Abstract
Campylobacter jejuni infection often results in bloody, inflammatory diarrhea, indicating bacterial disruption and invasion of the intestinal epithelium. While C. jejuni infection can be reproduced in vitro using intestinal epithelial cell (IEC) lines, low numbers of bacteria invading IECs do not reflect these clinical symptoms. Performing in vitro assays under atmospheric oxygen conditions neither is optimal for microaerophilic C. jejuni nor reflects the low-oxygen environment of the intestinal lumen. A vertical diffusion chamber (VDC) model system creates microaerobic conditions at the apical surface and aerobic conditions at the basolateral surface of cultured IECs, producing an in vitro system that closely mimics in vivo conditions in the human intestine. Ninefold increases in interacting and 80-fold increases in intracellular C. jejuni 11168H wild-type strain bacteria were observed after 24-h coculture with Caco-2 IECs in VDCs under microaerobic conditions at the apical surface, compared to results under aerobic conditions. Increased bacterial interaction was matched by an enhanced and directional host innate immune response, particularly an increased basolateral secretion of the proinflammatory chemokine interleukin-8 (IL-8). Analysis of the invasive ability of a nonmotile C. jejuni 11168H rpoN mutant in the VDC model system indicates that motility is an important factor in the early stages of bacterial invasion. The first report of the use of a VDC model system for studying the interactions of an invasive bacterial pathogen with IECs demonstrates the importance of performing such experiments under conditions that represent the in vivo situation and will allow novel insights into C. jejuni pathogenic mechanisms.
INTRODUCTION
The Gram-negative, microaerophilic organism Campylobacter jejuni is one of the most common causes of food-borne bacterial gastroenteritis in developed countries (6), with 500,000 and 2,500,000 predicted cases each year in the United Kingdom and the United States, respectively (27). The predominant route of transmission is consumption and handling of undercooked, contaminated poultry (20). Once ingested, C. jejuni can lead to symptoms ranging from mild, watery diarrhea to severe, bloody inflammatory diarrhea (1). The majority of C. jejuni infections are self-limiting; however, infection with C. jejuni can potentially lead to postinfectious sequelae, such as Guillain-Barré syndrome, reactive arthritis, and inflammatory bowel disease (8, 22, 38).
Despite the prevalence of C. jejuni as a causative agent of gastroenteritis, knowledge of the molecular basis of pathogenesis and interactions with host cells is still very limited compared to that for other enteropathogens, such as Salmonella species, Yersinia species, Shigella species, and pathogenic Escherichia coli (47). This knowledge gap can in part be attributed to the lack of a convenient, reproducible small-animal model system to study C. jejuni-host interactions (15). Although several animal models have been used, each one has major drawbacks. Animal models using either ferrets (4) or rhesus monkeys (40, 41) have been shown to closely mimic the disease observed in humans. However, the facilities required for the handling of such animals, the unavailability of host genetic manipulation techniques, and the relatively long generation time render these animals impractical for regular use in most laboratories. Chickens, as a natural host of C. jejuni, can easily be experimentally inoculated (13). However, although both chicks and chickens have been used successfully in various studies (18, 24), such studies reflect C. jejuni colonization, and the direct relevance of the chick and chicken models to human campylobacteriosis is debatable. The model organism most frequently used to study human pathogens is the mouse. Indeed, experimental inoculation of mice with C. jejuni has been performed for nearly 30 years (7). However, differences in the mouse strains used, the pretreatments of the mice, the routes of inoculation, and the inoculation loads have resulted in findings as diverse as noncolonization, nonsymptomatic carriage, or severe diarrhea (3, 8, 43, 48). Knockout mouse models of C. jejuni enteritis using mice deficient in NF-κB (19), MyD88 (45), interleukin-10 (IL-10) (33), and Nramp1 (9) have been reported. Additionally, infections of mice with limited enteric flora have been reported (10). The outcomes of C. jejuni infection in these models differ between genetically engineered mice, suggesting that a robust, reproducible, “gold standard” mouse model for C. jejuni infection remains elusive, and as such the C. jejuni research community has yet to adopt a defined mouse model for pathogenesis studies.
In the absence of a convenient, reproducible small-animal model, tissue culture assays represent a useful alternative. C. jejuni has been shown to adhere to and invade various polarized and nonpolarized intestinal epithelial cells (IECs) in vitro, including the Caco-2 (16), INT 407 (31), and T84 (35) cell lines. However, the reported adhesion and invasion interactions of C. jejuni with IECs are minimal compared to those of other enteric pathogens, with often less than 1% of the starting inoculum recovered intracellularly following gentamicin protection assays (21). This low level of adhesion and invasion does not correlate with the clinical presentation of C. jejuni infection in humans (21). One explanation for these low adhesion and invasion levels is that coculturing of C. jejuni with IECs is routinely performed under atmospheric oxygen conditions, as this is required for survival of the IECs. Even though the microaerophilic C. jejuni possesses several defense mechanisms against oxidative stress, such as the SodB superoxide dismutase (39) and the KatA catalase (14), it is likely that the bacterium behaves differently under atmospheric oxygen conditions than in the natural low-oxygen environment of the intestinal lumen.
