrolases, reduced release of eDNA, and a reduced capacity to form a biofilm, while mutation of the lrgAB operon has the opposite effects (13, 19). Additional results supporting this model include the fact that extracellular nuclease, whether applied exogenously or produced by S. aureus, limits biofilm formation, at least under certain in vitro conditions (3, 13, 22).
The production of extracellular nuclease has also been associated with reduced susceptibility to phagocytosis owing to an enhanced capacity to escape from neutrophil extracellular traps (NETs) (4). Thus, from a pathogenesis point of view, the production of staphylococcal extracellular nuclease potentially plays the opposing roles of promoting escape from NETs but limiting the ability to form a biofilm. These opposing roles may be largely hypothetical owing to the ability of S. aureus to regulate nuclease production such that it is produced under conditions when avoiding phagocytosis is the primary concern (e.g., in the bloodstream) but repressed during the process of colonization and biofilm formation. However, these two conditions are not mutually exclusive, as evidenced by the observation that neutrophils have been shown to penetrate S. aureus biofilms and ingest biofilm-associated staphylococcal cells (7). Thus, it is possible that these potentially opposing roles are clinically and/or therapeutically relevant. Based on this, we examined the impact of mutating each of two genes (SA0746 or nuc1 and SA1160 or nuc2) encoding extracellular nucleases on biofilm formation under in vitro and in vivo conditions with a specific emphasis on relative antibiotic susceptibility in the context of an established biofilm.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All experiments were done with the clinical osteomyelitis isolate UAMS-1 and its isogenic nuc1 (SA0746) and/or nuc2 (SA1160), nuc1 nuc2, and sarA mutants (3). The in vitro assay employed tryptic soy broth (TSB) supplemented with 3.0% NaCl and 0.5% glucose (1). The capacity of each strain to form a biofilm under in vitro conditions was assessed using a model of catheter-associated biofilm formation done with and without precoating of the substrate with 20% human plasma (23). Biofilms were allowed to form for 24 h before harvesting of catheters (n = 6) and processing to determine viable count as previously described (23). All in vitro experiments were done twice, with the results combined for statistical analysis. The relative daptomycin susceptibility of each strain was assessed by Etest (bioMérieux SA, Marcy l'Etoile, France) using Mueller-Hinton agar as the growth medium.
Nuclease assay.
Quantitative assays of nuclease activity were done using a fluorescence resonance energy transfer (FRET)-based assay (11). Briefly, supernatants from overnight cultures (16 h) grown in TSB with and without supplementation as described above were standardized, clarified by centrifugation, and filter sterilized. Aliquots of 25 μl were then mixed with an equal volume of FRET substrate diluted to 2 μM in buffer consisting of 20 mM Tris, pH 8.0, and 10 mM CaCl2. The FRET substrate was the same as previously described (11), except that the 5′ label was a 4,4,7,2′,4′,5′,7′-hexachloro-6-carboxyfluorescein (HEX) fluorophore. Results were assessed after a 10-min incubation at 30°C using a BioTek Synergy 2 apparatus (BioTek Instruments, Winooski, VT) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Results are reported as fluorescence units.
Assessment of biofilm formation and relative daptomycin susceptibility in vivo.
Biofilm formation was assessed in vivo using a murine model of catheter-associated biofilm formation (24). Briefly, uncoated catheters were implanted into each flank of NIH Swiss mice and inoculated with 105 CFU of the test strain in a total volume of 100 μl of phosphate-buffered saline (PBS) by direct injection into the lumen of each catheter. After 24 h, mice were randomly divided into experimental groups (n = 5). Because each mouse had two catheters implanted and because previous experiments have confirmed the absence of cross-contamination between catheters in opposite flanks of the same mouse (24), each catheter was treated as an independent data point (n = 10). In untreated mice, 100 μl of sterile PBS was injected in the lumen of each catheter at daily intervals. In the treated groups, 100 μl of sterile PBS containing 20× daptomycin (20 μg/ml) was injected into the lumen, also at daily intervals. This concentration corresponds to 20 times (20×) the concentration defined by the Clinical and Laboratory Standards Institute (CLSI) as the breakpoint MIC for S. aureus (≤1.0 μg/ml) (23). Treatment was continued for 7 days. All in vivo experiments were also done twice, with the results combined for statistical analysis.
