Abstract
Upon the invasion of the host by microorganisms, innate immunity is triggered through pathogen recognition by pattern recognition receptors (PRRs). Toll-like receptors (TLRs) are the best-studied class of PRRs, and they recognize specific pathogen-associated molecular patterns (PAMPs) from various microorganisms. A large number of studies have shown that genetic variation in TLRs may influence susceptibility to infections. We assessed the genetic variation of TLR2, which encodes one of the most important TLRs, in various populations around the globe and correlated it with changes in the function of the molecule. The three best-known nonsynonymous TLR2 polymorphisms (1892C>A, 2029C>T, and 2258G>A) were assessed in different populations from the main continental masses: Romanians, Vlax-Roma, Dutch (European populations), Han Chinese (East Asia), Dogon, Fulani (Africa), and Trio Indians (America). The 2029C>T polymorphism was absent in both European and non-European populations, with the exception of the Vlax-Roma, suggesting that this polymorphism most likely arose in Indo-Aryan people after migration into South Asia. The 1892C>A polymorphism that was found exclusively in European populations, but not in Asian, African, or American volunteers, probably occurred in proto-Indo-Europeans. Interestingly, 2258G>A was present only in Europeans, including Vlax-Roma, but at a very low frequency. The differential pattern of the TLR2 polymorphisms in various populations may explain some of the differences in susceptibility to infections between these populations.
INTRODUCTION
Innate immunity is the first line of defense against pathogenic microorganisms, and its activation is initiated by the recognition of microbial structures by pattern recognition receptors (PRRs) (24). The first and most studied class of PRRs is that of the Toll-like receptors (TLRs), named after the Toll receptor of Drosophila melanogaster, which has an essential role in the host defense of the fly against fungal infections (2, 22, 29). Ten human TLRs have been identified, with important roles in host defense against bacteria, viruses, and fungi (3, 28).
First described in 1998 (43), TLR2 is one of the most promiscuous members of the family, recognizing a large array of pathogen-associated molecular patterns (PAMPs), including peptidoglycan, lipoproteins, lipopeptides, phenol-soluble modulin, lipoteichoic acid, lipoarabinomannan, atypical lipopolysaccharides (LPSs), porins, glycoinositolphospholipids, glycolipids, and zymosan (38, 54). In recognizing this large panel of PAMPs, TLR2 forms either homodimers or heterodimers with TLR1 or TLR6.
TLR2 is encoded by the gene bearing the same name, and more than 175 single-nucleotide polymorphisms (SNPs) have been reported for the gene located on chromosome 4q32 (20, 27). TLR2 polymorphisms influence the susceptibilities of patients to several infections, such as leprosy, tuberculosis, staphylococcal infections, and even sepsis (5, 25, 31, 36, 56, 61). TLR2 and TLR9 cooperate in sensing mycobacteria, and they are crucial for the activation of an effective Th1 response against these infections (21). Similarly, TLR2 has been reported to be central to the recognition of lipoteichoic acid and peptidoglycans of Gram-positive bacteria (4, 48, 49, 52, 53), explaining its influence on susceptibility to these bacteria.
Interestingly, recent data have pointed out that TLR2 polymorphisms are associated with disseminated tuberculosis (10) or exert specific effects on susceptibility to certain mycobacterial strains, such as the Beijing strains of Mycobacterium tuberculosis (27). The Beijing strains have a clear geographical distribution (27, 40), raising the possibility that human TLR2 has coevolved in various populations depending on the type of infectious pressure in a particular region, similarly to what has been reported for polymorphisms in other innate immune genes such as TLR4 or Mal/TIRAP (17, 18).
In order to generate more insight into this hypothesis, we assessed the population distribution and functional consequences of the three most common nonsynonymous TLR2 polymorphisms: (i) a C/A substitution at nucleotide 1892 (1892C>A; rs5743704), predicted to replace proline with histidine at position 631 (Pro631His); (ii) a C/T substitution at nucleotide 2029 (2029C>T; rs121917864) from the start codon of TLR2, predicted to replace arginine with tryptophan at position 677 (Arg677Trp); and (iii) a G/A substitution at nucleotide 2258 (2258G>A; rs5743708) from the start codon of TLR2, predicted to replace arginine with glutamine at position 753 (Arg753Gln). These TLR2 SNPs have been reported to influence susceptibility to various infections, but little is known regarding their distribution in different geographical locations and whether this may explain, at least partly, differences in susceptibility to infections between various populations.
MATERIALS AND METHODS
Sample characteristics.