When the microaerophilic human pathogen Helicobacter pylori was cocultured with epithelial cells with microaerobic conditions at the apical surface and aerobic conditions at the basolateral surface using a vertical diffusion chamber (VDC), or Ussing chamber, the result was a significant increase in bacterial adherence under microaerobic conditions compared to the level under aerobic conditions (12). An increase in the expression of the H. pylori virulence factor CagA and changes in the host response were also observed (12). However, the VDC system has not previously been used to study an invasive enteric bacterial pathogen. The use of a similar VDC model system for C. jejuni infection of IECs will mimic the in vivo situation more closely than other tissue culture assays and as such should allow more-accurate investigations of host-pathogen interactions. In this study, a modified VDC system was used to allow the coculture of C. jejuni with IECs under microaerobic conditions in the apical compartment, resulting in an 80-fold increase in levels of bacterial invasion and an enhanced host innate immune response.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. jejuni wild-type strains used in this study were 11168H and 81-176. 11168H is a genetically stable hypermotile derivative (28, 30) of the original sequenced strain NCTC11168 (37). 11168H shows much higher colonization levels than the NCTC11168 strain in a chick colonization model (28) and is thus considered a better strain to use for host-pathogen interaction studies. 81-176 is a gastroenteritis isolate from a multistate outbreak from contaminated milk, widely used for C. jejuni infection and human volunteer studies (5). The C. jejuni 11168H rpoN mutant was obtained from the LSHTM Campylobacter Resource Facility (http://crf.lshtm.ac.uk/index.htm). C. jejuni strains were routinely cultured on blood agar (BA) plates supplemented with Campylobacter selective supplement (Oxoid, Basingstoke, United Kingdom) and 7% (vol/vol) horse blood (TCS Microbiology, Botolph Claydon, United Kingdom) at 37°C in a variable atmosphere incubator (VAIN) microaerobic chamber (Don Whitley Scientific, Sheffield, United Kingdom) containing 85% N2, 10% CO2, and 5% O2. Appropriate antibiotics were added as follows: ampicillin (100 μg/ml), kanamycin (50 μg/ml), and chloramphenicol (50 μg/ml for E. coli studies or 10 μg/ml for C. jejuni studies). All reagents were obtained from Invitrogen (Paisley, United Kingdom) unless otherwise stated.
Epithelial cell line and culture conditions.
The human Caco-2 IEC line was cultured in Dulbecco's modified essential medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum (FCS; Sigma-Aldrich, Poole, United Kingdom), 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% (vol/vol) nonessential amino acids and maintained at 37°C in 5% CO2 and 95% air. The human T84 IEC line was cultured in 1:1 DMEM–F-12 medium (Invitrogen) supplemented with 10% (vol/vol) FCS and 100 U/ml penicillin, 100 μg/ml streptomycin. For VDC experiments, 4 × 105 Caco-2 or T84 IECs were seeded into the upper compartment of a Snapwell filter (Corning Lifesciences, Amsterdam, Netherlands). To allow for the formation of a polarized monolayer, cells were grown for a minimum of 21 days for Caco-2 IECs or 14 days for T84 IECs. The growth medium was changed every 3 days.
Assembly of the vertical diffusion chamber model system.
Prior to assembly, the two compartments of the VDC (Harvard Apparatus, Holliston, MA) were sterilized by immersion in Haz-Tabs (Guest Medical, Ltd., Aylesford, United Kingdom) solution for 3 h, followed by three washes with sterile water. A Snapwell filter carrying a polarized monolayer of Caco-2 or T84 IECs was removed from the culture plate, washed three times with phosphate-buffered saline (PBS), and inserted between the two compartments of the VDC. The basolateral compartment was filled with 4 ml of the appropriate tissue culture medium supplemented with 1% (vol/vol) FCS and 1% (vol/vol) nonessential amino acids. The apical compartment was filled with 4 ml brucella broth (Oxoid). For infections, approximately 4 × 108 C. jejuni cells were harvested from a 24-h BA plate and added to the apical compartment. For aerobic coculturing, the VDC was maintained at 37°C in 5% CO2 and 95% air. For microaerobic coculturing, the VDC was maintained under microaerobic conditions (85% N2, 10% CO2, and 5% O2) in a VAIN. A gas mixture of 95% O2 and 5% CO2 was perfused through the basolateral compartment, while the apical compartment was left open to the microaerobic atmosphere in the VAIN (Fig. 1).
Fig 1.
Vertical diffusion chamber (VDC) model system. IECs were grown to polarization on Snapwell filters and placed between the two compartments of the VDC. The apical compartment was filled with brucella broth, and the basolateral compartment was filled with tissue culture medium. For infection studies, C. jejuni was added to the apical compartment of the VDC. For the aerobic controls, the VDC was left open and placed into a tissue culture incubator containing air enriched with 5% CO2. For microaerobic coculturing, the VDC was placed into a microaerobic incubator with the apical compartment open to the atmosphere within the incubator and the basolateral compartment closed and perfused with 95% O2 and 5% CO2.
Immunofluorescence analysis of cellular distribution of actin and occludin.
IECs were fixed with 2% (wt/vol) paraformaldehyde (Sigma-Aldrich) for 1 h at 4°C, permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 20 min, and then blocked with 1% (wt/vol) bovine serum albumin (BSA) in PBS for 1 h, both at room temperature. The filter was excised from the carrier and placed in a 12-well dish. For actin staining, IECs were incubated with Alexa Fluor 555-conjugated phalloidin (Invitrogen) (stock diluted 1:1,000 in PBS) for 1 h in the dark. For occludin staining, IECs were incubated with mouse antioccludin primary antibody (stock diluted 1:100 in PBS) (Invitrogen) for 1 h at room temperature, followed by Alexa Fluor 488-conjugated goat anti-mouse (Invitrogen) (stock diluted 1:200 in PBS) for 1 h at room temperature in the dark. Stained filters were mounted in Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; final concentration, 1.5 μg/ml) (Vector Laboratories, Peterborough, United Kingdom) on a coverslip (Fisher Scientific, Loughborough, United Kingdom) and examined with a Zeiss LSM510 confocal microscope (Carl Zeiss AG, Jena, Germany).
Fluorescent dextran diffusion assay.
IECs on Snapwell filters were washed three times with sterile PBS and placed back into the hanging support; 500 μl of 100 μM fluorescein isothiocyanate (FITC)-labeled dextran (Sigma-Aldrich) with an average molecular mass of 4 kDa in Ringer's solution (115 mM NaCl, 1 mM KCl, 1 mM CaCl2) was added to the apical side of the monolayer and incubated for 3 h at room temperature, with the basolateral side of the monolayer immersed in Ringer's solution. The amount of fluorescently labeled dextran on the basolateral side of the monolayer was determined postincubation by removal of the basolateral solution and measurement of the fluorescence intensity at 488 nm using a Gemini XPS fluorescence microplate reader (Molecular Devices, Sunnyvale, CA).
Measurement of Caco-2 monolayer TEER in the VDC.
