Abstract
This study describes a novel immunization strategy against avian coccidiosis using exosomes derived from Eimeria parasite antigen (Ag)-loaded dendritic cells (DCs). Chicken intestinal DCs were isolated and pulsed in vitro with a mixture of sporozoite-extracted Ags from Eimeria tenella, E. maxima, and E. acervulina, and the cell-derived exosomes were isolated. Chickens were nonimmunized or immunized intramuscularly with exosomes and subsequently noninfected or coinfected with E. tenella, E. maxima, and E. acervulina oocysts. Immune parameters compared among the nonimmunized/noninfected, nonimmunized/infected, and immunized/infected groups were the numbers of cells secreting Th1 cytokines, Th2 cytokines, interleukin-16 (IL-16), and Ag-reactive antibodies in vitro and in vivo readouts of protective immunity against Eimeria infection. Cecal tonsils, Peyer's patches, and spleens of immunized and infected chickens had increased numbers of cells secreting the IL-16 and the Th1 cytokines IL-2 and gamma interferon, greater Ag-stimulated proliferative responses, and higher numbers of Ag-reactive IgG- and IgA-producing cells following in vitro stimulation with the sporozoite Ags compared with the nonimmunized/noninfected and nonimmunized/infected controls. In contrast, the numbers of cells secreting the Th2 cytokines IL-4 and IL-10 were diminished in immunized and infected chickens compared with the nonimmunized/noninfected and the nonimmunized/infected controls. Chickens immunized with Ag-loaded exosomes and infected in vivo with Eimeria oocysts had increased body weight gains, reduced feed conversion ratios, diminished fecal oocyst shedding, lessened intestinal lesion scores, and reduced mortality compared with the nonimmunized/infected controls. These results suggest that successful field vaccination against avian coccidiosis using exosomes derived from DCs incubated with Ags isolated from Eimeria species may be possible.
INTRODUCTION
Coccidiosis is a complex intestinal disease of major economic importance in chickens that is caused by multiple species of the protozoan Eimeria (12). Infection by coccidial parasites has an enormous impact on worldwide poultry production due to the morbidity, mortality, and reduced body weight gain that the infection produces. Conventional disease control methods have relied on prophylactic administration of drugs with anticoccidial activity or on vaccination with live or attenuated parasites. However, alternative methods of disease mitigation are needed due to increasing government restrictions on the use of coccidiostats, the emergence of drug-resistant parasites, the high costs of new drug development, and the limited immunological cross-reactivity of parasite antigens (Ags) between Eimeria species. Particularly, there is an urgent need to develop a field vaccine against avian coccidiosis that is safe and effective against all relevant parasites.
One novel approach to vaccination against coccidiosis is to exploit dendritic cells (DCs) as an initial step in the development of a second-generation coccidiosis vaccine. DCs are Ag-presenting cells serving as immunologic sentinels that are critical for the induction of protective immunity against microbial pathogens (42). DC-based vaccination protocols are being undertaken to expand immune responses to viral, bacterial, parasitic, and fungal pathogens (6, 8, 27, 29). DC-mediated vaccination strategies rely on the technique of in vitro loading of DCs with pathogen-derived Ags. One of the proposed mechanisms through which DCs produce Ag-specific cellular and humoral immune responses involves the secretion of exosomes (10, 47). Exosomes secreted from antigen-presenting cells (APCs) have been suggested to play a functional role in mediating innate and adaptive immune responses to microbial pathogens (26). DC-derived exosomes expressing major histocompatibility complex class I (MHC-I), MHC-II, and costimulatory molecules induce and enhance Ag-specific T cell responses in vitro and in vivo (18). Because cytoplasmic vesicles are capable of becoming exosomes, among other fates, intracellular parasites, bacteria, and viruses that enter cells via an endocytotic pathway are prime candidates for DC-based exosome vaccines. Recent reports suggest that such an approach may be feasible for immunization against infection by Toxoplasma gondii (4), Leishmania major (38), Mycobacterium tuberculosis (5), and Salmonella enterica serovar Typhimurium (5). In this scenario, intracellular pathogens that are taken up by APCs are processed into MHC-II-binding peptide Ags, predominantly within endosomes. The Ag-loaded complexes are delivered to the cell surface through lipid bilayer fusion of the endosome and plasma membranes or, alternatively, are released extracellularly as intact exosomes (32). Thus, MHC-II–Ag complexes are presented to naïve T cells either on the surface of APCs or through their exosomes. Isolated APC-derived exosomes offer the additional advantage of amplifying Ag-specific T cell activation through the synergistic effects of direct T cell activation as well as indirect transfer of MHC-II–Ag complexes to DCs prior to antigen presentation (45). Remarkably, MHC barriers do not restrict exosome efficacy since syngeneic exosomes do not appear to work significantly better than allogeneic exosomes (4, 14). Because protective immunity against Eimeria infection is T cell dependent (37), coccidiosis vaccines based on DC exosomes are promising vehicles for activating parasite-specific T cells.