Statistical analysis.
Bacterial count data from in vitro plasma-coated and uncoated experiments were analyzed separately using a three-factor analysis of variance (ANOVA) model. The three factors included in the model were the mutation statuses of sarA, nuc1, and nuc2. For both coated and uncoated analyses, three-way and all two-way interactions were tested. Bacterial count data from in vivo harvested catheters were analyzed using a two-factor ANOVA model to assess the impact of sarA and nuc1 mutations for daptomycin-treated and untreated animals separately. Because no viable bacteria were detected on many catheters, P values were calculated using permutation tests. All statistical analyses were performed using R (version 2.7; The Foundation for Statistical Computing), with P values of ≤0.05 considered significant.
RESULTS
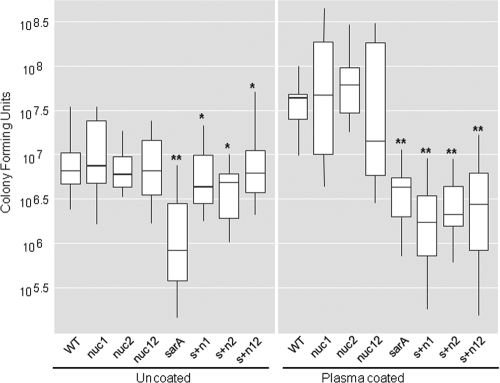
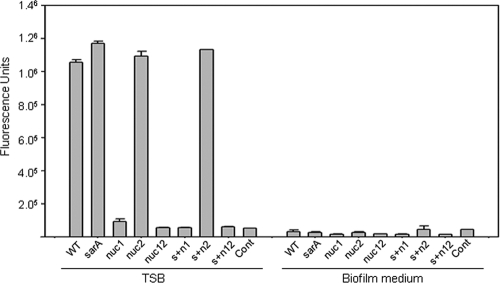
For the in vitro experiments, a catheter-associated model of biofilm formation was used with and without precoating of the substrate with 20% human plasma (23). In the clinical osteomyelitis isolate UAMS-1, mutation of nuc1 and/or nuc2 was found to have no significant impact on biofilm formation, irrespective of whether catheters were first coated with plasma proteins (Fig. 1). In contrast, mutation of sarA was found to limit biofilm formation to a statistically significant degree both with and without plasma coating. When the assay was repeated using nuc1 and nuc2 mutants generated in a UAMS-1 sarA mutant, which produces extracellular nucleases at elevated levels by comparison to the parent strain (3), mutation of either or both of the nuclease genes was found to enhance biofilm formation to a statistically significant degree, but only when the assay was done without coating with plasma proteins (Fig. 1). The fact that mutation of nuc2 had an impact on biofilm formation comparable to that observed with mutation of nuc1 was surprising, in that, as assessed using both DNase agar (3) and a FRET-based assay, mutation of nuc1 eliminated nuclease production even in a UAMS-1 sarA mutant, while mutation of nuc2 had no discernible effect (Fig. 2).
Fig 1.
In vitro biofilm formation in sarA and nuclease mutants with and without plasma coating. Biofilm formation was assessed using an in vitro model of catheter-associated biofilm formation with and without first coating the catheters with human plasma (23). Results are shown for UAMS-1 (wild type [WT]), its isogenic derivatives carrying mutations in nuc1, nuc2, or both (nuc12) with and without concomitant mutation of sarA (sarA). Strain designations: s+n1, sarA nuc1 double mutant; s+n2, sarA nuc2 double mutant; s+n12, sarA nuc1 nuc2 triple mutant. *, statistical significance (P < 0.05) by comparison to the results observed with the sarA mutant under the same assay condition; **, statistical significance (P < 0.05) by comparison to the wild-type strain.
Fig 2.
Production of extracellular nuclease. Nuclease production was assessed using a FRET-based assay with supernatants from cultures grown in TSB with (right) and without (left) supplementation of the medium with glucose and salt. Strain designations are the same as described in the Fig. 1 legend.