The transepithelial electrical resistance (TEER) of a Caco-2 monolayer in a VDC was measured by placing two voltage-sensing AgCl electrodes close to the cell monolayer on each side of the insert, passing a current through two further electrodes placed at the two distal ends of the VDC, and reading the voltage necessary to keep the current flowing. Resistance was calculated according to Ohm's law (R = V/I, where R = resistance, V = voltage, and I = current) and multiplied by the surface area of the monolayers (1.12 cm2).
Enumeration of interacting bacteria and intracellular bacteria.
At the desired time point of coculturing, the apical and basolateral supernatants were removed from the VDC and stored at −80°C for subsequent analysis. The Snapwell filter was removed from the VDC, washed three times with sterile PBS, and placed into a 6-well tissue culture dish. IECs were lysed by addition of 0.1% (vol/vol) Triton X-100 in PBS for 20 min at room temperature. The lysates were serially diluted in PBS, plated on BA plates, and incubated microaerobically for 72 h. CFU counts were determined, and the number of bacteria interacting with the IECs was calculated. Enumeration of intracellular bacteria was performed essentially as described above, with the following modification. Before lysis with Triton X-100, the IECs were incubated in DMEM containing 150 μg/ml gentamicin (Sigma-Aldrich) for 2 h at 37°C. This step kills extracellular, adherent bacteria and allows for the analysis of the number of intracellular bacteria present after coculturing. All VDC experiments were performed with at least two technical replicates and at least three biological replicates per experimental data set.
Cytokine analysis of the coculturing supernatants.
Coculturing supernatants were probed for the presence of interleukin-8 (IL-8) with a human IL-8 enzyme-linked immunosorbent assay (ELISA) development kit (Peprotech, London, United Kingdom), according to the manufacturers' instructions.
Microarray analysis of C. jejuni 11168H gene expression profiles.
Gene expression profiles of C. jejuni 11168H in the apical compartment of the VDC after coculture with Caco-2 IECs for 6 h or 24 h under either aerobic or microaerobic conditions were analyzed using an indirect-comparison method or type 2 experimental design (46). Replicate test sets of Cy5-labeled C. jejuni 11168H total RNA samples were combined with a common reference sample (Cy3-labeled C. jejuni 11168H genomic DNA) using methodology described previously (23). Whole-genome C. jejuni NCTC11168 microarrays printed on UltraGAPS glass slides (Corning Lifesciences), constructed by the BμG@S microarray group (http://www.bugs.sgul.ac.uk/), were used in this study (29). The microarray slides were scanned with an Affymetrix 418 array scanner (MWG Biotech, Ebersberg, Germany) according to the manufacturer's guidelines. Signal and local background intensity readings for each spot were quantified using ImaGene software v8.0 (BioDiscovery, El Segundo, CA). Quantified data were analyzed using GeneSpring GX software v7.3 (Agilent, Santa Clara, CA). Statistically significantly up- and downregulated genes were selected by comparing 11168H gene expression under microaerobic conditions against that under aerobic conditions using analysis of variance (ANOVA) with a Benjamini and Hochberg false-discovery rate as the multiple testing correction (2, 11).
Complementation of the C. jejuni 11168H rpoN mutant.
Complementation was performed by inserting a copy of the rpoN gene into the rpoN mutant chromosome with a C. jejuni NCTC11168 complementation vector (25), using previously described techniques (23). The complementation vector utilizes the constitutive chloramphenicol cassette promoter to express the rpoN gene and not the native rpoN promoter. The coding region for rpoN was amplified by PCR using proofreading Pfu polymerase (Fermentas, Sankt Leon-Rot, Germany) and ligated into the NcoI and NheI sites on the complementation vector. This construct was checked by sequencing (data not shown) and electroporated into the 11168H rpoN mutant. Putative clones were selected on BA plates containing kanamycin and chloramphenicol. Confirmation of the presence of copies of both rpoN and rpoN Kmr was performed by PCR and also by sequencing (data not shown).
Statistical analysis.
Data were statistically analyzed using Prism software (GraphPad Software, Inc., La Jolla, CA). Figures display means ± standard errors as well as the P values determined by unpaired Student t tests. All experiments represent at least three biological replicates performed in triplicate in each experiment.
Microarray data accession numbers.
The array design is available in BμG@Sbase (accession no. A-BUGS-9; http://bugs.sgul.ac.uk/A-BUGS-9) and also ArrayExpress (accession no. A-BUGS-9). Fully annotated microarray data have been deposited in BμG@Sbase (accession no. E-BUGS-125; http://bugs.sgul.ac.uk/E-BUGS-125) and also ArrayExpress (accession no. E-BUGS-125).
RESULTS
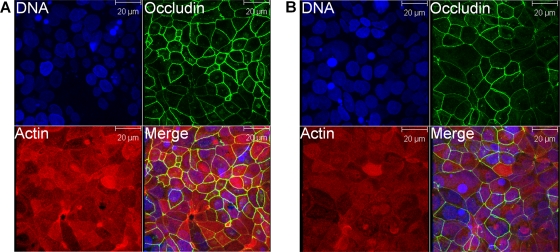
Cellular distribution of actin and occludin within IECs is not affected by culture in a VDC with microaerobic conditions at the apical surface.
Prior to using the VDC to coculture C. jejuni and IECs with microaerobic conditions at the apical surface, the effect of these conditions on polarized IECs was determined. The morphology of Caco-2 monolayers after 24 h of incubation in a VDC was analyzed by confocal laser microscopy. No difference in distribution of actin between IECs that had been maintained under microaerobic conditions (Fig. 2B) or under aerobic conditions (Fig. 2A) was detected. The fluorescence signal localized predominantly to the junctions between adjacent cells. Additionally, a strong localization of actin to the apical side of the Caco-2 IECs was noted, indicating the formation of a dense microvillus brush border. This indicated that the IECs were not adversely affected under microaerobic conditions and remained attached to the Snapwell filter. As the distribution of actin does not provide any information on the intactness of monolayers, the presence of intact tight junctions between the IECs was also analyzed. No difference in distribution of occludin between IECs that had been maintained under microaerobic conditions (Fig. 2B) or under aerobic conditions (Fig. 2A) was detected. Fluorescence was tightly localized to the cell-cell boundaries, indicating the presence of intact tight junctions. A similar distribution of actin and occludin was observed with T84 IECs (data not shown).