Our previous work demonstrated that a potent T cell-dependent protective immune response against Eimeria tenella infection in chickens was generated by in vivo administration of exosomes derived from DCs pulsed in vitro with E. tenella Ags (14). These results led us to further study the potential of Ag-loaded DC exosomes for vaccination against coccidiosis. It is well-known that under natural conditions several Eimeria species are nearly always present in chickens affected by coccidiosis (2, 23, 33). Therefore, a vaccine against that parasitic disease should induce protective immunity against economically important species of Eimeria (9). Particularly, we were interested in assessing the utility of vaccination with DC exosomes to protect against simultaneous infection by E. tenella, E. maxima, and E. acervulina, the three major coccidial species that are responsible for the majority of avian coccidiosis in commercial production facilities. Given that the immunizing capability of Eimeria sporozoite Ags is well documented (3, 7) and that the sporozoite stage of the parasite life cycle is a target of protective host immunity in vivo (20, 40, 44), the present study evaluated chicken DCs pulsed with sporozoite Ags from these denoted eimerian parasites.
MATERIALS AND METHODS
Animals.
White Leghorn chickens were hatched and reared under Eimeria-free conditions, with access to feed and water provided ad libitum. All experiments were performed in accordance with the guidelines approved by the University of Zaragoza Institutional Animal Care and Use Committee.
Parasites.
E. tenella and E. maxima strains were originally obtained from Merck, Sharp and Dome (Madrid, Spain) and the Beltsville Agricultural Research Center, respectively, and a Houghton E. acervulina strain was kindly provided by R. Marshall (Veterinary Laboratories Agency, Weybridge, United Kingdom). Oocysts were propagated, isolated, and sporulated using standard procedures (35). Only sporulated oocysts that had been stored for less than 4 weeks were used for infection.
Antibodies.
Anti-chicken CD45 (clone LT40), IgA (clone A-1), and IgG (clone G-1) antibodies (Abs) were from Southern Biotech (Birmingham, AL). Anti-chicken interleukin-2 (IL-2) Ab (clone 4F12) was from AbD Serotec (Oxford, United Kingdom). Anti-chicken IL-16 Ab was from Bethyl Laboratories (Montgomery, TX). Anti-chicken IL-4, IL-10, and gamma interferon (IFN-γ) antibodies were prepared as described previously (25, 48).
Isolation of cecal DCs.
