Abstract
Helicobacter pylori infection is thought to be involved in the development of several gastric diseases. Two H. pylori virulence factors (vacuolating cytotoxin A and cytotoxin-associated gene A) reportedly interact with lipid rafts in gastric epithelial cells. The role of Toll-like receptor (TLR)-mediated signaling in response to H. pylori infection has been investigated extensively in host cells. However, the receptor molecules in lipid rafts that are involved in H. pylori-induced innate sensing have not been well characterized. This study investigated whether lipid rafts play a role in H. pylori-induced ceramide secretion and TLR4 expression and thereby contribute to inflammation in gastric epithelial cells. We observed that both TLR4 and MD-2 mRNA and protein levels were significantly higher in H. pylori-infected AGS cells than in mock-infected cells. Moreover, significantly more TLR4 protein was detected in detergent-resistant membranes extracted from H. pylori-infected AGS cells than in those extracted from mock-infected cells. However, this effect was attenuated by the treatment of cells with cholesterol-usurping agents, suggesting that H. pylori-induced TLR4 signaling is dependent on cholesterol-rich microdomains. Similarly, the level of cellular ceramide was elevated and ceramide was translocated into lipid rafts after H. pylori infection, leading to interleukin-8 (IL-8) production. Using the sphingomyelinase inhibitor imipramine, we observed that H. pylori-induced TLR4 expression was ceramide dependent. These results indicate the mobilization of ceramide and TLR4 into lipid rafts by H. pylori infection in response to inflammation in gastric epithelial cells.
INTRODUCTION
Helicobacter pylori, a spiral Gram-negative microaerophilic bacterium, can colonize gastric epithelial cells and is thought to infect approximately half of the human population (37, 43). H. pylori infection is associated with several clinical pathologies, including gastritis, peptic ulcer diseases, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT) lymphoma (6, 44).
Persistent infection of the gastric mucosa by H. pylori can induce nuclear factor κB (NF-κB) activation and proinflammatory cytokine secretion, including interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) secretion (8, 21). Increased IL-8 secretion is associated with inflammatory severity in patients with H. pylori-induced gastritis (3). Furthermore, translocation of the protein encoded by H. pylori cytotoxin-associated gene A (CagA) leads to activation of IL-8 transcription through the NF-κB signaling pathway (8), suggesting that CagA plays a crucial role in H. pylori-induced inflammation in gastric epithelial cells. Moreover, several H. pylori virulence factors, including vacuolating cytotoxin (VacA), urease, and lipopolysaccharide (LPS), contribute to pathogenesis (10, 17, 36).
Among these bacterial virulence factors, VacA was the first to be isolated from detergent-resistant membranes (DRMs), commonly used to identify lipid rafts (16, 45, 48). Lipid rafts are microdomains within membranes that contain large amounts of cholesterol, phospholipids, and glycosylphosphatidylinositol (GPI)-anchored proteins (9, 22). Notably, cholesterol-rich microdomains are generally utilized by other bacterial toxins for entry or oligomerization (1, 42).
A recent study indicated that sphingomyelin is a novel VacA receptor in lipid rafts (20). Similarly, translocation of CagA is associated with lipid rafts and has been shown to be important for the CagA-induced pathogenesis of cells (30, 40). These studies suggest that cholesterol-rich membrane microdomains provide an essential ligand for toxin binding and may efficiently enhance H. pylori-induced pathogenesis of host cells.
Toll-like receptors (TLRs), as pattern recognition receptors, recognize conserved microbial components (2, 24, 39). For instance, TLR2 has been found to recognize lipoproteins and peptidoglycans in bacterial cell walls (49, 54). Moreover, TLR4 recognizes LPS of Gram-negative bacteria and activates NF-κB and activator protein-1 (AP-1) (13, 50, 62). Several reports have suggested that TLR2 contributes to H. pylori LPS-induced signaling (33, 57, 60). Other studies support the notion that H. pylori-induced signal transduction is mediated through TLR4 (25, 47, 53, 61). However, previous studies have shown that immune responses to intact H. pylori may not involve TLR4 (5, 15). Therefore, it remains unclear which TLRs mediate signal transduction during H. pylori infections.
Glycosphingolipids on host cells can be utilized as receptors for H. pylori adhesion (26, 46). A previous study showed that ceramide, the lipid portion of glycosphingolipids, is required for recognition of glycosphingolipids by H. pylori (55). Ceramide, generated from sphingomyelin by sphingomyelinase, localizes abundantly in membrane rafts and is involved in the regulation of apoptosis, cellular stress responses, and cell differentiation (19). Additionally, several pathogens generate ceramide-enriched membrane platforms from small primary rafts, and these platforms can serve as entry portals or otherwise facilitate pathogen infection (18). Although a number of molecules and various membrane compartments have been shown to participate in H. pylori-induced inflammation, the potential roles of TLR4 or ceramide mobilization into cell rafts, which may modulate inflammatory responses in H. pylori-infected cells, require further investigation.
In the present study, we examined whether lipid rafts are involved in the induction of ceramide secretion and TLR4/MD-2 expression during H. pylori infection of gastric adenocarcinoma epithelial cells. In addition, we investigated whether disruption of cholesterol-rich microdomains influences the levels of ceramide and TLR4 in the membrane, as well as H. pylori-induced IL-8 production. Our results provide insight into the molecular mechanisms underlying the function of lipid rafts, which serve as a platform for the clustering of ceramide and TLR4, crucial components for IL-8 secretion during H. pylori infection.
MATERIALS AND METHODS
Reagents and antibodies.
Rabbit anti-TLR4 polyclonal antibody (H80) and anti-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-MD-2 (ab24182) was purchased from Abcam (Cambridge, MA). Mouse anti-caveolin-1 and anti-transferrin receptor (anti-TfR; anti-CD71) monoclonal antibodies were purchased from BD Pharmingen (San Jose, CA). Mouse monoclonal anti-ceramide (15B4), fluorescein isothiocyanate (FITC)-conjugated LPS, lovastatin, methyl-β-cyclodextrin (MβCD), nystatin, and imipramine were purchased from Sigma-Aldrich (St. Louis, MO). Alexa Fluor 647-conjugated cholera toxin subunit B (CTX-B), Alexa Fluor 488-conjugated goat anti-rabbit IgG, Alexa Fluor 568-conjugated goat anti-mouse IgM, 4′,6-diamidino-2-phenylindole (DAPI), and Lipofectamine 2000 were purchased from Invitrogen (Carlsbad, CA). Luciferase substrate and a β-galactosidase expression vector were purchased from Promega (Madison, WI). An IL-8 promoter construct (IL-8/wt construct; nucleotides −162 to +44) was a kind gift of Chih-Hsin Tang of the Department of Pharmacology, China Medical University (14).
Cell and bacterial cultures.