Fig 2.
Microaerobic conditions and bacterial broth on the apical side of intestinal epithelial cells (IECs) in a vertical diffusion chamber (VDC) have no detrimental effect on Caco-2 IECs over 24 h. Caco-2 IECs were grown for 21 days on Snapwell filters and maintained in VDCs with brucella broth and either aerobic (A) or microaerobic (B) conditions in the apical compartment for 24 h. After the incubation, the IECs were processed for immunostaining and stained for occludin (green) and actin (red) and the nuclei counterstained with DAPI (blue). The images represent projections of a stack of Z-axis slices viewed from above.
The barrier function of polarized IECs is not affected by culture in a VDC with microaerobic conditions at the apical surface.
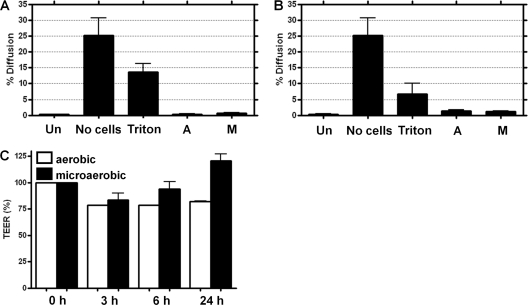
To quantitatively assess monolayer integrity after culture in a VDC, the diffusion of fluorescently labeled dextran across Caco-2 (Fig. 3A) and T84 (Fig. 3B) monolayers was determined. Approximately 25% of the labeled dextran diffused across an empty Snapwell filter after 3 h. However, there was no significant difference in dextran diffusion between IECs grown on Snapwell filters and IECs maintained in a VDC for 24 h with either microaerobic or aerobic conditions at the apical surface. Dextran diffusion was dramatically increased across IECs grown on Snapwell filters after permeabilization with Triton X-100, demonstrating that it is the barrier function of the polarized IECs that prevents dextran diffusion. Measurement of the TEER of an IEC monolayer on a permeable support is a direct, quantitative method for analysis of the polarization status of the IEC monolayer. There was no reduction in the TEER of a Caco-2 monolayer over a 24-h period under microaerobic conditions compared to the level under aerobic conditions (Fig. 3C).
Fig 3.
Analysis of the diffusion of a fluorescent marker across cellular monolayers on Snapwell filters following incubation in the vertical diffusion chamber (VDC). Caco-2 (A and C) or T84 (B) intestinal epithelial cells (IECs) were grown for 21 or 14 days, respectively, on Snapwell filters and maintained in VDCs with brucella broth and either aerobic (A) or microaerobic (M) conditions in the apical compartment for 24 h. VDCs were dismantled; 500 μl of 100 μM FITC-labeled dextran (average molecular mass of 4 kDa) in Ringer's solution was added to the apical side of the monolayer, and cells were incubated for 3 h at room temperature (A and B). After 3 h, the percentage of FITC-labeled dextran that had passed across the monolayer was determined from the relative fluorescence of the basolateral solution and the relative fluorescence of the input solution. An empty Snapwell filter (No cells), IECs grown for 21 days on Snapwell filters and permeabilized with 0.5% (vol/vol) Triton X-100 for 20 min at room temperature (Triton), and IECs grown for 21 days on Snapwell filters (Un) were used as controls. The transepithelial electrical resistance (TEER) was measured after assembly of the VDCs and set as 100% (C). After 3 h, 6 h, and 24 h postassembly, the TEER was measured and calculated as a percentage of the value obtained at time point 0.
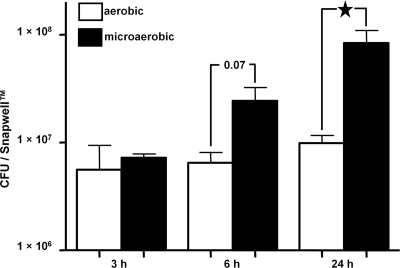
C. jejuni interactions with IECs are enhanced during coculture in a VDC under microaerobic conditions.
The C. jejuni 11168H wild-type strain was cocultured with Caco-2 IECs at a multiplicity of infection (MOI) of approximately 100:1 with either microaerobic or aerobic conditions in the apical compartment for 3, 6, and 24 h (Fig. 4). The numbers of bacteria interacting with the IECs under microaerobic conditions increased markedly over this period. After 24 h, a significant (P < 0.05) 9-fold increase in interacting C. jejuni 11168H bacteria was observed under microaerobic conditions compared to the number under aerobic conditions. It is possible that the increased numbers of interacting bacteria under microaerobic conditions may be the result of an increase in bacterial numbers during the 24-h assay due to increased proliferation of C. jejuni under microaerobic conditions. However, serial dilution plating of the contents of the apical compartment demonstrated equal numbers of bacteria present under microaerobic and aerobic conditions after 24 h of coculturing (data not shown). This indicated that the observed increase in C. jejuni interactions with IECs under microaerobic conditions was due to changes in bacterial activity, rather than an increase in bacterial numbers. Another possibility is that the microaerophilic conditions affect the activity and/or biology of the IECs, which then become more susceptible to C. jejuni infection.
Fig 4.
C. jejuni interactions with intestinal epithelial cells (IECs) are significantly increased under microaerobic conditions. C. jejuni 11168H wild-type strain bacteria were cocultured with Caco-2 IECs in a vertical diffusion chamber for 3 h, 6 h, and 24 h with either aerobic or microaerobic conditions in the apical compartment, and the numbers of interacting bacteria were assessed. ★, P < 0.05.