A total of 941 individuals were recruited and were classified into seven populations according to geographic and ethnic criteria: Romanians (n = 203) (Romania, Europe), Vlax-Roma (n = 175) (Romania, Europe), Dutch (n = 262) (Netherlands, Europe), Han Chinese (n = 89) (China, Asia), Dogon (n = 110) (Mali, Africa), Fulani (n = 48) (Mali, Africa), and Trio Indians (n = 54) (Suriname, South America). Blood samples were collected from unrelated healthy volunteers, and written informed consent was obtained from all individuals. All selected participants were self-reported third-generation natives. DNA samples were extracted from whole blood (collected into EDTA tubes) by use of the Puregene isolation kit (Gentra Systems, Minneapolis, MN) and the Maxwell 16 Blood DNA purification kit (Promega, Madison, WI).
Genotyping of TLR2 polymorphisms.
All primer sequences used to assess the 2029C>T and 2258G>A SNPs were designed by using Primer3 software (44) and are presented in Table 1. The genotyping of the 1892C>A polymorphism was performed by using a TaqMan SNP assay (Table 1) with the 7300 ABI real-time PCR system (both from Applied Biosystems, Foster City, CA) in 96-well plates. The results were confirmed by subsequent sequencing analysis. Based on the fact that TLR2 has a noncoding exon 3 duplication with 93% homology that can lead to false-positive results for the TLR2 2029C>T polymorphism, we chose to verify our first P1 gene-specific primer set results with a second P2 gene-specific primer set. The pseudogene region of interest was amplified with a P4 pseudogene-specific primer set. A P3 primer set was used to identify the presence of the 2258G>A polymorphism.
Table 1.
Oligonucleotide sequences used for genotyping of TLR2a
| Target | Primer pair | Direction | Sequence |
|---|---|---|---|
| TLR2 gene | |||
| 2029C>T (677Arg→Trp) | P1 | Forward | 5′-ATTTGTTTCTTACAGTGAGCGGG-3′ |
| Reverse | 5′-GGAAATGGGAGAAGTCCAGTTC-3′ | ||
| P2 | Forward | 5′-CCTCCCTCTTACCCATGTTACTA-3′ | |
| Reverse | 5′-TGCACCACTCACTCTTCACA-3′ | ||
| 2258G>A (753Arg→Gln) | P3 | Forward | 5′-TTCAAGTTGTGTCTTCATAAGCG-3′ |
| Reverse | 5′-CAGATTTACCCAAAATCCTTCC-3′ | ||
| TLR2 pseudogene (exon 3 noncoding duplication) | P4 | Forward | 5′-TTCCCCTTTGAGAAGAAGCA-3′ |
| Reverse | 5′-GAGAGTTCCCTCACCCCTTC-3′ |
The genotyping method used for the 1892C>A (631 Pro→His) TLR2 polymorphism was a TaqMan C_25607736_10 SNP assay.
The PCR products for the P1 to P4 primer sets were analyzed on a 2% agarose gel stained with ethidium bromide prior to direct sequencing. For sequencing, we used BigDye Terminator Ready reaction mix with an ABI Prism 3100 automated sequencer (Applied Biosystems, Foster City, CA). The results were assembled with ABI Prism software, version 5.1, and analyzed by using Chromas software, version 2.33.
Role of TLR2 1892C>A and 2258G>A polymorphisms in induction of the proinflammatory cytokines interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and IL-17 in peripheral blood mononuclear cells.
Blood was drawn from the antecubital veins of 102 healthy Dutch subjects. The isolation of peripheral blood mononuclear cells (PBMCs) was performed as described elsewhere previously (15). Briefly, cells were isolated by density centrifugation of blood diluted 1:1 in pyrogen-free saline over Ficoll-Paque (Pharmacia Biotech, Pittsburgh, PA). Cells were washed twice in saline and suspended in RPMI culture medium (Invitrogen, Carlsbad, CA) supplemented with 10 μg/ml gentamicin, 10 mM l-glutamine, and 10 mM pyruvate. Cells were counted with a Coulter Counter (Coulter Electronics, Brea, CA), and the number of cells was adjusted to 5 × 106 cells/ml. A total of 5 × 105 mononuclear cells in a 100-μl volume were added to round-bottom 96-well plates (Greiner, Monroe, NC) and incubated with either 100 μl of culture medium (negative control) or various stimuli, including P3C (Pam3Cys) (10 μg/ml; EMC Microcollections, Tübingen, Germany), LPS (10 ng/ml; Sigma, St. Louis, MO), Mycobacterium tuberculosis sonicate (1 μg/ml), Staphylococcus aureus (1 × 106 microorganisms/ml), or Escherichia coli (1 × 106 microorganisms/ml). All stimuli used, except for LPS itself, were checked for contamination with LPS with a Limulus amoebocyte lysate (LAL) assay, and LPS concentrations were found to be below the detection limit.