Human AGS cells (ATCC CRL 1739) were cultured in F12 medium (Invitrogen). MKN45 cells were cultured in Dulbecco's minimum essential medium (Invitrogen). TSGH9201 and SC-M1 cells were cultured in RPMI 1640 medium (Invitrogen). All culture media were supplemented with 10% complement-inactivated fetal bovine serum (HyClone, Logan, UT) and maintained at 37°C. For transient transfection, AGS cells were cultured in 12-well plates and incubated with 500 μl Opti-MEM (Invitrogen), 1 μg reporter gene, and 1 μl Lipofectamine 2000 (Invitrogen) for 6 h at 37°C. Transfected cells were then cultured in complete medium for 24 h before further analysis. H. pylori 26695 (ATCC 700392) was recovered from frozen stocks on brucella agar plates (Becton Dickinson, Franklin Lakes, NJ) containing 10% defibrinated sheep blood. H. pylori was cultured as described previously (31).
Quantitative real-time reverse transcription-PCR.
Total RNA was isolated from cells by use of TRIzol reagent (Invitrogen), and 1 μg of total RNA was reverse transcribed into cDNA by use of an oligo(dT) primer. Quantitative real-time PCR using SYBR green I master mix and a model 7900 sequence detection system was conducted according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). After preincubation at 50°C for 2 min and 95°C for 10 min, PCR was performed with 40 cycles of 95°C for 10 s and 60°C for 1 min. The threshold was set above the nontemplate control background and within the linear phase of target gene amplification in order to calculate the cycle number at which the transcript was detected (the threshold cycle [CT]). The oligonucleotide primers used corresponded to human TLR4 (forward, 5′-ACAACCTCCCCTTCTCAACC-3′; and reverse, 5′-TGAGATGTCCAATGGGGAAG-3′) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward, 5′-CCCCCAATGTATCCGTTGTG-3′; and reverse, 5′-TAGCCCAGGATGCCCTTTAGT-3′). All oligonucleotide primers were synthesized by Invitrogen.
Isolation and analysis of proteins in detergent-resistant fractions.
To isolate detergent-soluble and -resistant fractions, AGS cells were lysed with ice-cold buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA) containing 1% (vol/vol) Triton X-100 and incubated at 4°C for 30 min. Cell lysates were centrifuged at 18,000 × g at 4°C for 30 min to separate detergent-soluble and -resistant fractions as described previously (51). Each fraction was assessed by Western blotting.
Western blot analysis.
H. pylori-infected cells were washed three times with phosphate-buffered saline (PBS) and then boiled in SDS-PAGE sample buffer for 10 min. The samples were then resolved by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA). The membranes were incubated with rabbit anti-TLR4 or mouse anti-caveolin-1 or anti-TfR (anti-CD71) at room temperature for 1 h. The blots were washed and then incubated with horseradish peroxidase-conjugated secondary antibody (Millipore). The proteins of interest were detected using ECL Western blotting detection reagents (GE Healthcare, Piscataway, NJ) and visualized using X-ray film (Kodak, Rochester, NY).
Flow cytometry analysis.
Cells infected at various multiplicities of infection (MOIs) with H. pylori at different times points were harvested and fixed with ice-cold 70% ethanol for 1 h. Cells were then stained with 200 ng/ml FITC-conjugated LPS (B4), anti-ceramide, and an isotype control. The stained cells were subjected to flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and were analyzed using Cell Quest software (Becton Dickinson). All samples were examined in triplicate for at least three independent experiments.
Reporter activity assay.
AGS cells were grown to 90% confluence in 12-well plates and transfected with the IL-8/wt reporter construct by use of Lipofectamine 2000 (Invitrogen). After 16 h, cells were infected with H. pylori in the absence or presence of MβCD, lovastatin, anti-TLR4, or anti-ceramide for 6 h. To prepare total cell lysates, 100 μl of reporter lysis buffer (Promega) was added to each well, and cells were scraped from dishes. Equal volumes of luciferase substrate (Promega) were added to all samples, and luminescence was measured using a microplate luminometer (Biotek, Winooski, VT). Luciferase activity was normalized to transfection efficiency, which was determined by the β-galactosidase activity generated from a cotransfected β-galactosidase expression vector (Promega).
Transfection of siRNA.
TLR4 (On-Targetplus siRNA 7099) and control (sc-37007) small interfering RNAs (siRNAs) were purchased from Thermo Fisher Scientific (Lafayette, CO) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. AGS cells were transfected with siRNAs (50 nM) by use of Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Immunofluorescence labeling and confocal microscopic analysis.
To visualize the localization of H. pylori, TLR4, and ceramide in lipid rafts of cells, AGS cells (0.2 × 106) were seeded on coverslips in six-well plates and incubated for 16 h. Cells were infected with H. pylori at an MOI of 100 for 6 h and then washed three times with PBS and fixed with 3.7% paraformaldehyde for 1 h. The cells were then permeabilized with 0.1% Triton X-100 for 30 min and stained with Alexa Fluor 647-conjugated CTX-B. To label TLR4 and ceramide, samples were incubated for 30 min with rabbit polyclonal anti-TLR4 and mouse monoclonal anti-ceramide followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 568-conjugated goat anti-mouse IgM, respectively. The stained cells were then analyzed under a confocal laser scanning microscope (LSM 510; Carl Zeiss, Göttingen, Germany) with a 100× objective (oil immersion; numerical aperture, 1.3). The quantification of fluorescence intensity was performed by ImageJ (7).
Determination of IL-8 secretion.
The concentration of IL-8 was determined by enzyme-linked immunosorbent assay (ELISA). AGS cells were pretreated with MβCD, lovastatin, anti-TLR4, or anti-ceramide and then infected with H. pylori at an MOI of 100 for 24 h. The IL-8 concentration in AGS cell culture supernatants was determined using a sandwich ELISA kit (R&D Systems) according to the manufacturer's instructions (30).
Statistical analysis.
Student's t test was used to calculate the statistical significance of experimental differences between two groups. Multiple testing results were corrected using the Bonferroni correction. The colocalization of TLR4 and GM1 was analyzed by ImageJ (7) and quantified using Pearson's correlation coefficient. The difference was considered significant when the P value was <0.05. Statistical analyses were carried out using the SPSS program (version 11.0; SPSS Inc., Chicago, IL).