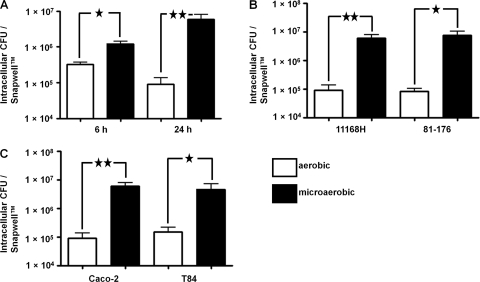
C. jejuni invasion of IECs is dramatically enhanced during coculture in a VDC under microaerobic conditions.
The C. jejuni 11168H wild-type strain was cocultured with Caco-2 IECs at an MOI of approximately 100:1 with either microaerobic or aerobic conditions in the apical compartment for 6 and 24 h, and the numbers of intracellular bacteria were determined (Fig. 5A). There was a significant (P < 0.05) 5-fold increase in the numbers of intracellular C. jejuni bacteria after 6 h under microaerobic conditions. After 24 h, there was a significant (P < 0.01) 80-fold increase in intracellular C. jejuni bacteria recovered under microaerobic conditions. To confirm that the observed increase in numbers of intracellular C. jejuni bacteria after 24 h of coculturing in the VDC under microaerobic conditions was not a specific effect of the bacterial strain or IEC line used, two further experiments were performed. The C. jejuni 81-176 wild-type strain was cocultured for 24 h with Caco-2 IECs with either aerobic or microaerobic conditions in the apical compartment (Fig. 5B). After 24 h, there was a significant (P < 0.05) 89-fold increase in intracellular C. jejuni 81-176 bacteria recovered from Caco-2 cells under microaerobic conditions. Additionally, the C. jejuni 11168H wild-type strain was cocultured for 24 h with T84 IECs with either aerobic or microaerobic conditions in the apical compartment (Fig. 5C). After 24 h, there was a significant (P < 0.05) 41-fold increase in intracellular C. jejuni 11168H bacteria recovered from T84 cells under microaerobic conditions.
Fig 5.
C. jejuni invasion of intestinal epithelial cells (IECs) is significantly increased under microaerobic conditions. C. jejuni wild-type strains were cocultured with IECs in a vertical diffusion chamber with either aerobic or microaerobic conditions in the apical compartment, and the numbers of intracellular bacteria were assessed. (A) 11168H cocultured with Caco-2 IECs for 6 h and 24 h. (B) 11168H or 81-176 cocultured with Caco-2 IECs for 24 h. (C) 11168H cocultured with Caco-2 or T84 IECs for 24 h. ★, P < 0.05; ★★, P < 0.01.
Increased C. jejuni interactions with IECs during coculture in a VDC result in an increased, polarized innate immune response.
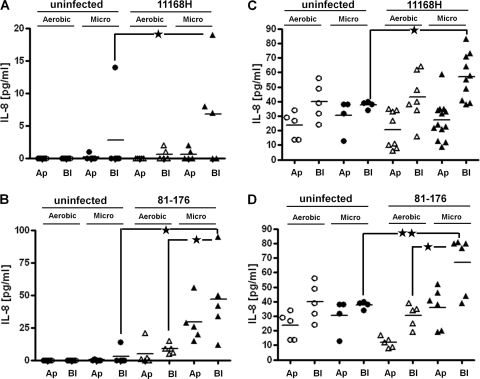
The neutrophil chemoattractant interleukin-8 (IL-8) has been shown to be involved in the host innate immune response to C. jejuni in both IECs and primary human tissue (26). Caco-2 IECs have been shown to secrete only low levels of IL-8 in response to C. jejuni infection, while T84 IECs have been shown to secrete much higher levels of IL-8 (32). Also, the C. jejuni 81-176 wild-type strain has been shown to induce a stronger IL-8 response than the 11168H wild-type strain from both Caco-2 and HEp-2 cells (49). IL-8 secretion from Caco-2 or T84 IECs cocultured with C. jejuni 11168H or 81-176 in a VDC under either microaerobic or aerobic conditions was assessed. Supernatants from both apical and basolateral VDC compartments were probed separately for the presence of IL-8 (Fig. 6). There was a significant increase in IL-8 secretion from Caco-2 IECs under microaerobic conditions during infection with either 11168H or 81-176, and IL-8 secretion into the basolateral compartment was significantly higher than that into the apical compartment during infection with 81-176 (Fig. 6B). 81-176 induced higher levels of IL-8 secretion from Caco-2 IECs than 11168H. The levels of IL-8 secreted by T84 IECs in response to either 11168H or 81-176 were much higher than those secreted by Caco-2 IECs under both microaerobic and aerobic conditions (Fig. 6C and D). However, the highest levels of IL-8 secretion were into the basolateral compartment under microaerobic conditions, suggesting that analogous to Caco-2 IECs, T84 IECs respond to increased numbers of interacting C. jejuni bacteria by mounting an increased, polarized innate immune response.
Fig 6.
Increased C. jejuni interactions with and invasion of intestinal epithelial cells (IECs) are mirrored by an enhanced host response. After either Caco-2 (A and B) or T84 (C and D) IECs were cocultured with C. jejuni 11168H (A and C) or 81-176 (B and D) wild-type strains with either aerobic (Aerobic) or microaerobic (Micro) conditions in the apical compartment, supernatants from apical (Ap) and basolateral (Bl) compartments of a vertical diffusion chamber (VDC) were probed for the presence of the proinflammatory chemokine IL-8 by enzyme-linked immunosorbent assay. Uninfected IECs were used included as controls. ★, P < 0.05; ★★, P < 0.01.
Analysis of C. jejuni 11168H gene expression after coculture with Caco-2 IECs in the apical compartment of a VDC under either aerobic or microaerobic conditions.
In order to investigate bacterial factors involved in the observed increased bacterial interaction and invasion of IECs after coculture in a VDC under microaerobic conditions, gene expression profiles of the C. jejuni 11168H wild-type strain in the apical compartment of the VDC after 6 and 24 h of coculture with Caco-2 IECs were analyzed using standard microarray techniques. Based on ANOVA selection methodology, a total of 67 genes were differentially expressed after 6 h under microaerobic conditions compared to aerobic conditions, with 43 genes upregulated and 24 genes downregulated (see Tables S1 and S2 in the supplemental material). Of most significance was the upregulation of fdhA, petA, and Cj0414, suggesting the activation of a different respiratory pathway during coculture under microaerobic conditions. After 24 h, a total of 132 genes were differentially expressed under microaerobic conditions compared to aerobic conditions, with 73 genes upregulated and 59 genes downregulated under microaerobic conditions (see Tables S3 and S4 in the supplemental material). The recN, mfd, rarA, and ruvA genes, encoding DNA repair proteins, were upregulated under aerobic conditions, suggesting greater levels of DNA damage under aerobic conditions. Also, the Cj1425c, Cj1440c, and kpsT genes in the capsular polysaccharide (CPS) locus were downregulated under microaerobic conditions. A recent study demonstrated the downregulation of CPS genes when in contact with IECs in vitro (11). Downregulation of CPS genes may lead to greater exposure of C. jejuni surface structures that may be involved in mediating bacterial interactions with the IECs. However, the expression of genes encoding many C. jejuni virulence factors is unchanged. It is possible that changes in the regulation of bacterial factors involved in the observed increased bacterial interaction and invasion of IECs after coculturing in a VDC under microaerobic conditions occur at the posttranscriptional level and are not reflected in these results.