Cecal tonsils (CTs) from E. tenella-infected chickens were homogenized for 1 h at 37°C in phosphate-buffered saline (PBS; 25 mM Na2HPO4, 5.5 mM NaH2PO4, 137 mM NaCl, 5.5 mM KCl), pH 7.2, containing 11 mM d-glucose (GKN buffer) (43) and supplemented with 0.2% bovine serum albumin (BSA), 1.0 mg/ml collagenase A (Roche, Mannheim, Germany), and 750 U/ml bovine pancreatic DNase I (type IV; Sigma, St. Louis, MO) with continuous agitation (15). The released cells were filtered through a 250-μm-mesh-size mesh screen, resuspended, washed in GKN buffer containing 5.0 mM EDTA, and passed through a 70-μm-pore-size cell strainer (BD Falcon, Franklin Lakes, NJ). The DC-containing cells were purified from dead cells, erythrocytes, and epithelial cells by Percoll density gradient centrifugation (1.075 g/ml, high density; Amersham Pharmacia Biotech, Uppsala, Sweden) at 950 × g for 20 min. Cell viability was >90% by trypan blue exclusion. Eimeria Ag-positive, CD45+ DCs were isolated by fluorescence-activated cell sorting and magnetic bead selection as described previously (15). Briefly, the cells (1.0 × 107) were sequentially incubated on ice for 30 min with 0.5 ml of E. tenella antiserum (1:100), followed by fluorescein-conjugated goat anti-rabbit IgG secondary Ab. Cell sorting was performed using an Epics Elite flow cytometer (Beckman Coulter, Fullerton, CA) equipped with an argon ion laser set at 488 nm with 20 mW, and fluorescence was detected with a band-pass filter (525 ± 15 nm). Data files were analyzed with software provided with the flow cytometer (version 4.02). Each experiment included cells incubated with nonimmune rabbit IgG or in the absence of primary Ab as negative controls to discriminate the labeled cell population from background debris. Sorted cells were incubated with biotinylated anti-chicken CD45 Ab (1:100) for 45 min, washed, and isolated using streptavidin-conjugated magnetic beads (Dynabeads FlowComp) according to the manufacturer's guidelines (Invitrogen, Carlsbad, CA). Postseparation cell viability was >90%. CD45+ DCs were cultured in RPMI 1640 medium (Sigma) supplemented with 2.0 mM l-glutamine, 1.0 mM sodium pyruvate, 40 mg/ml gentamicin, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% heat-inactivated chicken serum (Sigma).
Eimeria Ag purification and DC loading.
Freshly excysted E. tenella, E. maxima, or E. acervulina sporozoites were separately purified from oocysts and sporocyst debris and sonicated on ice (14). The sonicates were centrifuged at 300 × g for 5 min, and the pellets were resuspended in 25 mM Tris-HCl, pH 7.6, containing 150 mM NaCl, 5.0 mM EDTA, 1.0% Triton X-100, and protease inhibitor cocktail at 4°C. The samples were centrifuged at 100,000 × g for 1 h at 4°C, and the pellets were resuspended in the same buffer for 1 h at 4°C and recentrifuged. The pellets were collected and resuspended, and protein concentrations were determined according to the procedure described by Lowry et al. (28) using BSA as standard and stored at −20°C. Purified DCs (5.0 × 105) were pulsed at 39°C for 1 day with an equal protein mixture containing 200 μg of sporozoite Ag from each Eimeria species (600 μg total).
Purification of Ag-pulsed, DC-derived exosomes.
Eimeria Ag-pulsed DCs were exposed to a nonlethal heat shock at 43.5°C for 30 min, followed by a recovery period at 41°C (the basal temperature of the chicken) for 2 h (41). Cell debris was removed from culture supernatants by centrifugation at 300 × g for 10 min at 4°C. Culture supernatants were filtered through 0.22-μm-pore-size cellulose acetate filters (Millipore, Billerica, MA), and the filtrate was centrifuged at 10,000 × g for 30 min. Supernatants were collected, and exosomes were pelleted at 100,000 × g for 1 h at 4°C in a Beckman L80M ultracentrifuge equipped with a 70.1Ti rotor, washed with cold PBS, resuspended in PBS at 1/100 of the original volume, and stored at −20°C in 10-μg aliquots until use. All procedures were performed under aseptic conditions.
Immunization and parasite challenge infection.