After 24 h of incubation at 37°C, the supernatants were collected and stored at −70°C until cytokine assays were performed. Cytokine concentrations were measured by using commercial enzyme-linked immunosorbent assay (ELISA) kits. The experiments were performed in triplicate.
Statistical analysis.
Deviation from the Hardy-Weinberg equilibrium (HWE) for each locus was tested by using the function HWE.test of the open-source statistical environment R (available at www.r-project.org). Genotype frequencies between populations were compared by using Fisher's exact test with 10,000 permutations, as implemented by the Fisher test in the stats package (55). The P value was accepted as significant when the it was <0.05.
Differences in cytokine production capacities between groups were analyzed by using the Mann-Whitney U test. Differences were considered statistically significant at a P value of <0.05.
RESULTS
The TLR2 genotype distributions are shown in Table 2. None of the three analyzed SNPs deviated from Hardy-Weinberg equilibrium. The 1892A allele variant was found exclusively in European populations and not in Asian, African, or American volunteers.
Table 2.
TLR2 genotype distributions
| Population | Total no. of individuals | 1892C>A (631 Pro→His) |
2029C>T (677 Arg→Trp) |
2258G>A (753 Arg→Gln) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of individuals with genotype |
Allele frequency |
No. of individuals with genotype |
Allele frequency |
No. of individuals with genotype |
Allele frequency |
||||||||||||||
| CC | CA | AA | C | A | SD | CC | CT | TT | C | T | SD | GG | GA | AA | G | A | SD | ||
| Romanians | 203 | 190 | 12 | 0 | 0.97 | 0.03 | ±0.008 | 203 | 0 | 0 | 1 | 0 | ±0.0 | 195 | 8 | 0 | 0.98 | 0.02 | ±0.007 |
| Vlax-Roma | 175 | 163 | 12 | 0 | 0.97 | 0.03 | ±0.010 | 170 | 5 | 0 | 0.986 | 0.014 | ±0.006 | 173 | 2 | 0 | 0.994 | 0.006 | ±0.004 |
| Dutch | 262 | 247 | 15 | 0 | 0.97 | 0.03 | ±0.007 | 262 | 0 | 0 | 1 | 0 | ±0.0 | 242 | 19 | 1 | 0.96 | 0.04 | ±0.009 |
| Han Chinese | 89 | 89 | 0 | 0 | 1 | 0 | ±0.0 | 89 | 0 | 0 | 1 | 0 | ±0.0 | 89 | 0 | 0 | 1 | 0 | ±0.0 |
| Dogon | 110 | 110 | 0 | 0 | 1 | 0 | ±0.0 | 110 | 0 | 0 | 1 | 0 | ±0.0 | 110 | 0 | 0 | 1 | 0 | ±0.0 |
| Fulani | 48 | 48 | 0 | 0 | 1 | 0 | ±0.0 | 48 | 0 | 0 | 1 | 0 | ±0.0 | 48 | 0 | 0 | 1 | 0 | ±0.0 |
| Trio Indians | 54 | 54 | 0 | 0 | 1 | 0 | ±0.0 | 54 | 0 | 0 | 1 | 0 | ±0.0 | 54 | 0 | 0 | 1 | 0 | ±0.0 |
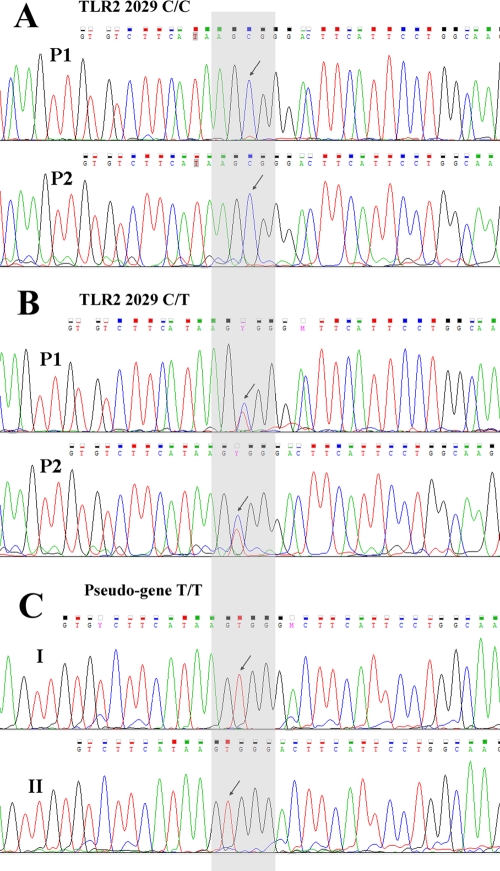
The 2029T polymorphism was absent in both European and non-European populations, with the exception of the Vlax-Roma population. Because the 2029C>T functional mutation was previously suspected to be an artifact due to the presence (approximately 23 kb upstream) of a noncoding pseudogene homologous to the third exon of TLR2 with 93% sequence identity (33), we chose to avoid false-positive results by using three primer pairs: two gene specific (P1 and P2) and one pseudogene specific (P4). All the samples were genotyped by the method of sequencing using capillary electrophoresis. The 2029T minor allele was present in five Vlax-Roma individuals and was absent in all other populations. Examples of sequencing-derived electropherograms for P1 and P2 that show the wild-type and heterozygous statuses for TLR2 position 2029 are depicted in Fig. 1A and B. When we sequenced the pseudogene locus of interest using P4, we found only the T/T homozygous variant, both in heterozygous 2029C/T and in wild-type 2029C/C samples (Fig. 1C).