A nonmotile C. jejuni 11168H rpoN mutant lacks the ability for enhanced interactions with IECs during coculture in a VDC under microaerobic conditions.
Motility has previously been demonstrated to be important for C. jejuni interaction and invasion of the intestinal epithelium (36, 44). Despite the lack of significant changes in the expression of flagellar biosynthesis genes observed in the microarray studies, the effect of motility on the observed increased bacterial interaction and invasion of IECs after coculture in a VDC under microaerobic conditions was investigated. A nonmotile 11168H rpoN mutant was cocultured with Caco-2 IECs in a VDC with either microaerobic or aerobic conditions in the apical compartment for 6 h. Significantly lower numbers of rpoN mutant bacteria than 11168H wild-type bacteria were able to interact with (Fig. 7A) and invade (Fig. 7B) Caco-2 IECs under both microaerobic and aerobic conditions. Most importantly, the rpoN mutant had lower levels of interaction with and invasion of Caco-2 IECs under microaerobic conditions than under aerobic conditions, in contrast with the wild-type strain. A complemented 11168H rpoN mutant strain was generated by reinsertion of a functional copy of the gene into a predicted pseudogene on the chromosome (25). Successful complementation was demonstrated by restoration of wild-type autoagglutination and motility phenotypes (data not shown). The 11168H rpoN complement also partially restored the wild-type phenotype, demonstrating enhanced interaction with and invasion of Caco-2 IECs under microaerobic conditions (Fig. 7A and B).
Fig 7.
A C. jejuni 11168H rpoN mutant demonstrates reduced interactions with and invasion of Caco-2 intestinal epithelial cells (IECs) under microaerobic conditions. C. jejuni 11168H wild-type (11168H wt), rpoN mutant (rpoN mut), or complemented rpoN (comp) bacteria were cocultured with Caco-2 IECs in a vertical diffusion chamber for 6 h with either aerobic or microaerobic conditions in the apical compartment. The numbers of interacting (A) and intracellular (B) bacteria were assessed. ★, P < 0.05; ★★★, P < 0.001.
DISCUSSION
C. jejuni is one of the most prevalent causes of food-borne gastroenteritis worldwide. However, despite the prevalence of this human pathogen, the molecular basis of pathogenicity remains poorly understood in comparison to that for other enteric pathogens. This is partly due to the lack of a convenient, reproducible small-animal model and major drawbacks with the widely used in vitro tissue culture cell models (15, 21). To date, in vitro tissue culture assays have indicated only very low levels of C. jejuni invasion, which does not correlate with the observed clinical symptoms of bloody, inflammatory diarrhea that suggest infection by an invasive enteric pathogen. One of the drawbacks with in vitro tissue culture cell assays used to study C. jejuni interactions with host cells is the coculturing of the microaerophilic C. jejuni with IECs under aerobic conditions, which are likely to result in changes in the ability of the bacteria to interact with IECs. These assay conditions are also not reflective of the very-low-oxygen environment in the gut lumen encountered by enteric pathogens during the initial stages of in vivo infection of IECs (34). In this study, a modified VDC system was used to allow the coculture of C. jejuni with IECs under microaerobic conditions, to provide a more relevant model of the conditions under which in vivo infection occurs.
After establishing that both Caco-2 and T84 IECs could be maintained in the VDC with microaerobic conditions in the apical compartment for at least 24 h without any apparent detrimental effects, the effect of coculturing C. jejuni 11168H wild-type strain bacteria with IECs under these conditions was assessed. A time-dependent increase in the numbers of both interacting and intracellular C. jejuni 11168H wild-type strain bacteria was demonstrated under microaerobic conditions. These results were confirmed using a second C. jejuni wild-type strain (81-176) as well as using a second IEC line (T84) to rule out possible strain- or cell line-specific effects. The increased levels of bacterial interaction and invasion were demonstrated to lead to an increased, polarized innate immune response from the IECs. Significantly more IL-8 was detected after coculturing under microaerobic conditions, suggesting that IECs are able to sense and respond to the increased bacterial challenge. In addition, significantly more IL-8 was detected in the basolateral supernatants than in the apical supernatants. This suggests that the IL-8 secretion occurs in a polarized fashion, with the chemokine secreted from the basolateral surface. This concurs with the biological function of IL-8 as a neutrophil attractant, which would be of limited use in the intestinal lumen. In agreement with previous reports (32), a marked difference in amount of secreted IL-8 was detected between the two IEC lines used, with the T84 IECs demonstrating higher levels of secretion than the Caco-2 IECs. Both C. jejuni wild-type strains induced similar levels of IL-8 secretion from T84 IECs but differed in ability to induce an IL-8 response from the Caco-2 IECs, despite demonstrating similar numbers of interacting and invading bacteria. This suggests that both C. jejuni strain-specific factors and IEC line specific-factors contribute to the level of the innate immune response observed in these experiments.
A C. jejuni NCTC11168 rpoN mutant is completely aflagellate, nonmotile, and unable to secrete the CiaB protein (17). In this study, a 11168H rpoN mutant exhibited lower numbers of interacting and intracellular bacteria than the wild-type strain when cocultured with Caco-2 IECs in the VDC under either microaerobic or aerobic conditions after 6 h of coculturing. However, lower numbers of intracellular 11168H rpoN mutant bacteria were recovered after coculturing under microaerobic conditions. This is in contrast to the C. jejuni 11168H wild-type strain, where fewer intracellular bacteria were recovered after coculturing under aerobic conditions. These data suggest that bacterial motility is not just important for the interaction and invasion of C. jejuni per se but is also an important factor involved in mediating the increased interaction and invasion of C. jejuni when cocultured with Caco-2 IECs in the VDC under microaerobic conditions.