RESULTS
H. pylori infection induces TLR4 expression in AGS cells.
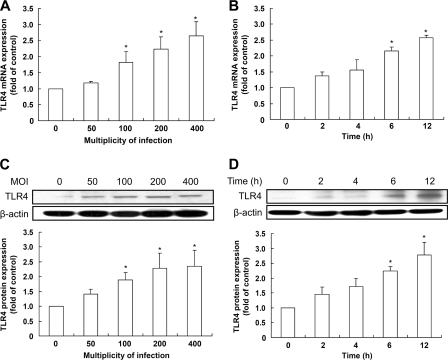
To examine whether H. pylori infection induced TLR4 expression in our experimental system, human AGS cells were cocultured with H. pylori. Induction of TLR4 mRNA expression was dependent on the MOI and duration of H. pylori infection, as determined by quantitative real-time reverse transcription-PCR (Fig. 1A and B). Furthermore, as expected, H. pylori-induced TLR4 protein expression increased following infection, in an MOI- and time-dependent manner (Fig. 1C and D). After adjusting the results using the Bonferroni correction, no significant differences in TLR4 expression were observed at low MOIs (0 to 50) or for short durations (0 to 4 h) of H. pylori infection. These results suggest that H. pylori infection of gastric epithelial cells induces TLR4 expression and that this induction requires a sufficient bacterial load and duration of infection.
Fig 1.
H. pylori induces TLR4 expression in gastric epithelial cells. AGS cells were infected with H. pylori for 6 h at different MOIs (A and C) or at an MOI of 100 for the indicated times (B and D). Total RNA and cell lysates were prepared to measure TLR4 mRNA and protein expression levels by quantitative real-time PCR (A and B) and Western blot analysis (C and D), respectively. (A and B) TLR4 mRNA expression was measured by quantitative real-time PCR, and GAPDH was used as an internal control. (C and D) Protein expression levels were quantified by densitometric analysis and normalized to the β-actin level. The data are presented as means ± standard deviations for 3 independent experiments. Statistical significance was evaluated using Student's t test (*, P < 0.05).
Since TLR4-mediated signaling is reportedly coupled to MD-2 expression (50), we performed Western blot analysis to determine the protein expression of MD-2 during infection. As shown in Fig. 2, infection of cells with H. pylori increased the expression of MD-2 in AGS cells, in an MOI- and time-dependent manner. After Bonferroni correction was applied to these values to determine significance, no significant changes (the P value must be less than 0.0125) were observed in cells infected with H. pylori at low MOIs (0 to 50) or for short durations (0 to 4 h), similar to the trend observed in Fig. 1.
Fig 2.
Involvement of MD-2 expression in response to H. pylori infection in AGS cells. AGS cells were infected with H. pylori at various MOIs from 0 to 400 for 6 h (A) or at an MOI of 100 for the indicated times (B). Expression levels of MD-2 were determined by Western blot analysis. Protein expression levels were quantified using densitometric analysis, normalized to the β-actin level, and presented as means ± standard deviations for 3 independent experiments. Statistical significance was determined using Student's t test (*, P < 0.05).
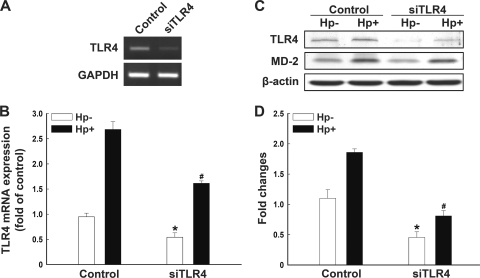
Cells were then transfected with TLR4 or control siRNA for 24 h. As indicated in Fig. 3A, the transfection of cells with TLR4 siRNA significantly reduced the level of TLR4 mRNA. Quantitative real-time PCR and Western blot analyses showed that H. pylori-induced TLR4 RNA and protein expression was suppressed by transfection with TLR4 siRNA (Fig. 3). Our data therefore confirm the involvement of TLR4/MD-2 expression in the signaling cascade induced by H. pylori infection.
Fig 3.
Infection of H. pylori induces TLR4 and MD-2 expression. AGS cells were transfected with TLR4 or control siRNA for 24 h, followed by infection (Hp+) or no infection (Hp−) with H. pylori for 6 h. TLR4 mRNA levels were assessed using semiquantitative reverse transcriptase PCR (A) and quantitative real-time PCR (B). (C) The protein levels of TLR4 and MD-2 were examined using Western blot analysis. (D) Protein expression levels of TLR4 were quantified by densitometric analysis and normalized to the β-actin level. Results are expressed as means ± standard deviations. *, P < 0.05 compared to control siRNA-treated, mock-infected group; #, P < 0.05 compared to control siRNA-treated, H. pylori-infected group.
Involvement of lipid rafts in H. pylori-induced TLR4 expression.
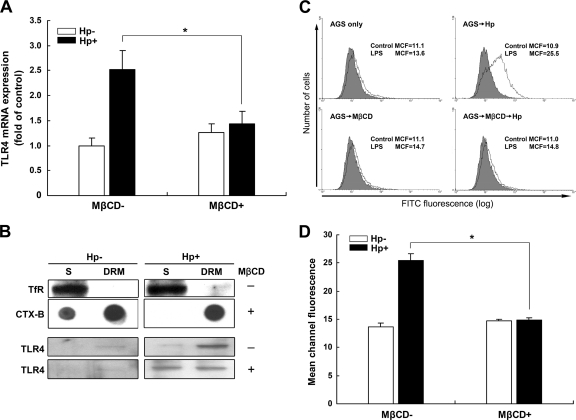
We then explored whether lipid rafts play a crucial role in H. pylori-induced TLR4 expression. AGS cells were pretreated with or without MβCD, followed by infection with H. pylori for 6 h. As shown in Fig. 4A, pretreatment of cells with MβCD inhibited H. pylori-induced TLR4 mRNA expression. We then examined whether H. pylori-enhanced TLR4 expression occurs in cholesterol-rich membrane microdomains, also called lipid rafts. Immunoblotting showed that a non-raft-associated protein, TfR, was enriched in the soluble fraction (S) of the membrane, whereas a raft-associated protein, CTX-B, which binds to the ganglioside GM1, was enriched in the DRM fraction (Fig. 4B). Upon infection with H. pylori, TLR4 was abundantly localized in the DRM fraction. However, a portion of TLR4 translocated from the DRM fraction to the detergent-soluble fraction after pretreatment of cells with MβCD (Fig. 4B).
Fig 4.
Involvement of lipid rafts in H. pylori-induced TLR4 expression in gastric epithelial cells. AGS cells were pretreated with medium alone or 5 mM MβCD at 37°C for 30 min. Cells were then washed and incubated with H. pylori at an MOI of 100 for 6 h. (A) The TLR4 mRNA level was evaluated by quantitative real-time PCR, and GAPDH was used as an internal control. (B) Detergent-resistant (DRM) and detergent-soluble (S) membrane fractions were prepared from cells that were subjected to cold detergent extraction using 1% Triton X-100 followed by centrifugation. Each fraction was subjected to Western blot or dot blot analysis using antibodies against transferrin receptor (TfR) or cholera toxin subunit B (CTX-B), conjugated to horseradish peroxidase, or an antibody specific to TLR4. (C) After infection with H. pylori for 6 h, cells were probed with control FITC-conjugated anti-mouse IgG (gray histograms) or FITC-conjugated LPS (white histograms). The FITC fluorescence level was analyzed by flow cytometry. (D) The numbers represent the mean channel fluorescence (MCF), and the quantitative results represent the means and standard deviations for 3 independent experiments. *, P < 0.05 compared to untreated MβCD control group, as determined by Student's t test.