Fig 1.
(A) Electropherogram pattern for no mutation at TLR2 position 2029. The wild-type state (C allele) is indicated by the black arrows at nucleotide position 2029 when analyzed by using either the P1 or P2 gene-specific primer pair. (B) Electropherogram pattern for 2099C>T heterozygous samples (Vlax-Roma). The heterozygous state is indicated by the black arrows and the Y ambiguity code (IUPAC nucleotide ambiguity codes) when analyzed by using either the P1 or P2 gene-specific primer pair. (C) Sequence analysis of the region upstream of the TLR2 gene with 93% sequence identity to the third exon. The black arrows indicate the T/T homozygous variant present in both wild-type (2029C/C) (I) and heterozygous (2029C/T) (II) samples.
The TLR2 2258A variant allele was present only in Europeans, with a very low frequency in Vlax-Roma individuals (0.006).
All the studied polymorphisms had relatively low frequencies in the populations in which they were present (Table 2).
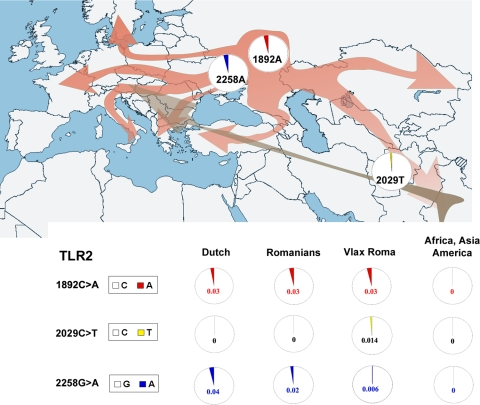
Figure 2 shows the allele frequency differences for the TLR2 SNPs between the evaluated populations as well as the possible order (and location) in which the mutations of interest may have occurred.
Fig 2.
Worldwide distribution of the analyzed nonsynonymous TLR2 polymorphisms in human populations. Circles indicate allele frequencies (major allele frequencies are depicted in white, and minor allele frequencies are shown in red for 1892A, yellow for 2029T, and blue for 2258A). The map shows the Indo-European migration routes (red arrows) as well as the subsequent Roma migration from India to Europe (brown arrow).
Effect of the 1892C>A and 2258G>A TLR2 polymorphisms on IL-6 production.
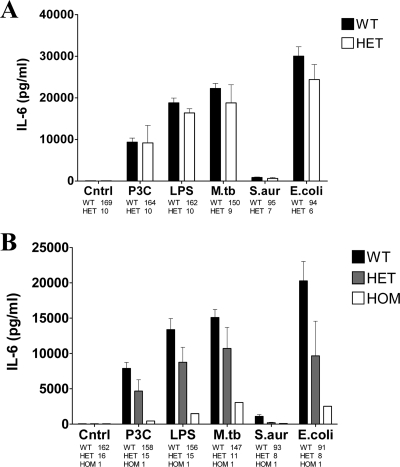
A trend toward lower IL-6 production levels for the mononuclear cells (MNCs) exposed to various stimuli was detected when blood samples were collected from individuals heterozygous for the TLR2 1892A polymorphism (Fig. 3A) as well as individuals bearing the TLR2 2258A (Fig. 3B) minor allele (Fig. 3). However, these differences were also seen for the TLR4 ligand LPS, and they did not reach statistical significance. Furthermore, no differences in the capacities of PBMCs obtained from donors with different TLR2 genotypes to produce IL-1β, TNF-α, IFN-γ, and IL-17 were observed (data not shown).