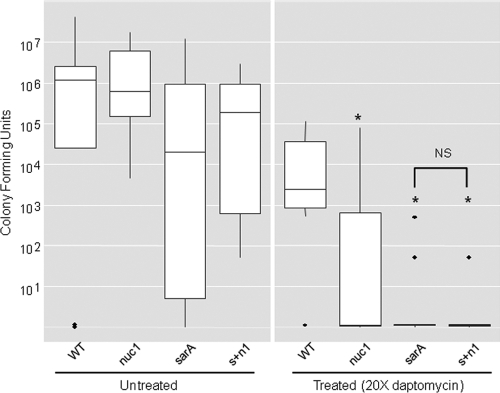
Importantly, our FRET-based assay also demonstrated that nuclease production is dramatically reduced in our biofilm medium by comparison to TSB (Fig. 2). This suggests that the impact of eliminating nuclease production is likely to be minimized in our in vitro catheter assay. This limitation is difficult to overcome in vitro because UAMS-1 does not form a robust biofilm unless the medium is supplemented with both NaCl and glucose (1). Both for this reason and, more importantly, to address the discrepancy associated with plasma coating in our in vitro assays, we compared UAMS-1 nuc1, sarA, and sarA nuc1 mutants in vivo using a murine model of catheter-associated biofilm formation (24). Because reduced antibiotic susceptibility is a defining feature of S. aureus biofilms (23, 24), we also assessed the relative antibiotic susceptibility of established biofilms using daptomycin as the test antibiotic. Importantly, as assessed in vitro, none of the mutants examined in this study exhibited altered susceptibility to daptomycin (Fig. 3). As with our in vitro experiments employing plasma-coated catheters, mutation of nuc1 had little impact on biofilm formation in UAMS-1 (Fig. 4). However, by comparison to UAMS-1, the nuc1 mutant did exhibit increased susceptibility to daptomycin. We also attempted to complement the nuc1 mutation in trans but were unable to do so because the complementing plasmid was unstable in vivo (data not shown). However, we previously confirmed complementation of the nuc1 mutation under in vitro conditions (22), thus confirming that the increased susceptibility of the nuc1 mutant observed here is unlikely to be due to polar effects unrelated to the production of extracellular nuclease. The increased susceptibility of a UAMS-1 sarA mutant was also apparent even by comparison to the isogenic nuc1 mutant (Fig. 4), which is consistent with our hypothesis that the impact of mutating sarA on biofilm formation in S. aureus extends beyond its impact on the production of extracellular nucleases (3, 22).
Fig 3.
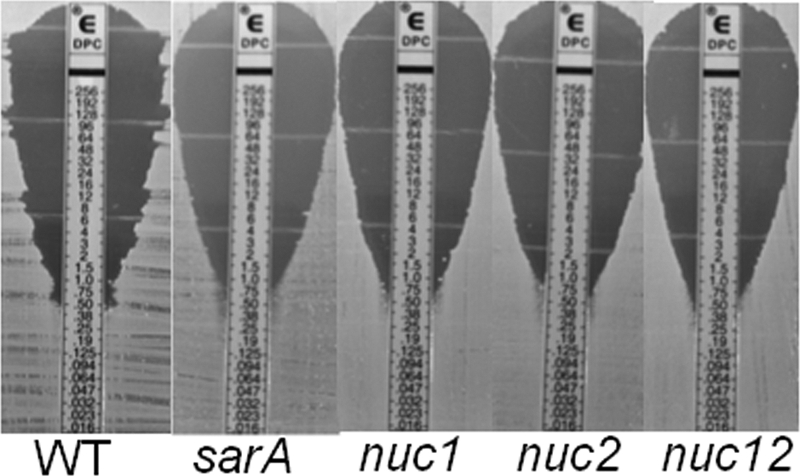
Impact of sarA and nuc mutations on daptomycin susceptibility in vitro. Relative susceptibility to daptomycin was assessed by Etest using Mueller-Hinton agar as the growth medium.