We then analyzed whether H. pylori-induced TLR4 expression required cholesterol-rich membrane microdomains. As shown in Fig. 4C and D, H. pylori infection elicited an increase in TLR4 expression; the mean channel fluorescence (MCF) for FITC-conjugated LPS was 25.5. After treatment of cells with MβCD followed by H. pylori infection, the expression of TLR4 was significantly reduced, to the baseline level (MCF = 14.8). These data show that disrupting lipid rafts by pretreating AGS cells with MβCD led to a reduction in H. pylori-induced TLR4 expression in the DRM fraction, suggesting that lipid rafts play an important role in H. pylori-triggered TLR4 expression.
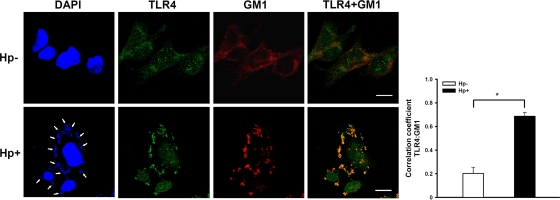
We then directly analyzed whether TLR4 colocalized in lipid rafts at sites of H. pylori infection by confocal microscopy. The analysis of the distribution of fluorescence intensity signals showed that faint TLR4 staining colocalized with the raft marker GM1 in the absence of H. pylori infection (Fig. 5, upper panels). However, remarkably greater mobilization of TLR4 and GM1 was observed at sites of H. pylori infection (Fig. 5, lower panels). The colocalization of TLR4 and GM1-raft signals showed significant correlation for H. pylori-infected cells (Pearson coefficient of 0.71; P < 0.01) (Fig. 5, right panel). Our data clearly indicate that H. pylori infection and TLR4 expression colocalized with the raft marker GM1, which stained the cell membrane. These results further support the hypothesis that reciprocal elicitation and mobilization of TLR4 into membrane rafts occur in response to H. pylori infection.
Fig 5.
Mobilization of TLR4 into cholesterol-rich microdomains at sites of H. pylori infection. AGS cells were infected with H. pylori (or not infected) at 37°C for 6 h. Cells were fixed and stained with anti-TLR4 (green), with Alexa Fluor 647-conjugated CTX-B to visualize GM1 (red), or with DAPI (blue) to visualize bacteria (arrows) and the cell nucleus. Samples were analyzed by confocal microscopy. The colocalization of TLR4 with GM1 appears yellow in the overlay. The colocalization of fluorescence for TLR4 and GM1 signals was quantified using the Pearson correlation coefficient, and the data are presented in the right panel. Statistical significance was determined for 3 independent experiments. *, P < 0.01. Bars, 10 μm. Hp−, not treated with H. pylori; Hp+, treated with H. pylori.
H. pylori-induced TLR4 expression is dependent on ceramide release.
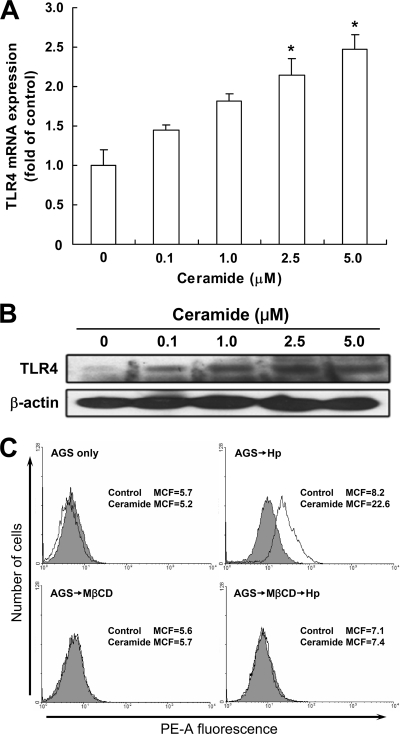
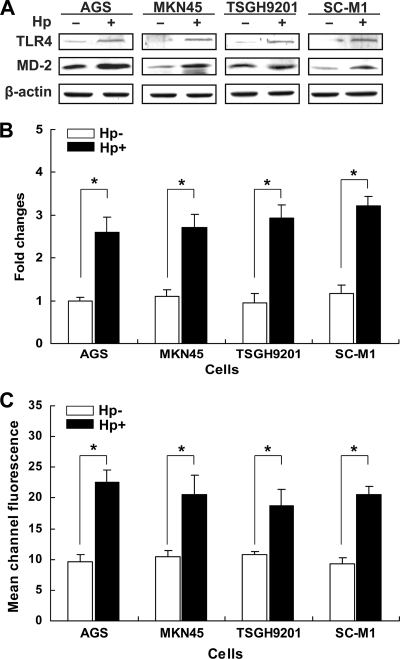
To examine whether H. pylori-elicited TLR4 signaling depends on ceramide secretion, AGS cells were treated with exogenous C2-ceramide or H. pylori. C2-ceramide increased both TLR4 mRNA and protein expression, in a dose-dependent manner (Fig. 6A and B). Furthermore, flow cytometry showed that infection of AGS cells with H. pylori increased ceramide release (Fig. 6C). We further analyzed the expression of ceramide and TLR4 following H. pylori infection in AGS and 3 other gastric epithelial cell lines (MKN45, TSGH9201, and SC-M1). The effects of H. pylori on TLR4 and MD2 protein expression were evaluated by Western blot analysis. Infection with H. pylori induced different levels of TLR4 and MD-2 expression in different gastric epithelial cells (Fig. 7A and B). In parallel, H. pylori significantly elevated ceramide expression in these cells, as determined by flow cytometric analysis (Fig. 7C). These results suggest that H. pylori infection induces TLR4/MD-2 and ceramide expression not only in AGS cells but also in other gastric epithelium-derived cell lines.
Fig 6.
H. pylori activates ceramide release in lipid rafts. AGS cells were treated with various concentrations of ceramide for 24 h. Total RNA and cell lysates were prepared to measure TLR4 mRNA and protein expression levels by quantitative real-time PCR (A) and Western blot analysis (B), respectively. The mRNA and protein expression levels were normalized to GAPDH and β-actin, respectively. (C) AGS cells were pretreated with medium alone or 5 mM MβCD for 30 min and then incubated with H. pylori at an MOI of 100 for 6 h. Cells were then stained with anti-ceramide (white histograms) or isotype control IgM (gray histograms), followed by Alexa Fluor 568-conjugated goat anti-mouse IgM, and the fluorescence intensity was assessed by flow cytometry. Results are expressed as means ± standard deviations. *, P < 0.05 compared with untreated control. MCF, mean channel fluorescence; MβCD, methyl-β-cyclodextrin.