Fig 3.
Capacity for production of the cytokine IL-6 by PBMCs obtained from wild-type (WT) (1892C/C) and heterozygous (HET) (1892C/A) individuals (A) and wild-type individuals (2258G/G), heterozygous (HET) individuals (2258G/A), and individuals homozygous for the mutant-type allele (HOM) (2258A/A) after stimulation for 24 h with P3C, LPS, M. tuberculosis, S. aureus, or E. coli.
DISCUSSION
TLR2 is an important pattern recognition receptor that recognizes a relatively large number of microbial ligands and, by extension, is important for the recognition of various classes of microorganisms. TLR2 polymorphisms have been reported to influence susceptibility to various microorganisms. In the present study, we assessed the distribution of the three main TLR2 SNPs in various populations, in order to investigate whether these polymorphisms may be partly responsible for differences in disease susceptibility between these populations.
By assessing TLR2 polymorphisms in populations from all major continental masses, we found that the 2029C>T polymorphism is absent among the European populations studied. These data are supported by previous reports of populations from Germany (39, 47, 59), Turkey (6, 14), Iran (1), and the Netherlands (58). A clear difference, however, was present in the Vlax-Roma, gypsies living in Bosnia-Herzegovina, Romania, Albania, and Hungary (30), in which a small number of individuals were heterozygous for this polymorphism. The Vlax-Roma account for a substantial proportion of the overall Romani population (11). The Roma population is an Eastern Indo-European population that, after splitting from the Western Indo-Europeans, established themselves in North India (26, 41) before migrating into Europe relatively late (900 to 1,100 years ago) (19, 42). Our findings suggest that this polymorphism most likely arose in Vlax-Roma people after separation from the Western Indo-Europeans and possibly even after they left India. This last assumption is supported by the absence of this polymorphism from other populations in India (7). Due to the low prevalence (1.4%) of this mutation/polymorphism, functional studies with primary cells of individuals with the variant allele have not been feasible. However, taking into account that individuals of Roma ethnicity are greatly affected by tuberculosis (9), future studies to evaluate whether the TLR2 2029T minor allele may be a genetic susceptibility factor in this population are warranted.
One major aspect to be considered when data from the literature are reviewed for the presence of the SNP at position 2029 is the presence of the noncoding exon 3 duplication of TLR2 at approximately 23 kb upstream of the TLR2 gene. Several studies reporting its presence did not exclude this possibility, such as the association of the 2029C>T polymorphism with tuberculosis in Tunisian patients (5) and with leprosy in Korean patients (25), and these data could therefore be the result of technical artifacts. This may also explain the discrepancy between those two studies and the absence of a correlation between this polymorphism and leprosy in patients from India (33) and Japan (34). We failed to find this polymorphism in populations from China or Trio Indians (who arrived in America via North-East Asia), findings supported by previous studies in China (12, 60), South Korea (45, 62), and India (7). Similarly, we also did not find this polymorphism in the Dogon and Fulani populations from Mali, Africa, and others have failed to find it in populations from Ethiopia (8).
The TLR2 1892C>A polymorphism was found exclusively in Indo-European populations, both from Europe and Vlax-Roma. Similar data were obtained by studies from Belgium (35) and Croatia (16). The presence of this polymorphism in all Indo-European populations studied, but not in other populations from around the globe, suggests an older origin in proto-Indo-Europeans. The variant polymorphic allele was associated with a protective role against meningococcal meningitis (16, 50), while it was associated with increasing susceptibility to tuberculosis (16), and more studies are warranted before a definitive assessment of its importance in susceptibility to infections can be made.
Probably the most investigated TLR2 polymorphism is the 2258G>A polymorphism, which in studies performed with European populations has been reported to be associated with several important infections, such as Candida infections (20), severe bacterial infections (31), late-stage Lyme disease (46), acute rheumatic fever (6), urinary tract infections (51), tuberculosis (36), and autoinflammatory diseases such as Behcet disease (13) and familial Mediterranean fever (37). The TLR2 2258G>A SNP was also reported to be involved in the development of arthritis and extraarticular features following infection with Salmonella enterica serovar Enteritidis (57). The same polymorphism was also found in a New Zeeland cohort of individuals of European ancestry (23). In our study, the 2258A allele was present only in Europeans and not in other populations. These data are supported by data from previous studies showing that this SNP is not present in Asian populations, such as those from Taiwan (12), South Korea (45, 62), and India (7); in a Chinese Han population (32); in one African cohort from Ethiopia (8); and in one American cohort from Colombia (63). In a southeastern Chinese population, the TLR2 2258A allele was found at a very low frequency and was not associated with susceptibility to tuberculosis (60).