Fig 4.
Impact of sarA and nuclease production on susceptibility of UAMS-1 to daptomycin in vivo. Biofilm formation and relative susceptibility to daptomycin were assessed using an in vivo model of catheter-associated biofilm formation (24). Results are shown for UAMS-1 (wild type [WT]) and its isogenic derivatives carrying mutations in sarA and/or SA0746 (nuc1). Asterisks indicate statistical significance (P < 0.05) by comparison to the results observed with the wild-type strain under the same assay condition. The NS above the bracket indicates the lack of statistical significance between the sarA and sarA nuc1 mutants. Solid dots represent outlying observations.
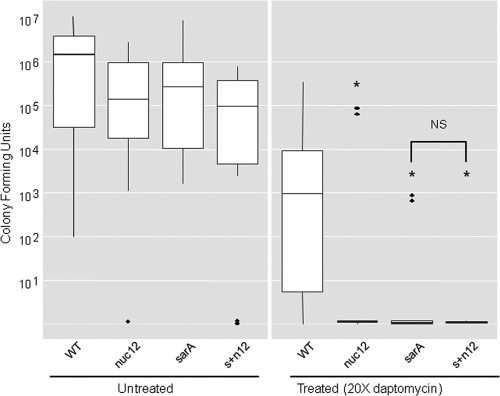
At the same time, our in vitro experiments indicated that mutation of nuc2 also has an impact on the ability of a sarA mutant to form a biofilm, at least in the absence of coating with plasma proteins (Fig. 1). Based on this, we also examined the impact of mutating both nuc1 and nuc2 (nuc12) in UAMS-1 and its sarA mutant. In the parent strain, the results were comparable with those observed with the nuc1 mutant (Fig. 4). However, the UAMS-1 nuc12 mutant exhibited increased susceptibility to daptomycin to a degree that was comparable to that of the isogenic sarA mutant and exceeded that of the nuc1 mutant (Fig. 5). These results demonstrate that the extracellular nuclease encoded by nuc2 is functional under in vivo conditions. However, the more important point is that, under in vivo conditions, mutation of either nuclease gene was associated with decreased biofilm formation, at least as defined by relative antibiotic susceptibility, while the opposite was true when biofilm formation was assessed in vitro.
Fig 5.
Impact of sarA and nuclease production on susceptibility of UAMS-1 to daptomycin in vivo. Biofilm formation and relative susceptibility to daptomycin were assessed using an in vivo model of catheter-associated biofilm formation (24). Asterisks indicate statistical significance (P < 0.05) by comparison to the results observed with the wild-type strain under the same assay condition. The NS above the bracket indicates the lack of statistical significance between the sarA and sarA nuc12 mutants. Solid dots represent outlying observations.
DISCUSSION
Biofilm formation in S. aureus is a complicated process impacted by multiple factors (5, 12, 14, 20). The cumulative data suggest that these factors include eDNA, surface-associated proteins, and the polysaccharide intercellular adhesin (PIA), with the relative impact of each being dependent on both the strain under study and the methods used to assess biofilm formation (1, 3, 10, 15, 21, 22). Much of our work has focused on the staphylococcal accessory gene regulator (sarA), mutation of which has been shown to limit biofilm formation in all of the strains that we have examined, other than those known to carry defects that directly impact either these regulatory circuits or the production of specific effector molecules like the fibronectin-binding proteins (1, 3, 22–24). Mutation of sarA results in the increased production of extracellular proteases and nucleases (3) and decreased production of PIA (2), any or all of which could contribute to the biofilm-deficient phenotype of sarA mutants. Our studies have led us to conclude that it is the increased production of extracellular proteases that plays the primary role in this regard (3, 22), and independent studies from other laboratories have provided strong support for the hypothesis that extracellular proteases are important in S. aureus biofilm formation (5, 6, 8, 13). This is consistent with the observation that specific surface-associated proteins known to be sensitive to protease-mediated degradation, including the fibronectin-binding proteins (FnbA and FnbB) and protein A (Spa), have been shown to promote biofilm formation in S. aureus (14, 15). It is also consistent with the observation that biofilm formation was enhanced in our in vitro assay when the substrate was coated with plasma proteins. However, most of these studies, including our own, were limited to in vitro experiments that may or may not reflect the more therapeutically relevant in vivo conditions.