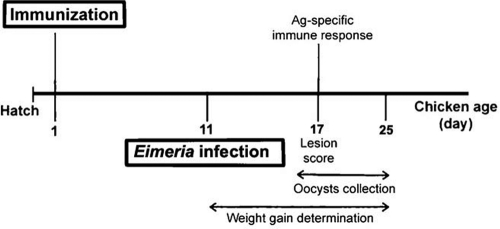
The experimental design is illustrated in Fig. 1. One-day-old chickens were randomly divided into 3 groups of 100 chickens each with equal mean body weights. Group I served as nonimmunized and noninfected controls, group II served as nonimmunized and infected controls, and group III served as the immunized and infected experimental group. Chickens in groups I and II received injections intramuscularly (i.m.) into the superficial breast muscle, the pectoral muscle, with 100 μl of sterile PBS. Chickens in group III were immunized i.m. with 100 μl of purified exosomes (10 μg/bird) from Ag-pulsed DCs. At 10 days postimmunization, chickens in groups II and III were infected with 1.0 × 104 E. tenella, 2.5 × 105 E. acervulina, and 2.5 × 104 E. maxima sporulated oocysts per bird by oral inoculation into the crop (39). Body weights and feed conversion ratios were measured at 0 and 14 days postinfection. Fecal oocyst numbers were counted at between 5 and 14 days postinfection using a McMaster chamber as described previously (24). At 6 days postinfection, 20 chickens per treatment group were killed for intestinal lesion score and immune response quantification. In these 20 chickens, the intestinal lesion scores were determined on a graded scale from 0 (none) to 4 (high) in a blinded fashion by two independent observers as described previously (22). Ten out of those 20 chickens were used for immune response quantification. It is to be noted that Ag-specific proliferative responses and the number of Ag-specific cytokine- and Ab-secreting cells were determined in each of those 10 chickens. The percent mortality in all groups was recorded at 14 days postinfection. At this time point, the live chickens in each of the groups were used for evaluation of zootechnical parameters. All experiments were repeated three times.
Fig 1.
Schematic illustration of the experimental design.
Quantification of Ag-specific cytokine- and Ab-secreting cells.
CTs, jejunal Peyer's patches (PPs), and spleens were harvested at 6 days postinfection, pressed through 250-μm-mesh-size mesh screens, and resuspended and washed in GKN buffer containing 5.0 mM EDTA. Single-cell suspensions were obtained by filtering through a 70-μm-pore-size cell strainer (BD Falcon) and were purified from dead cells, erythrocytes, and epithelial cells by Percoll density gradient centrifugation as described above. For quantification of cytokine-producing cells, 1.0 × 105 cells were cultured for 3 days in the presence or absence of E. tenella, E. maxima, or E. acervulina Ags (100 μg/ml) in 0.2 ml of RPMI 1640 medium. The cells were washed and added to microplates that had previously been coated overnight at 4°C with anti-chicken IL-2, IL-4, IL-16, or IFN-γ Abs, blocked at room temperature for 2 h with PBS containing 10 μg/ml of BSA and 0.05% Tween 20 (PBS/BSA/T), and washed three times with PBS; and cytokine enzyme-linked immunosorbent spot (ELISPOT) assays were performed as described previously (15). For quantification of Ag-specific, Ab-secreting cells, 1.0 × 105 cells were cultured for 10 days with 10 ng/ml of Escherichia coli lipopolysaccharide (Sigma) in the presence of E. tenella, E. maxima, or E. acervulina Ags (100 μg/ml) in 0.2 ml of RPMI 1640 medium. The cells were added to microtiter plates precoated with E. tenella, E. maxima, or E. acervulina Ags and blocked as described above, and IgG and IgA ELISPOT assays were performed as described previously (16). Control wells for cytokine and Ab ELISPOT assays contained cells incubated with medium alone in the absence of Eimeria Ags.
Ag-specific proliferative responses.