A VDC model system was used to investigate the interactions of the microaerophilic gastric pathogen H. pylori with epithelial cells under low-oxygen conditions in the apical compartment, demonstrating increased numbers of adherent bacteria when cocultured with Caco-2 IECs under microaerobic conditions (12). Caco-2 IECs were used as no polarized gastric epithelial cell line was available. A more recent study using a similar VDC model to analyze the interaction of the facultative anaerobe enterohemorrhagic E. coli (EHEC) with IECs under anaerobic conditions demonstrated increased interactions of the bacteria when cocultured with anaerobic/microaerobic conditions in the apical compartment of the VDC (42). This suggests that the behavior not only of microaerobic bacteria but also of bacteria that are capable of proliferating at atmospheric oxygen concentration is changed when cocultured with IECs under microaerobic or anaerobic conditions. Using the VDC model with C. jejuni allows for the first time the interactions of an invasive enteric bacterial pathogen to be studied under low-oxygen coculture conditions. The data suggest that the VDC model is very useful for analysis of the host-pathogen interactions of a wide range of pathogenic bacteria under conditions more closely resembling the in vivo situation in the human intestinal lumen. However, it should be noted that VDC models, like other in vitro cell culture models, are limited in the extent to which they model the complexity of real tissue. Further steps need to be taken to more closely represent the complexity of the intestinal epithelium, especially in terms of mucous secretion and different cell types present in the human intestine.
Even though it has been demonstrated that C. jejuni can invade and survive within IECs in vitro, the fate of the bacteria postinfection has been very difficult to assess due to the small amounts of invasion observed under standard in vitro tissue culture conditions (21). Performing such coculture experiments in the VDC model system increased by 80-fold the number of intracellular bacteria observed after 24 h. This allows for a much more detailed analysis of mechanisms of IEC invasion by C. jejuni, as invasion is no longer a rare event. Furthermore, the intracellular fate of C. jejuni is more easily traceable. Methods, such as analysis of gene transcription from intracellular bacteria, that have not been possible to date due to the amounts of recoverable RNA being below a useful threshold should now be possible. Furthermore, as the compartment in which the bacteria are incubated is separate from the one supporting the IECs with nutrients and oxygen, it is more amenable to manipulations than classical coculturing in tissue culture plates without interfering with the IECs. This means that it will be easier to test the effects of different substances on the invasive behavior of the bacteria. Substances, like bile salts, that have been shown to increase expression of C. jejuni virulence genes can be added to the bacterial suspension and their effect on bacterial invasion, host response, or monolayer disruption analyzed.
Using the VDC model system to coculture C. jejuni with IECs under microaerobic conditions resulted in dramatic changes in the host-pathogen interactions observed. This model provides an improved mimic of the in vivo situation encountered by C. jejuni in the human intestinal lumen. IECs are not negatively affected by microaerobic conditions at the apical surface over 24 h. A time-dependent increase in the numbers of both interacting and intracellular C. jejuni bacteria was demonstrated after coculturing with Caco-2 IECs in the VDC under microaerobic conditions. This increased interaction of C. jejuni with the IECs was mirrored by an increased innate immune response. Taken together, these results indicate that use of the VDC model system provides an improved model to investigate C. jejuni-host cell interactions and the elucidation of the molecular basis of pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
Dominic C. Mills was supported by a Bloomsbury Colleges Ph.D. studentship (2007 to 2010) awarded to N.D. and P.W.T.
We thank Dennis Linton (University of Manchester, United Kingdom) for providing the C. jejuni NCTC11168 complementation vector. We acknowledge BμG@S (the Bacterial Microarray Group at St. George's, University of London) for supply of the microarray and advice and The Wellcome Trust for funding this multicollaborative microbial pathogen microarray facility under the Functional Genomics Resources Initiative.
Footnotes
Published ahead of print 21 February 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Allos BM. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201–1206 [DOI] [PubMed] [Google Scholar]
- 2. Bacon J, et al. 2004. The influence of reduced oxygen availability on pathogenicity and gene expression in Mycobacterium tuberculosis. Tuberculosis (Edinb.) 84:205–217 [DOI] [PubMed] [Google Scholar]
- 3. Baqar S, et al. 1996. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect. Immun. 64:4933–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell JA, Manning DD. 1990. A domestic ferret model of immunity to Campylobacter jejuni-induced enteric disease. Infect. Immun. 58:1848–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472–479 [DOI] [PubMed] [Google Scholar]
- 6. Blaser MJ. 1997. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 176:S103–S105 [DOI] [PubMed] [Google Scholar]
- 7. Blaser MJ, Duncan DJ, Warren GH, Wang WL. 1983. Experimental Campylobacter jejuni infection of adult mice. Infect. Immun. 39:908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blaser MJ, et al. 1986. Extraintestinal Campylobacter jejuni and Campylobacter coli infections: host factors and strain characteristics. J. Infect. Dis. 153:552–559 [DOI] [PubMed] [Google Scholar]
- 9. Champion OL, et al. 2008. A murine intraperitoneal infection model reveals that host resistance to Campylobacter jejuni is Nramp1 dependent. Microbes Infect. 10:922–927 [DOI] [PubMed] [Google Scholar]
- 10. Chang C, Miller JF. 2006. Campylobacter jejuni colonization of mice with limited enteric flora. Infect. Immun. 74:5261–5271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corcionivoschi N, et al. 2009. Campylobacter jejuni cocultured with epithelial cells reduces surface capsular polysaccharide expression. Infect. Immun. 77:1959–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cottet S, Corthesy-Theulaz I, Spertini F, Corthesy B. 2002. Microaerophilic conditions permit to mimic in vitro events occurring during in vivo Helicobacter pylori infection and to identify Rho/Ras-associated proteins in cellular signaling. J. Biol. Chem. 277:33978–33986 [DOI] [PubMed] [Google Scholar]