An interesting aspect is the consequences of mutations on TLR2 function. The presence of the 1892A and 2258A minor alleles tended to result in a reduced capacity for IL-6 production in response to TLR ligands as well as whole microorganisms. This change in TLR2 function is in line with the observed effect of the polymorphism on susceptibility to infections (see above). However, these differences in healthy individuals did not reach statistical significance, and larger studies (including patient cohorts) are needed in order to be able to draw definitive conclusions. The lack of enough individuals bearing the variant allele for the polymorphism at position 2029 has precluded us from assessing its function.
In conclusion, the studies presented here demonstrate a specific pattern of TLR2 polymorphisms in various human populations around the globe, in which Indo-Europeans seem to harbor a more polymorphic TLR2 gene, at least with regard to three of the major known SNPs, than other populations. This argues for a relatively late appearance of these mutations during human evolutionary history, after the split of the populations in the Middle East subsequent to the migration out of Africa. For some of these SNPs, an association with susceptibility to various infections has been suggested, adding to the arguments that genetic variation in TLRs may contribute to the differential infection susceptibilities of different populations.
ACKNOWLEDGMENTS
M.I. was supported by the Sectoral Operational Programme Human Resources Development (SOP HRD), financed by the European Social Fund and by the Romanian Government under contract number POSDRU/89/1.5/S/64109. B.F. was supported by a Rubicon grant of the Netherlands Organization for Scientific Research (NWO). M.M. was supported by a European Union FP6 (BioMalPar) fellowship. M.G.N. was supported by a Vici grant of the NWO. R.C. was supported by a Vidi grant of the NWO.
We declare no conflict of interest.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Ajdary S, Ghamilouie MM, Alimohammadian MH, Hosseini M, Pakzad SR. 2010. Lack of association of Toll-like receptor 2 Arg753Gln with cutaneous leishmaniasis. Parasitol. Int. 59:466–468 [DOI] [PubMed] [Google Scholar]
- 2. Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. 4:499–511 [DOI] [PubMed] [Google Scholar]
- 3. Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- 4. Aliprantis AO, et al. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736–739 [DOI] [PubMed] [Google Scholar]
- 5. Ben-Ali M, Barbouche MR, Bousnina S, Chabbou A, Dellagi K. 2004. Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clin. Diagn. Lab. Immunol. 11:625–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berdeli A, Celik HA, Ozyurek R, Dogrusoz B, Aydin HH. 2005. TLR-2 gene Arg753Gln polymorphism is strongly associated with acute rheumatic fever in children. J. Mol. Med. (Berlin) 83:535–541 [DOI] [PubMed] [Google Scholar]
- 7. Biswas D, Gupta SK, Sindhwani G, Patras A. 2009. TLR2 polymorphisms, Arg753Gln and Arg677Trp, are not associated with increased burden of tuberculosis in Indian patients. BMC Res. Notes 2:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bochud PY, et al. 2008. Toll-like receptor 2 (TLR2) polymorphisms are associated with reversal reaction in leprosy. J. Infect. Dis. 197:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casals M, Pila P, Langohr K, Millet JP, Cayla JA. Incidence of infectious diseases and survival among the Roma population: a longitudinal cohort study. Eur. J. Public Health, in press [DOI] [PubMed] [Google Scholar]
- 10. Caws M, et al. 2008. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 4:e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaix R, Austerlitz F, Morar B, Kalaydjieva L, Heyer E. 2004. Vlax Roma history: what do coalescent-based methods tell us? Eur. J. Hum. Genet. 12:285–292 [DOI] [PubMed] [Google Scholar]
- 12. Cheng PL, Eng HL, Chou MH, You HL, Lin TM. 2007. Genetic polymorphisms of viral infection-associated Toll-like receptors in Chinese population. Transl. Res. 150:311–318 [DOI] [PubMed] [Google Scholar]
- 13. Cosan F, et al. 2009. No association of the TLR2 gene Arg753Gln polymorphism with rheumatic heart disease and Behcet's disease. Clin. Rheumatol. 28:1385–1388 [DOI] [PubMed] [Google Scholar]