Single cells from CT, PP, and spleen cells (1.0 × 105) were cultured for 3 days in the presence or absence of E. tenella, E. maxima, or E. acervulina Ags (100 μg/ml) in 0.2 ml of RPMI 1640 medium in round-bottomed wells of microtiter plates. 5-Bromo-2′-deoxyuridine (BrdU; Boehringer Mannheim, Mannheim, Germany) was added to the cultures on day 4, and proliferation was measured on day 5 by determination of the absorbance at 450 nm using horseradish peroxidase-conjugated anti-BrdU Ab and tetramethylbenzidine substrate according to the manufacturer's instructions. Control wells contained cells incubated with medium alone in the absence of Eimeria Ags.
Statistical analysis.
All data were expressed as mean ± standard deviation (SD) values. Duncan's multiple-range test was used to evaluate the differences between treatment groups. Differences between mean values were considered statistically significant at P values of <0.05.
RESULTS
Ag-stimulated cytokine-producing cells.
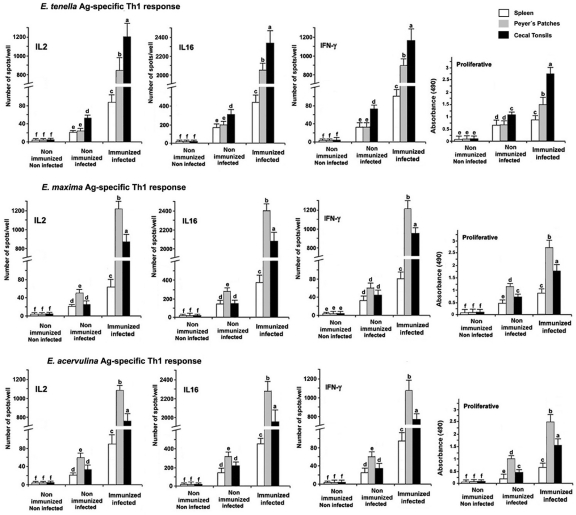
Initially, we assessed the IL-16 and Th1 cytokine responses of chickens immunized with DC exosomes pulsed with a mixture of E. tenella, E. maxima, and E. acervulina Ags and subsequently coinfected with the three parasite species. CT, PP, and spleen cells of immunized and infected chickens that had been stimulated in vitro with E. tenella, E. maxima, or E. acervulina sporozoite Ags had significantly increased numbers of IL-16-producing cells together with IL-2- and IFN-γ-producing cells compared both with the nonimmunized and noninfected and with the nonimmunized and infected controls (P < 0.05) (Fig. 2). Similarly, immunization with the DC-pulsed exosomes increased Ag-stimulated proliferation of CT, PP, and spleen cells from the infected chickens compared with the nonimmunized/noninfected and nonimmunized/infected controls (P < 0.05). Both the numbers of IL-16- and Th1 cytokine-producing cells and cell proliferation were greater in CT-derived cells stimulated with E. tenella Ag than in PP cells treated with E. tenella Ag. In contrast, these parameters were greater in PP-derived cells stimulated with E. maxima or E. acervulina Ags than in CT cells treated with E. maxima or E. acervulina Ags. This differential profile of cell response to the different Eimeria species Ags correlates with the respective sites of infection in the intestine by these three parasites: the cecum for E. tenella and the jejunum and duodenum for E. maxima and E. acervulina.
Fig 2.
Eimeria Ag-specific IL-16- and Th1 cytokine-secreting cells and proliferative responses. Chickens were nonimmunized and noninfected (group I), nonimmunized and infected (group II), or immunized with Ag-pulsed DC-derived exosomes at day 1 and orally coinfected with sporulated oocysts of E. tenella, E. maxima, and E. acervulina at day 11 (group III). Eimeria Ag-stimulated IL-16-, IL-2-, and IFN-γ-secreting cells in the spleen, cecal tonsils, and Peyer's patches at 6 days postinfection were quantified by ELISPOT assay. Ag-stimulated proliferative responses were measured by BrdU incorporation and quantified by enzyme-linked immunosorbent assay. Each bar represents the mean ± SD value from three independent experiments. Within each graph, bars with different letters are significantly different (P < 0.05) according to Duncan's multiple-range test.