Extracellular nuclease has also been shown to have a negative impact on biofilm formation (9, 13, 17, 19), and this suggests that exogenous nuclease could be used to limit biofilm formation and thereby reduce the therapeutic recalcitrance of biofilm-associated S. aureus infections. In contrast, nuclease production has also been shown to promote the ability of S. aureus to cause disease by promoting escape from neutrophil extracellular traps (NETs) (4, 16). This brings up the possibility that the therapeutic use of exogenous nucleases could have the adverse effect of promoting the escape of S. aureus from phagocytes. In this study, we attempted to address these potentially opposing roles by examining the impact of extracellular nucleases on biofilm formation under both in vitro and in vivo conditions. Under in vitro conditions, limiting nuclease production was shown to enhance biofilm formation, but only in a UAMS-1 sarA mutant, which produces increased amounts of extracellular nucleases by comparison to the parent strain (3) and only when the assay was done without coating the substrate with plasma proteins. This is in contrast to reports demonstrating that exonucleases, including those produced by S. aureus, limit biofilm formation even in assays done with a plasma-coated substrate (13, 19). It was also demonstrated using the same murine model employed here that a UAMS-1 cidA mutant releases reduced amounts of DNA and has a reduced capacity to form a biofilm in vivo that is comparable to that observed with the isogenic sarA mutant (19). However, while highly suggestive, this does not prove a cause-and-effect relationship between DNA release and biofilm formation.
Thus, to date, the role of S. aureus exonucleases in biofilm-associated infection remains unclear. To address this, we extended our in vitro studies to include an in vivo model of biofilm formation. Unlike previous reports, we also included consideration of relative antibiotic susceptibility in vivo, based on the realization that it is the reduced susceptibility of biofilm-associated infections that constitutes the greatest clinical concern. The results of these experiments led us to two conclusions that significantly contrast with those obtained using in vitro assays, including our own. The first and arguably most important is that limiting the production of extracellular nucleases in S. aureus does not enhance biofilm formation in vivo but rather has the opposite effect, particularly when assessed on the basis of relative susceptibility to daptomycin. Indeed, while few studies examining the role of S. aureus nucleases in biofilm formation have taken the step of considering antibiotic susceptibility, a recent report did demonstrate that, under in vitro conditions, an S. aureus nuc1 nuc2 mutant was capable of forming a biofilm to an extent that could be associated with dramatically reduced susceptibility to daptomycin (10). Thus, our results demonstrating increased susceptibility in nuc mutants provide an additional contrast regarding the role of extracellular nucleases under in vitro versus in vivo conditions. The second is that, despite the fact that mutation of nuc2 had no discernible impact on nuclease production in vitro, it did further enhance this susceptibility even in a nuc1 mutant. This is important, in that many previous studies were limited to the analysis of nuc1 on the basis of the absence of a nuclease-deficient phenotype with a nuc2 mutant under in vitro conditions (3, 13, 22).
It is interesting to note that, when both nuc1 and nuc2 were mutated, the degree of increased daptomycin susceptibility was comparable to that of a sarA mutant. This was a surprising result, in that sarA mutants produce increased amounts of extracellular nuclease (3, 22). If the increased production of extracellular nucleases were responsible for the decreased capacity of a sarA mutant to form a biofilm and its increased antibiotic susceptibility (3, 23, 24), then mutation of the nuc genes would be expected to result in decreased rather than increased susceptibility. Thus, these results are consistent with our hypothesis that the primary impact of sarA on biofilm formation is mediated through factors other than its impact on the production of extracellular nucleases. One possible explanation is that exonucleases do in fact serve a protective role in vivo that is unrelated to biofilm formation. Whether or not this is true, the results that we present clearly demonstrate that the production of extracellular nucleases by S. aureus does not limit biofilm formation under in vivo conditions. This is not to say, however, that nuclease production is not important. For instance, one of the factors contributing to the ability of S. aureus to cause disease is its ability to persist in the environment, and that may well be enhanced by both the cidA-mediated release of extracellular DNA and reduced DNA degradation. Nor is it to say that exogenous nucleases could not be used to therapeutic advantage, particularly since they could be applied in nonphysiological amounts. At the same time, these results do suggest caution in utilizing such an approach, as it could potentially have an adverse therapeutic effect.