Fig 7.
Infection of H. pylori enhances ceramide and TLR4 expression. Cells from the indicated lines were infected (or not infected) with H. pylori for 6 h at 37°C. (A) Cell lysates were prepared to analyze TLR4 and MD-2 protein expression levels by Western blotting. (B) Protein expression levels of TLR4 were quantified by densitometric analysis and normalized to the β-actin level. (C) The cells were fixed and stained with anti-ceramide and assessed by flow cytometry to determine fluorescence intensities. *, P < 0.05 compared to each uninfected H. pylori group, as determined by Student's t test. Hp−, not treated with H. pylori; Hp+, treated with H. pylori.
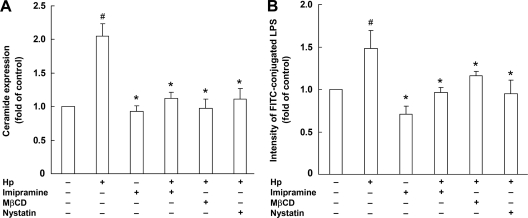
The involvement of lipid rafts in the induction of ceramide and TLR4 by H. pylori infection was further investigated. Pretreatment of cells with imipramine, an acid sphingomyelinase inhibitor, resulted in significantly attenuated H. pylori-induced ceramide release (P < 0.05) (Fig. 8A) and reduced TLR4 expression (Fig. 8B). These phenomena were evidently dependent on lipid raft integrity, as disruption of lipid rafts with either of 2 raft disruption agents, MβCD and nystatin, significantly attenuated H. pylori-stimulated ceramide release and reduced TLR4 expression (Fig. 8). These results suggest that H. pylori infection of AGS cells stimulates TLR4 expression via ceramide release, which is dependent on lipid rafts.
Fig 8.
Disruption of lipid rafts reduces H. pylori-induced ceramide and TLR4 expression. AGS cells were pretreated with imipramine (10 μM), MβCD (2.5 μM), or nystatin (10 μg/ml) for 1 h, followed by infection with H. pylori at an MOI of 100 for 6 h. The cells were fixed and stained with anti-ceramide (A) or anti-TLR4 (B), and the fluorescence intensities were then determined by flow cytometry. Results are expressed as means ± standard deviations. #, P < 0.05 compared with untreated control group; *, P < 0.05 compared with H. pylori-infected group.
Ceramide and TLR4 are mobilized into lipid rafts upon H. pylori infection.
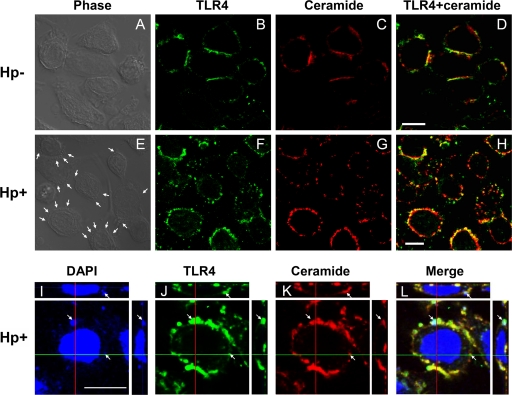
The localization of ceramide and TLR4 in H. pylori-infected AGS cells was then visualized by confocal microscopy. The colocalization of ceramide and TLR4 around the cell surface was evident but decreased in noninfected AGS cells compared to infected cells (Fig. 9A to D). In H. pylori-infected AGS cells, more apparent punctate aggregates of ceramide and TLR4 were noted at sites where H. pylori adhered to the cell surface (Fig. 9E to H, arrows). Adhered H. pylori cells (stained with DAPI) colocalized with ceramide and TLR4, as shown in merged DAPI-TLR4-ceramide images obtained by confocal microscopy z-section analysis (Fig. 9I to L). These results indicate that both ceramide and TLR4 are mobilized into lipid rafts upon H. pylori infection.
Fig 9.
H. pylori-stimulated ceramide and TLR4 cluster in lipid rafts. AGS cells were infected with H. pylori (or not infected) at 37°C for 6 h. Cells were fixed and stained with anti-TLR4 (green) (B, F, and J), anti-ceramide (red) (C, G, and K), or DAPI (blue) to visualize bacteria and the cell nucleus (I) and then analyzed by confocal microscopy or were analyzed by phase-contrast microscopy to visualize bacteria (A and E). Arrows indicate the colocalization of TLR4, ceramide, and H. pylori on infected cells. Confocal z-section images are shown for bacteria colocalized with TLR4 and ceramide (I to L). Bars, 10 μm. Hp−, not treated with H. pylori; Hp+, treated with H. pylori.
Mobilization of ceramide and TLR4 into lipid rafts by H. pylori induces IL-8 activation.
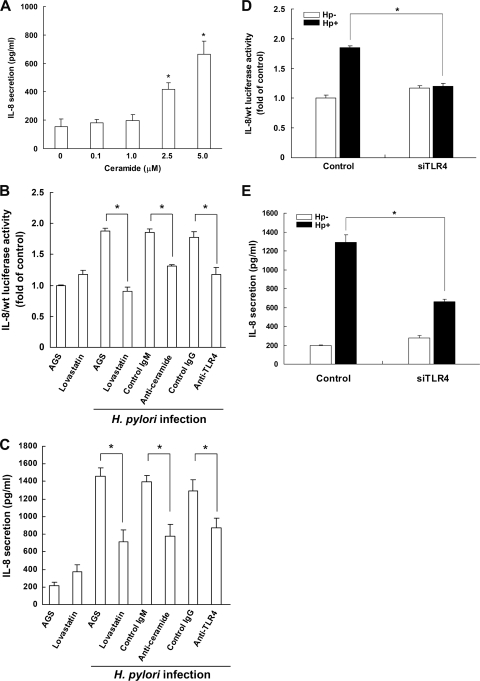
IL-8 secretion was significantly increased on exposure of AGS cells to C2-ceramide at 2.5 to 5 μM (Fig. 10A). This finding remained significant after adjustment by the Bonferroni correction for multiple testing (P < 0.0125). This is consistent with a previous report by Masamune et al. that ceramide plays a crucial role in H. pylori-induced IL-8 expression (38). We then tested whether the 3 components—TLR4, ceramide, and lipid raft integrity—were essential for IL-8 secretion in H. pylori-infected cells. A human IL-8 promoter-Luc reporter construct (IL-8/wt construct) containing both AP-1 and NF-κB sites was transfected into AGS cells. Following transfection, the cells were infected with H. pylori and then subjected to luciferase activity assays. Culture supernatants of H. pylori-infected cells were harvested for IL-8 secretion analysis. As shown in Fig. 10B and C, IL-8 promoter activity and IL-8 secretion were strongly induced in H. pylori-infected AGS cells.