- 14. Duzgun N, Duman T, Haydardedeoglu FE, Tutkak H. 2007. The lack of genetic association of the Toll-like receptor 2 (TLR2) Arg753Gln and Arg677Trp polymorphisms with rheumatic heart disease. Clin. Rheumatol. 26:915–919 [DOI] [PubMed] [Google Scholar]
- 15. Endres S, Ghorbani R, Lonnemann G, van der Meer JW, Dinarello CA. 1988. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin. Immunol. Immunopathol. 49:424–438 [DOI] [PubMed] [Google Scholar]
- 16. Etokebe GE, et al. 2010. Toll-like receptor 2 (P631H) mutant impairs membrane internalization and is a dominant negative allele. Scand. J. Immunol. 71:369–381 [DOI] [PubMed] [Google Scholar]
- 17. Ferwerda B, et al. 2009. Functional and genetic evidence that the Mal/TIRAP allele variant 180L has been selected by providing protection against septic shock. Proc. Natl. Acad. Sci. U. S. A. 106:10272–10277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferwerda B, et al. 2007. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc. Natl. Acad. Sci. U. S. A. 104:16645–16650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fraser A. 1992. The gypsies. Blackwell Publishers, Oxford, United Kingdom [Google Scholar]
- 20. Georgel P, Macquin C, Bahram S. 2009. The heterogeneous allelic repertoire of human Toll-like receptor (TLR) genes. PLoS One 4:e7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerold G, Zychlinsky A, de Diego JL. 2007. What is the role of Toll-like receptors in bacterial infections? Semin. Immunol. 19:41–47 [DOI] [PubMed] [Google Scholar]
- 22. Hashimoto C, Hudson KL, Anderson KV. 1988. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52:269–279 [DOI] [PubMed] [Google Scholar]
- 23. Hong J, et al. 2007. TLR2, TLR4 and TLR9 polymorphisms and Crohn's disease in a New Zealand Caucasian cohort. J. Gastroenterol. Hepatol. 22:1760–1766 [DOI] [PubMed] [Google Scholar]
- 24. Janeway CA, Jr, Medzhitov R. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216 [DOI] [PubMed] [Google Scholar]
- 25. Kang TJ, Chae GT. 2001. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol. Med. Microbiol. 31:53–58 [DOI] [PubMed] [Google Scholar]
- 26. Kivisild T, et al. 1999. Deep common ancestry of Indian and western-Eurasian mitochondrial DNA lineages. Curr. Biol. 9:1331–1334 [DOI] [PubMed] [Google Scholar]
- 27. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. 2011. Innate immune recognition of Mycobacterium tuberculosis. Clin. Dev. Immunol. 2011:405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar H, Kawai T, Akira S. 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388:621–625 [DOI] [PubMed] [Google Scholar]
- 29. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973–983 [DOI] [PubMed] [Google Scholar]
- 30. Lewis MP. 2009. Ethnologue: languages of the world, 16th ed SIL International, Dallas, TX [Google Scholar]
- 31. Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. 2000. A novel polymorphism in the Toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect. Immun. 68:6398–6401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma MJ, et al. 2010. Toll-like receptors, tumor necrosis factor-alpha, and interleukin-10 gene polymorphisms in risk of pulmonary tuberculosis and disease severity. Hum. Immunol. 71:1005–1010 [DOI] [PubMed] [Google Scholar]
- 33. Malhotra D, Relhan V, Reddy BS, Bamezai R. 2005. TLR2 Arg677Trp polymorphism in leprosy: revisited. Hum. Genet. 116:413–415 [DOI] [PubMed] [Google Scholar]
- 34. Mikita N, et al. 2009. No involvement of non-synonymous TLR2 polymorphisms in Japanese leprosy patients. J. Dermatol. Sci. 54:48–49 [DOI] [PubMed] [Google Scholar]
- 35. Moens L, et al. 2007. Toll-like receptor 2 and Toll-like receptor 4 polymorphisms in invasive pneumococcal disease. Microbes Infect. 9:15–20 [DOI] [PubMed] [Google Scholar]
- 36. Ogus AC, et al. 2004. The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur. Respir. J. 23:219–223 [DOI] [PubMed] [Google Scholar]
- 37. Ozen S, et al. 2006. Arg753Gln TLR-2 polymorphism in familial Mediterranean fever: linking the environment to the phenotype in a monogenic inflammatory disease. J. Rheumatol. 33:2498–2500 [PubMed] [Google Scholar]