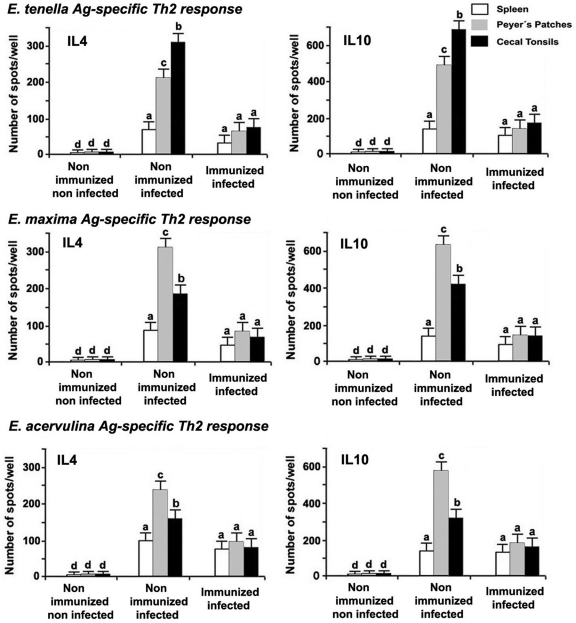
Immunization of chickens with Eimeria Ag-loaded DC exosomes significantly decreased the numbers of IL-4- and IL-10-producing cells in the CTs and PPs but not in the spleens of infected animals compared with the nonimmunized and infected controls (P < 0.05) (Fig. 3). However, compared with the nonimmunized and noninfected controls, both types of cytokine-secreting cells were increased in all three types of tissues examined from the immunized and infected group.
Fig 3.
Eimeria Ag-specific Th2 cytokine-secreting cells. Chickens were nonimmunized or immunized with Ag-loaded DC exosomes and noninfected or infected with E. tenella, E. maxima, and E. acervulina as described in Fig. 1. Eimeria Ag-stimulated IL-4- and IL-10-secreting cells in the spleen, cecal tonsils, and Peyer's patches at 6 days postinfection were quantified by ELISPOT assay. Each bar represents the mean ± SD value from three independent experiments. Within each graph, bars with different letters are significantly different (P < 0.05) according to Duncan's multiple-range test.
Ag-reactive IgG- and IgA-producing cells.
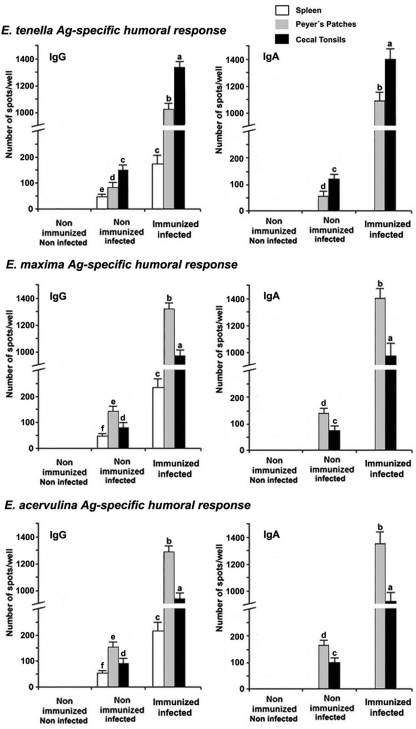
Immunization of chickens with Eimeria Ag-loaded DC exosomes significantly increased the numbers of E. tenella, E. maxima, or E. acervulina Ag-reactive IgG-secreting cells in the CTs, PPs, and spleens of infected animals compared with the nonimmunized/noninfected and the nonimmunized/infected control groups (P < 0.05) (Fig. 4). Similarly, greater numbers of Ag-reactive IgA-producing cells were present in the CTs and PPs but not in the spleens of the experimental versus control groups. As with the IL-16- and Th1 cytokine-producing cells (Fig. 2), increased levels of IgG- and IgA-producing cells were again seen in CT-derived cells stimulated with E. tenella Ag compared with PP cells treated with E. tenella Ag, while greater numbers of Ab-secreting cells were observed in PP cells stimulated with E. maxima or E. acervulina Ags than in CT cells treated with E. maxima or E. acervulina Ags.