Fig 10.
Activation of IL-8 by H. pylori is attenuated by inhibition of ceramide and TLR4 and disruption of rafts. (A) Ceramide stimulates IL-8 secretion in AGS cells. Cells were treated with ceramide at the indicated concentrations for 24 h. The level of IL-8 in the culture supernatant was determined by a standard ELISA method. (B to E) H. pylori-induced IL-8 activity and IL-8 secretion were influenced by pretreatment with lovastatin, anti-ceramide, anti-TLR4, and TLR4 siRNA. AGS cells were transfected with IL-8/wt Luc reporter plasmid in the presence of lovastatin (50 μM), anti-ceramide, or anti-TLR4 prior to infection of cells with H. pylori at an MOI of 100. Cell lysates were subjected to luciferase activity assays to determine IL-8 activity (B), and IL-8 levels in the cell culture supernatants were determined by ELISA (C). Cells were transfected with TLR4 or control siRNA for 24 h, followed by infection with H. pylori, and the IL-8 activity (D) and IL-8 production (E) were analyzed using a luciferase activity assay and ELISA, respectively. Data represent the means ± standard deviations for 3 independent experiments. Statistical significance was evaluated using Student's t test (*, P < 0.05).
Our results indicated that MβCD stimulated NF-κB reporter activity as well as IL-8 production. Previous studies have used lovastatin, an inhibitor of 3-hydroxy-3-methyl-glutaryl–coenzyme A (HMG-CoA) reductase, to reduce the concentration of endogenous cholesterol and thereby to disrupt rafts, which has no effect on NF-κB or IL-8 activation (30, 40). Hence, lovastatin was employed in the following experiments. To examine whether lipid raft integrity was crucial for H. pylori-induced IL-8 production, AGS cells were pretreated with lovastatin prior to infection with H. pylori. Lovastatin treatment significantly attenuated IL-8 promoter activity and IL-8 secretion in H. pylori-infected cells (Fig. 10B and C). Similarly, the attenuation occurred when cells were pretreated with either anti-ceramide or anti-TLR4 followed by H. pylori infection. Moreover, both IL-8 promoter activity (Fig. 10D) and IL-8 secretion (Fig. 10E) were significantly reduced by transfection of cells with TLR4 siRNA prior to infection with H. pylori. Thus, these results suggest that modulation of cellular cholesterol levels reduces the mobilization of ceramide and TLR4 into cholesterol-rich microdomains, thereby attenuating H. pylori-induced IL-8 activation.
DISCUSSION
TLRs are transmembrane proteins that have been found to play a critical role in the recognition of microbial components (2, 24, 39). LPS derived from Gram-negative bacteria can be recognized by TLR4 and activated MD-2 to signal cell responses (50). Several studies have shown that H. pylori and LPS alone have the capacity to upregulate TLR4 expression in gastric epithelial cells (27, 53). In addition, patients with H. pylori infection reportedly exhibit definite TLR4 expression in the gastric mucosa (4, 47). Together, these reports suggest that TLR4 may contribute to inflammatory responses induced by H. pylori. Consistent with these findings, our results showed that TLR4 and MD-2 expression in gastric epithelial cells was upregulated upon H. pylori infection (Fig. 1 and 2). Transfection of cells with TLR4 siRNA followed by infection with H. pylori reduced TLR4 expression (Fig. 3) and attenuated IL-8 activation (Fig. 10).
Controversy remains, however, over which TLRs are involved in H. pylori-induced pathogenesis. Several studies have reported that immune responses to intact H. pylori may not involve TLR4 (5, 15). In addition, LPS derived from H. pylori reportedly induces TLR2 rather than TLR4 signaling (52, 60). The reasons for these discrepancies may potentially be explained by variations in the bacterial strains or experimental systems employed in the studies. We used the laboratory strain H. pylori 26695 (56), unlike various other studies that have used clinical isolates (5, 15, 52, 60), as substantial heterogeneity exists across clinical isolates. In addition, experimental parameters such as the multiplicity of infection, the cell lines assessed, and the infection times utilized varied among reports. In this study, we used H. pylori at an MOI of 100 and infected AGS cells for 6 h. Notably, our experimental approach was very similar to that reported by Su et al., who employed AGS cells as an assay platform and suggested that H. pylori contributes to activation of TLR4 expression (53). Furthermore, we employed 3 additional gastric epithelium-derived cell lines (MKN45, TSGH9201, and SC-M1) to validate that H. pylori can upregulate TLR4 and MD-2 expression (Fig. 7), and we obtained data in accordance with findings previously reported by Ishihara et al. (25).
In this study, we demonstrated that upregulation of TLR4 by H. pylori was associated with increased secretion of ceramide (Fig. 6), which has been shown to activate NF-κB and IL-8 production upon H. pylori infection (38). Ceramide plays an important role in several signaling pathways by triggering a coalescence of receptor molecules in membrane rafts (19). Strikingly, it was found that H. pylori harbors sphingomyelinase activity, which can hydrolyze sphingomyelin and elevate cellular ceramide levels (35). Increased ceramide levels lead to the formation of ceramide-enriched membrane rafts and the activation of specific signaling pathways, including proinflammatory cytokine release (18). These studies support our finding that H. pylori infection induces mobilization of ceramide into lipid rafts, whereby it may activate TLR4 signaling and contribute to induction of IL-8 secretion.
The DRM fractionation data in our current study provide evidence for the localization of TLR4 with the raft marker CTX-B, suggesting that infection by H. pylori triggered the coalescence of TLR4 into lipid rafts. We further observed that TLR4 expression was diminished after pretreatment of cells with imipramine (Fig. 8), which acts as an acid sphingomyelinase inhibitor, indicating that ceramide stimulated TLR4 expression in gastric epithelial cells upon H. pylori infection. Our finding was consistent with previous reports that ceramide acts as a signaling intermediate between TLR4 and sphingolipid receptors, which localize abundantly in lipid rafts (12). It remains unclear, however, whether ceramide acts as a TLR4 signaling agonist upon H. pylori infection. Details of the connection between H. pylori-induced ceramide/TLR4 and lipid rafts require further research.