- 13. Davis L, DiRita V. 2008. Experimental chick colonization by Campylobacter jejuni. Curr. Protoc. Microbiol. 8A:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Day WA, Jr, Sajecki JL, Pitts TM, Joens LA. 2000. Role of catalase in Campylobacter jejuni intracellular survival. Infect. Immun. 68:6337–6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorrell N, Wren BW. 2007. The second century of Campylobacter research: recent advances, new opportunities and old problems. Curr. Opin. Infect. Dis. 20:514–518 [DOI] [PubMed] [Google Scholar]
- 16. Everest PH, et al. 1992. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37:319–325 [DOI] [PubMed] [Google Scholar]
- 17. Fernando U, Biswas D, Allan B, Willson P, Potter AA. 2007. Influence of Campylobacter jejuni fliA, rpoN and flgK genes on colonization of the chicken gut. Int. J. Food Microbiol. 118:194–200 [DOI] [PubMed] [Google Scholar]
- 18. Flanagan RC, Neal-McKinney JM, Dhillon AS, Miller WG, Konkel ME. 2009. Examination of Campylobacter jejuni putative adhesins leads to the identification of a new protein, designated FlpA, required for chicken colonization. Infect. Immun. 77:2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox JG, et al. 2004. Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72:1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedman CR, et al. 2004. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin. Infect. Dis. 38:S285–S296 [DOI] [PubMed] [Google Scholar]
- 21. Friis LM, Pin C, Pearson BM, Wells JM. 2005. In vitro cell culture methods for investigating Campylobacter invasion mechanisms. J. Microbiol. Methods 61:145–160 [DOI] [PubMed] [Google Scholar]
- 22. Gradel KO, et al. 2009. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 137:495–501 [DOI] [PubMed] [Google Scholar]
- 23. Gundogdu O, et al. 2011. The Campylobacter jejuni transcriptional regulator Cj1556 plays a role in the oxidative and aerobic stress response and is important for bacterial survival in vivo. J. Bacteriol. 193:4238–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hendrixson DR, DiRita VJ. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52:471–484 [DOI] [PubMed] [Google Scholar]
- 25. Hitchen P, et al. 2010. Modification of the Campylobacter jejuni flagellin glycan by the product of the Cj1295 homopolymeric-tract-containing gene. Microbiology 156:1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu L, Hickey TE. 2005. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect. Immun. 73:4437–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janssen R, et al. 2008. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin. Microbiol. Rev. 21:505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones MA, et al. 2004. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect. Immun. 72:3769–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamal N, et al. 2007. Deletion of a previously uncharacterized flagellar-hook-length control gene fliK modulates the sigma54-dependent regulon in Campylobacter jejuni. Microbiology 153:3099–3111 [DOI] [PubMed] [Google Scholar]
- 30. Karlyshev AV, et al. 2004. The Campylobacter jejuni general glycosylation system is important for attachment to human epithelial cells and in the colonization of chicks. Microbiology 150:1957–1964 [DOI] [PubMed] [Google Scholar]
- 31. Konkel ME, Hayes SF, Joens LA, Cieplak W., Jr 1992. Characteristics of the internalization and intracellular survival of Campylobacter jejuni in human epithelial cell cultures. Microb. Pathog. 13:357–370 [DOI] [PubMed] [Google Scholar]
- 32. MacCallum AJ, Harris D, Haddock G, Everest PH. 2006. Campylobacter jejuni-infected human epithelial cell lines vary in their ability to secrete interleukin-8 compared to in vitro-infected primary human intestinal tissue. Microbiology 152:3661–3665 [DOI] [PubMed] [Google Scholar]
- 33. Mansfield LS, et al. 2007. C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect. Immun. 75:1099–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marteyn B, et al. 2010. Modulation of Shigella virulence in response to available oxygen in vivo. Nature 465:355–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monteville MR, Konkel ME. 2002. Fibronectin-facilitated invasion of T84 eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect. Immun. 70:6665–6671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novik V, Hofreuter D, Galan JE. 2010. Identification of Campylobacter jejuni genes involved in its interaction with epithelial cells. Infect. Immun. 78:3540–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parkhill J, et al. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668 [DOI] [PubMed] [Google Scholar]
- 38. Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. 2007. Campylobacter reactive arthritis: a systematic review. Semin. Arthritis Rheum. 37:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Purdy D, Cawthraw S, Dickinson JH, Newell DG, Park SF. 1999. Generation of a superoxide dismutase (SOD)-deficient mutant of Campylobacter coli: evidence for the significance of SOD in Campylobacter survival and colonization. Appl. Environ. Microbiol. 65:2540–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Russell RG, Blaser MJ, Sarmiento JI, Fox J. 1989. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect. Immun. 57:1438–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Russell RG, O'Donnoghue M, Blake DC, Jr, Zulty J, DeTolla LJ. 1993. Early colonic damage and invasion of Campylobacter jejuni in experimentally challenged infant Macaca mulatta. J. Infect. Dis. 168:210–215 [DOI] [PubMed] [Google Scholar]
- 42. Schuller S, Phillips AD. 2010. Microaerobic conditions enhance type III secretion and adherence of enterohaemorrhagic Escherichia coli to polarized human intestinal epithelial cells. Environ. Microbiol. 12:2426–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stanfield JT, McCardell BA, Madden JM. 1987. Campylobacter diarrhea in an adult mouse model. Microb. Pathog. 3:155–165 [DOI] [PubMed] [Google Scholar]
- 44. Wassenaar TM, Bleumink-Pluym NM, van der Zeijst BA. 1991. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 10:2055–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watson RO, Novik V, Hofreuter D, Lara-Tejero M, Galan JE. 2007. A MyD88-deficient mouse model reveals a role for Nramp1 in Campylobacter jejuni infection. Infect. Immun. 75:1994–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang IV, et al. 2002. Within the fold: assessing differential expression measures and reproducibility in microarray assays. Genome Biol. 3:research0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Young KT, Davis LM, Dirita VJ. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5:665–679 [DOI] [PubMed] [Google Scholar]
- 48. Yrios JW, Balish E. 1986. Pathogenesis of Campylobacter spp. in athymic and euthymic germfree mice. Infect. Immun. 53:384–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zilbauer M, et al. 2005. Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infect. Immun. 73:7281–7289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.