Fig 4.
Eimeria Ag-specific IgG- and IgA-secreting cells. Chickens were nonimmunized or immunized with Ag-loaded DC exosomes and noninfected or infected with E. tenella, E. maxima, and E. acervulina as described in Fig. 1. Eimeria Ag-stimulated IgG- or IgA-secreting cells in the spleen, cecal tonsils, and Peyer's patches at 6 days postinfection were quantified by ELISPOT assay. Each bar represents the mean ± SD value from three independent experiments. Within each graph, bars with different letters are significantly different (P < 0.05) according to Duncan's multiple-range test.
Effect of immunization with DC exosomes on in vivo parameters of protective immunity against Eimeria infection.
Chickens immunized with Eimeria Ag-loaded DC exosomes had increased body weight gains, reduced feed conversion ratios, diminished fecal oocyst shedding, decreased intestinal lesion scores, and lessened mortality compared with the nonimmunized and infected controls (P < 0.05) (Table 1). Indeed, weight gains, feed conversion ratios, and mortality in the immunized and infected experimental group were equal to those in the noninfected controls.
Table 1.
Effect of immunization with Ag-pulsed DC-derived exosomes on in vivo parameters of protective immunity against E. tenella, E. maxima, and E. acervulina coinfectiona
| Treatment group | Body wt gain (g/chicken) | Feed conversion ratio (g of feed/g of chicken) | Fecal oocyst output (103/chicken) | Lesion score |
Mortality (%) | ||
|---|---|---|---|---|---|---|---|
| UI | MI | C | |||||
| Unimmunized, uninfected | 670 ± 18a | 1.52 ± 0.08a | 0a | 0a | 0a | 0a | 0a |
| Unimmunized, infected | 243 ± 29b | 4.95 ± 0.12b | 12,853 ± 0.68b | 2.8 ± 0.6b | 2.7 ± 0.6b | 2.6 ± 0.5b | 16 ± 2.8b |
| Immunized, infected | 634 ± 21a | 1.55 ± 0.09a | 593 ± 0.71c | 0.9 ± 0.7c | 0.9 ± 0.6c | 1.1 ± 0.7c | 0a |
Each value represents the mean ± SD of three independent immunization/infection trials, with each trial containing 100 chickens/group. Within each group, values with different superscripts are significantly different (P < 0.05) according to Duncan's multiple-range test. The lesion score ranges from 1 (none) to 4 (high). UI, upper intestine; MI, middle intestine; C, ceca.
DISCUSSION
This study demonstrates the efficacy of immunization of chickens with DC exosomes preloaded with E. tenella, E. maxima, and E. acervulina Ags on increasing in vitro parameters of Th1 immunity and on mediating in vivo protection against simultaneous infection with these Eimeria species. The CTs, PPs, and spleens of immunized and infected chickens had greater numbers of Eimeria sporozoite Ag-stimulated IL-16-, IL-2-, and IFN-γ-producing cells, increased Ag-stimulated proliferative responses, and augmented numbers of Ag-reactive IgG- and IgA-secreting cells compared with the nonimmunized/noninfected and nonimmunized/infected controls. In contrast, the numbers of IL-4- and IL-10-secreting cells were diminished in immunized and infected chickens compared with the two control groups. Chickens vaccinated with Eimeria Ag-pulsed DC exosomes and infected in vivo with parasite oocysts also had increased resistance to coccidiosis compared with the nonimmunized and infected controls, as evidenced by greater body weight gains, decreased feed conversion ratios, reduced fecal oocyst output, diminished gut lesions, and reduced mortality. To our knowledge, this is the first report demonstrating successful induction of protective immunity against simultaneous experimental infection with E. tenella, E. maxima, and E. acervulina using DC exosomes pulsed with Ags isolated from these coccidial species.