Our results indicate that H. pylori infection of AGS cells induces not only TLR4 mRNA and protein expression but also secretion of the proinflammatory cytokine IL-8. We found that treatment of AGS cells with anti-TLR4 or TLR4 siRNA inhibited H. pylori-induced IL-8 promoter activity and IL-8 production (Fig. 10D and E). The IL-8 promoter contains NF-κB and AP-1 binding sites (14), and thus our results implicate a possible connection between H. pylori infection and NF-κB-mediated activation of IL-8. However, IL-8 secretion was not completely impaired by the pretreatment of cells with anti-TLR4 or TLR4 siRNA. This may be explained by another pathway involved in H. pylori CagA-induced IL-8 production (8). Although this is not direct evidence for TLR4 as the major receptor for signaling pathways induced by H. pylori, close associations between H. pylori, TLR4, and IL-8 secretion suggest the possible involvement of TLR4 in H. pylori-induced responses. Constitutive expression of TLR4 contributes by allowing TLR4 to serve as a receptor for H. pylori binding (53) and also to act as a potent receptor involved in the response to H. pylori LPS-induced activation of cytoplasmic NF-κB and the subsequent activation of the IL-8 promoter (25). Together, these data support our hypothesis that TLR4 is crucial for inducing IL-8 activation in response to H. pylori infection.
Several studies have reported that bacteria utilize lipid rafts or raft-associated molecules for adhesion to or invasion of host cells (11, 29, 34, 59). With H. pylori, previous studies have shown that cholesterol-rich microdomains are required for VacA binding, delivery, and intoxication of cells (20, 28, 41, 48). Our previous studies demonstrated that translocation of CagA and the internalization of H. pylori in gastric epithelial cells are reduced significantly upon treatment of cells with MβCD, thereby decreasing the pathogenesis induced by this bacterium (30, 32). Moreover, we recently reported that the glucosylation of cholesterol in H. pylori is crucial for efficient cag type 4 secretion system (TFSS)-associated activity, which may due to the reorganization of membrane architecture (58). Here we showed that H. pylori-induced release of ceramide and expression of TLR4, as well as secretion of IL-8, were attenuated upon depletion of cholesterol or by inhibition of cholesterol synthesis. These data support the notion that the integrity of lipid rafts is essential for H. pylori-induced pathogenesis of host cells, as suggested by previous studies (23, 30, 40).
In conclusion, we have demonstrated that infection of gastric epithelial cells with H. pylori induces TLR4/MD-2 mRNA and protein expression. Furthermore, H. pylori facilitates the mobilization of ceramide and TLR4 into lipid rafts, where both contribute to NF-κB activation and IL-8 production. Together, these results shed light on the molecular mechanisms of H. pylori-induced pathogenesis of host cells.
ACKNOWLEDGMENTS
This work was supported by the National Science Council, Taiwan (NSC99-2314-B-038-034-MY2 and NSC100-2313-B-039-003), the China Medical University (CMU99-ASIA-21 and CMU99-S-09), Clinical Trial and Research Center of Excellence funds (DOH101-TD-B-111-004) from the Taiwanese Department of Health, and the Tomorrow Medicine Foundation.
We thank Shu-Chen Shen (Agricultural Biotechnology Research Center, Academia Sinica) for confocal microscopy analysis.
We have no conflicts of interest to declare for this work.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Abrami L, van Der Goot FG. 1999. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J. Cell Biol. 147:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aderem A, Ulevitch RJ. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782–787 [DOI] [PubMed] [Google Scholar]
- 3. Ando T, et al. 1996. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am. J. Gastroenterol. 91:1150–1156 [PubMed] [Google Scholar]
- 4. Asahi K, et al. 2007. Helicobacter pylori infection affects Toll-like receptor 4 expression in human gastric mucosa. Hepatogastroenterology 54:1941–1944 [PubMed] [Google Scholar]
- 5. Backhed F, et al. 2003. Gastric mucosal recognition of Helicobacter pylori is independent of Toll-like receptor 4. J. Infect. Dis. 187:829–836 [DOI] [PubMed] [Google Scholar]
- 6. Blaser MJ. 1990. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 161:626–633 [DOI] [PubMed] [Google Scholar]
- 7. Bolte S, Cordelieres FP. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224:213–232 [DOI] [PubMed] [Google Scholar]
- 8. Brandt S, Kwok T, Hartig R, Konig W, Backert S. 2005. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. U. S. A. 102:9300–9305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown DA, London E. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136 [DOI] [PubMed] [Google Scholar]
- 10. Cover TL, Blanke SR. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3:320–332 [DOI] [PubMed] [Google Scholar]
- 11. Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN. 2004. Bacterial penetration of bladder epithelium through lipid rafts. J. Biol. Chem. 279:18944–18951 [DOI] [PubMed] [Google Scholar]
- 12. Fischer H, et al. 2007. Ceramide as a TLR4 agonist; a putative signalling intermediate between sphingolipid receptors for microbial ligands and TLR4. Cell. Microbiol. 9:1239–1251 [DOI] [PubMed] [Google Scholar]
- 13. Fitzgerald KA, Rowe DC, Golenbock DT. 2004. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 6:1361–1367 [DOI] [PubMed] [Google Scholar]
- 14. Fong YC, et al. 2008. Osteoblast-derived TGF-beta1 stimulates IL-8 release through AP-1 and NF-kappaB in human cancer cells. J. Bone Miner. Res. 23:961–970 [DOI] [PubMed] [Google Scholar]
- 15. Garza-Gonzalez E, et al. 2008. mRNA levels of TLR4 and TLR5 are independent of H pylori. World J. Gastroenterol. 14:5306–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gauthier NC, et al. 2004. Glycosylphosphatidylinositol-anchored proteins and actin cytoskeleton modulate chloride transport by channels formed by the Helicobacter pylori vacuolating cytotoxin VacA in HeLa cells. J. Biol. Chem. 279:9481–9489 [DOI] [PubMed] [Google Scholar]
- 17. Gobert AP, et al. 2002. Cutting edge: urease release by Helicobacter pylori stimulates macrophage inducible nitric oxide synthase. J. Immunol. 168:6002–6006 [DOI] [PubMed] [Google Scholar]
- 18. Gulbins E, Dreschers S, Wilker B, Grassme H. 2004. Ceramide, membrane rafts and infections. J. Mol. Med. 82:357–363 [DOI] [PubMed] [Google Scholar]
- 19. Gulbins E, Kolesnick R. 2003. Raft ceramide in molecular medicine. Oncogene 22:7070–7077 [DOI] [PubMed] [Google Scholar]
- 20. Gupta VR, et al. 2008. Sphingomyelin functions as a novel receptor for Helicobacter pylori VacA. PLoS Pathog. 4:e1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PR, Smythies LE, Smith PD, Dubois A. 2000. Inflammatory cytokine mRNA expression during early and persistent Helicobacter pylori infection in nonhuman primates. J. Infect. Dis. 181:783–786 [DOI] [PubMed] [Google Scholar]
- 22. Hooper NM. 1999. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review). Mol. Membr. Biol. 16:145–156 [DOI] [PubMed] [Google Scholar]