ACKNOWLEDGMENTS
This work was supported by grant AI074935 (to M.S.S.). Support was also obtained from resources provided through the Clinical and Translational Sciences Award (RR0298884) to the University of Arkansas for Medical Sciences.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Beenken KE, Blevins Smeltzer JSS. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 71:4206–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beenken KE, et al. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beenken KE, et al. 2010. Epistatic relationships between sarA and agr in Staphylococcus aureus biofilm formation. PLoS One 5:e10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berends ET, et al. 2010. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J. Innate Immun. 2:576–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fitzpatrick F, Humphreys H, O'Gara JP. 2005. The genetics of staphylococcal biofilm formation—will a greater understanding of pathogenesis lead to better management of device-related infection? Clin. Microbiol. Infect. 11:967–973 [DOI] [PubMed] [Google Scholar]
- 6. Geoghegan JA, et al. 2010. Role of surface protein SasG in biofilm formation by Staphylococcus aureus. J. Bacteriol. 192:5663–5673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Günther F, et al. 2009. Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN). Mol. Immunol. 46:1805–1813 [DOI] [PubMed] [Google Scholar]
- 8. Houston P, Rowe SE, Pozzi C, Waters EM, O'Gara JP. 2011. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect. Immun. 79:1153–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huseby MJ, et al. 2010. Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms. Proc. Natl. Acad. Sci. U. S. A. 107:14407–14412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. John AK, Schmaler M, Khanna N, Landmann R. 2011. Reversible daptomycin tolerance of adherent staphylococci in an implant infection model. Antimicrob. Agents Chemother. 55:3510–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiedrowski MR, et al. 2011. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One 6:e26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mack D, et al. 2004. Mechanisms of biofilm formation in Staphylococcus epidermidis and Staphylococcus aureus: functional molecules, regulatory circuits, and adaptive responses. Int. J. Med. Microbiol. 294:203–212 [DOI] [PubMed] [Google Scholar]
- 13. Mann EE, et al. 2009. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Gara JP. 2007. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 270:179–188 [DOI] [PubMed] [Google Scholar]
- 15. O'Neill E, et al. 2008. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 190:3835–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pilsczek FH, et al. 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185:7413–7425 [DOI] [PubMed] [Google Scholar]
- 17. Ranjit DK, Endres JL, Bayles KW. 2011. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J. Bacteriol. 193:2468–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rice KC, Bayles KW. 2008. Molecular control of bacterial death and lysis. Microbiol. Mol. Biol. Rev. 72:85–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rice KC, et al. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 104:8113–8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Speziale P, et al. 2008. Prevention and treatment of Staphylococcus biofilms. Curr. Med. Chem. 15:3185–3195 [DOI] [PubMed] [Google Scholar]
- 21. Tang J, et al. 2011. The staphylococcal nuclease prevents biofilm formation in Staphylococcus aureus and other biofilm-forming bacteria. Sci. China Life Sci. 54:863–869 [DOI] [PubMed] [Google Scholar]
- 22. Tsang LH, Cassat JE, Shaw LN, Beenken KE, Smeltzer MS. 2008. Factors contributing to the biofilm-deficient phenotype of Staphylococcus aureus sarA mutants. PLoS One 3:e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiss EC, Spencer HJ, Daily SJ, Weiss BD, Smeltzer MS. 2009. Impact of sarA on antibiotic susceptibility of Staphylococcus aureus in a catheter-associated in vitro model of biofilm formation. Antimicrob. Agents Chemother. 53:2475–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weiss EC, et al. 2009. Impact of sarA on daptomycin susceptibility of Staphylococcus aureus biofilms in vivo. Antimicrob. Agents Chemother. 53:4096–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]