- 38. Ozinsky A, et al. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. U. S. A. 97:13766–13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pabst S, et al. 2009. Toll-like receptor 2 gene polymorphisms Arg677Trp and Arg753Gln in chronic obstructive pulmonary disease. Lung 187:173–178 [DOI] [PubMed] [Google Scholar]
- 40. Parwati I, van Crevel R, van Soolingen D. 2010. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect. Dis. 10:103–111 [DOI] [PubMed] [Google Scholar]
- 41. Quintana-Murci L, et al. 1999. Genetic evidence of an early exit of Homo sapiens sapiens from Africa through eastern Africa. Nat. Genet. 23:437–441 [DOI] [PubMed] [Google Scholar]
- 42. Rochow I, Matschke K. 1998. Neues zu den zigeunern im Byzantinischen reich um die wende von 13. Zum 14. Jahrhundert. Jahrbuch der O sterreichischen Byzantinistik, p 241-254 VÖAW, Vienna, Austria [Google Scholar]
- 43. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. U. S. A. 95:588–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rozen S, Skaletsky H. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365–386 [DOI] [PubMed] [Google Scholar]
- 45. Ryu YJ, et al. 2006. Toll-like receptor 2 polymorphisms and nontuberculous mycobacterial lung diseases. Clin. Vaccine Immunol. 13:818–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schroder NW, et al. 2005. Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J. Immunol. 175:2534–2540 [DOI] [PubMed] [Google Scholar]
- 47. Schroder NW, et al. 2003. High frequency of polymorphism Arg753Gln of the Toll-like receptor-2 gene detected by a novel allele-specific PCR. J. Mol. Med. (Berlin) 81:368–372 [DOI] [PubMed] [Google Scholar]
- 48. Schroder NW, et al. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587–15594 [DOI] [PubMed] [Google Scholar]
- 49. Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406–17409 [DOI] [PubMed] [Google Scholar]
- 50. Smirnova I, et al. 2003. Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc. Natl. Acad. Sci. U. S. A. 100:6075–6080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tabel Y, Berdeli A, Mir S. 2007. Association of TLR2 gene Arg753Gln polymorphism with urinary tract infection in children. Int. J. Immunogenet. 34:399–405 [DOI] [PubMed] [Google Scholar]
- 52. Takeda K, Takeuchi O, Akira S. 2002. Recognition of lipopeptides by Toll-like receptors. J. Endotoxin Res. 8:459–463 [DOI] [PubMed] [Google Scholar]
- 53. Takeuchi O, et al. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443–451 [DOI] [PubMed] [Google Scholar]
- 54. Takeuchi O, et al. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933–940 [DOI] [PubMed] [Google Scholar]
- 55. Team RDC 2010. R: a language and environment for statistical computing. Team RDC, Vienna, Austria [Google Scholar]
- 56. Texereau J, et al. 2005. The importance of Toll-like receptor 2 polymorphisms in severe infections. Clin. Infect. Dis. 41(Suppl. 7):S408–S415 [DOI] [PubMed] [Google Scholar]
- 57. Tsui FW, et al. 2008. Toll-like receptor 2 variants are associated with acute reactive arthritis. Arthritis Rheum. 58:3436–3438 [DOI] [PubMed] [Google Scholar]
- 58. van der Graaf CA, et al. 2003. Candida-specific interferon-gamma deficiency and Toll-like receptor polymorphisms in patients with chronic mucocutaneous candidiasis. Neth. J. Med. 61:365–369 [PubMed] [Google Scholar]
- 59. Woehrle T, et al. 2008. Pathogen specific cytokine release reveals an effect of TLR2 Arg753Gln during Candida sepsis in humans. Cytokine 41:322–329 [DOI] [PubMed] [Google Scholar]
- 60. Xue Y, et al. 2010. Toll-like receptors 2 and 4 gene polymorphisms in a southeastern Chinese population with tuberculosis. Int. J. Immunogenet. 37:135–138 [DOI] [PubMed] [Google Scholar]
- 61. Yim JJ, et al. 2006. The association between microsatellite polymorphisms in intron II of the human Toll-like receptor 2 gene and tuberculosis among Koreans. Genes Immun. 7:150–155 [DOI] [PubMed] [Google Scholar]
- 62. Yoon HJ, et al. 2006. Lack of Toll-like receptor 4 and 2 polymorphisms in Korean patients with bacteremia. J. Korean Med. Sci. 21:979–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zafra G, et al. 2008. Polymorphisms of Toll-like receptor 2 and 4 genes in Chagas disease. Mem. Inst. Oswaldo Cruz 103:27–30 [DOI] [PubMed] [Google Scholar]