- 23. Hutton ML, et al. 2010. Helicobacter pylori exploits cholesterol-rich microdomains for induction of NF-kappaB-dependent responses and peptidoglycan delivery in epithelial cells. Infect. Immun. 78:4523–4531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imler JL, Hoffmann JA. 2001. Toll receptors in innate immunity. Trends Cell Biol. 11:304–311 [DOI] [PubMed] [Google Scholar]
- 25. Ishihara S, et al. 2004. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J. Immunol. 173:1406–1416 [DOI] [PubMed] [Google Scholar]
- 26. Kamisago S, et al. 1996. Role of sulfatides in adhesion of Helicobacter pylori to gastric cancer cells. Infect. Immun. 64:624–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawahara T, et al. 2001. Type I Helicobacter pylori lipopolysaccharide stimulates Toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect. Immun. 69:4382–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuo CH, Wang WC. 2003. Binding and internalization of Helicobacter pylori VacA via cellular lipid rafts in epithelial cells. Biochem. Biophys. Res. Commun. 303:640–644 [DOI] [PubMed] [Google Scholar]
- 29. Lafont F, Tran Van Nhieu G, Hanada K, Sansonetti P, van der Goot FG. 2002. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 21:4449–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lai CH, et al. 2008. Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA-induced responses in AGS cells. Infect. Immun. 76:3293–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai CH, et al. 2006. Association of antibiotic resistance and higher internalization activity in resistant Helicobacter pylori isolates. J. Antimicrob. Chemother. 57:466–471 [DOI] [PubMed] [Google Scholar]
- 32. Lai CH, et al. 2011. Helicobacter pylori CagA-mediated IL-8 induction in gastric epithelial cells is cholesterol-dependent and requires the C-terminal tyrosine phosphorylation-containing domain. FEMS Microbiol. Lett. 323:155–163 [DOI] [PubMed] [Google Scholar]
- 33. Lepper PM, Triantafilou M, Schumann C, Schneider EM, Triantafilou K. 2005. Lipopolysaccharides from Helicobacter pylori can act as antagonists for Toll-like receptor 4. Cell. Microbiol. 7:519–528 [DOI] [PubMed] [Google Scholar]
- 34. Lin M, Rikihisa Y. 2003. Obligatory intracellular parasitism by Ehrlichia chaffeensis and Anaplasma phagocytophilum involves caveolae and glycosylphosphatidylinositol-anchored proteins. Cell. Microbiol. 5:809–820 [DOI] [PubMed] [Google Scholar]
- 35. Lin YL, Liu JS, Chen KT, Chen CT, Chan EC. 1998. Identification of neutral and acidic sphingomyelinases in Helicobacter pylori. FEBS Lett. 423:249–253 [DOI] [PubMed] [Google Scholar]
- 36. Mai UE, et al. 1992. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 175:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marshall B. 2002. Helicobacter pylori: 20 years on. Clin. Med. 2:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Masamune A, et al. 1999. Helicobacter pylori-dependent ceramide production may mediate increased interleukin 8 expression in human gastric cancer cell lines. Gastroenterology 116:1330–1341 [DOI] [PubMed] [Google Scholar]
- 39. Medzhitov R, Janeway CA., Jr 1998. An ancient system of host defense. Curr. Opin. Immunol. 10:12–15 [DOI] [PubMed] [Google Scholar]
- 40. Murata-Kamiya N, Kikuchi K, Hayashi T, Higashi H, Hatakeyama M. 2010. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe 7:399–411 [DOI] [PubMed] [Google Scholar]
- 41. Nakayama M, et al. 2006. Clustering of Helicobacter pylori VacA in lipid rafts, mediated by its receptor, receptor-like protein tyrosine phosphatase beta, is required for intoxication in AZ-521 cells. Infect. Immun. 74:6571–6580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Orlandi PA, Fishman PH. 1998. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parsonnet J. 1998. Helicobacter pylori. Infect. Dis. Clin. North Am. 12:185–197 [DOI] [PubMed] [Google Scholar]
- 44. Parsonnet J, et al. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127–1131 [DOI] [PubMed] [Google Scholar]
- 45. Patel HK, et al. 2002. Plasma membrane cholesterol modulates cellular vacuolation induced by the Helicobacter pylori vacuolating cytotoxin. Infect. Immun. 70:4112–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saitoh T, et al. 1991. Identification of glycolipid receptors for Helicobacter pylori by TLC-immunostaining. FEBS Lett. 282:385–387 [DOI] [PubMed] [Google Scholar]
- 47. Schmausser B, et al. 2004. Expression and subcellular distribution of Toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin. Exp. Immunol. 136:521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schraw W, Li Y, McClain MS, van der Goot FG, Cover TL. 2002. Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J. Biol. Chem. 277:34642–34650 [DOI] [PubMed] [Google Scholar]
- 49. Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406–17409 [DOI] [PubMed] [Google Scholar]
- 50. Shimazu R, et al. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simons K, Toomre D. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1:31–39 [DOI] [PubMed] [Google Scholar]
- 52. Smith MF, Jr, et al. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J. Biol. Chem. 278:32552–32560 [DOI] [PubMed] [Google Scholar]
- 53. Su B, Ceponis PJ, Lebel S, Huynh H, Sherman PM. 2003. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect. Immun. 71:3496–3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takeuchi O, et al. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443–451 [DOI] [PubMed] [Google Scholar]
- 55. Tang W, et al. 2001. Requirement of ceramide for adhesion of Helicobacter pylori to glycosphingolipids. FEBS Lett. 504:31–35 [DOI] [PubMed] [Google Scholar]
- 56. Tomb JF, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539–547 [DOI] [PubMed] [Google Scholar]
- 57. Triantafilou M, et al. 2007. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell. Microbiol. 9:2030–2039 [DOI] [PubMed] [Google Scholar]
- 58. Wang HJ, Cheng WC, Cheng HH, Lai CH, Wang WC. 2012. Helicobacter pylori cholesteryl glucosides interfere with host membrane phase and affect type IV secretion system function during infection in AGS cells. Mol. Microbiol. 83:67–84 [DOI] [PubMed] [Google Scholar]
- 59. Wang M, Hajishengallis G. 2008. Lipid raft-dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages. Cell. Microbiol. 10:2029–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yokota S, et al. 2007. Highly-purified Helicobacter pylori LPS preparations induce weak inflammatory reactions and utilize Toll-like receptor 2 complex but not Toll-like receptor 4 complex. FEMS Immunol. Med. Microbiol. 51:140–148 [DOI] [PubMed] [Google Scholar]
- 61. Yokota S, Okabayashi T, Rehli M, Fujii N, Amano K. 2010. Helicobacter pylori lipopolysaccharides upregulate Toll-like receptor 4 expression and proliferation of gastric epithelial cells via the MEK1/2-ERK1/2 mitogen-activated protein kinase pathway. Infect. Immun. 78:468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang G, Ghosh S. 2001. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 107:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]