Increased numbers of IL-16-, IL-2-, and IFN-γ-producing cells in the immunized and infected chickens compared with the nonimmunized and infected group demonstrate that vaccination with parasite Ag-loaded DC exosomes induced a robust primary Th1 immune response. These results confirm and extend previous observations in chickens (14) and mammals (1, 4, 38). Further, the induction of Th1 cytokine-producing cells is correlated with a marked reduction in the numbers of Th2 cytokine-synthesizing cells (6, 29), suggesting that the immune response is polarized toward a Th1 response following immunization with Ag-loaded exosomes. IL-16 was selected and included in the present study to evaluate the efficacy of the immunization since Ag-loaded exosomes stimulate T cells (4, 14, 19) and IL-16 has two major effects on T cells: promotion of Th1 cell migration (30) and inhibition of Th2 cell activation (13). While some reports indicated that a host Th2 mucosal immune response is associated with the control of some protozoan infections (34, 46), other studies revealed that Th1 immunity was more efficient in conferring protection against infection by apicomplexan parasites (4, 19, 21). Depending upon the particular pathogen and host species, the local balance between Th1 and Th2 cytokine production is crucial for disease outcome (31, 36, 38). Because in the present study a low level of Th2 cytokine-producing cells was detected in the immunized and infected group, it is possible that these cells may play a role, to some extent, in the host's Ag-specific response against the coccidial parasites.
With regard to the nonimmunized and infected chickens, our findings also demonstrated a relatively small, but statistically significant, increase in the numbers of Th1 cytokine-producing cells and a more dramatic increase in the numbers of Th2-secreting cells compared with the noninfected controls. These results show apparent discrepancy with the data published by other investigators who analyzed the expression of genes encoding IL-16, Th1, and Th2 cytokines following primary infection of chickens with either a single or multiple Eimeria species. Hong et al. (21) reported that the expression levels of IL-2 and IL-16 gene transcripts were equal when comparing E. tenella-infected chickens with noninfected controls after primary infection with this parasite. Similarly, Cornelissen et al. (11) showed that the levels of IL-2 mRNA were unchanged following primary E. maxima infection compared with those in noninfected chickens. These differences from our results may arise from dissimilarities in the types of cytokine-producing cells investigated in the aforementioned studies, i.e., intestinal intraepithelial lymphocytes (IELs) (21) and cells from the duodenal, cecal, and jejunal mucosae (11). We used cells from intestinal CTs and PPs that form part of the larger gut-associated lymphoid tissues (GALT). Because avians, unlike mammals, do not possess encapsulated lymph nodes (17), primary Ag processing likely occurs in the mucosa-associated lymphoid tissue of the chicken CTs and PPs. Therefore, we speculate that the population of immunocompetent cells within the chicken GALT is functionally distinct from that ascribed to IELs and mucosal cell populations.
Eimeria parasites not only are host specific but also exhibit tissue tropism in the chicken gut, with E. tenella colonizing the ceca, E. maxima the jejunum, and E. acervulina the duodenum. Nevertheless, host immunity against these parasites is initiated as a local response in the lamina propria by lymphoid cells originating in the CTs and PPs (49). In this regard, the present findings showed that vaccination with Ag-loaded exosomes induced a stronger response in cells from the CTs and PPs than in spleen cells, which correlates with the protective role that these chicken lymphoid tissues play in resistance to Eimeria infection. Consequently, strategies for vaccination against avian coccidiosis should be based on stimulation of these gut-associated lymphoid organs. Because exosomes are capable of migrating to the intestinal mucosae and present Ags to immunocompetent cells (1), it seems possible that administered Ag-pulsed DC exosomes similarly interact with T and B lymphocytes within the gut CTs and PPs to stimulate cellular and humoral immunities.
The use of exosomes in chicken immunization against eimerian parasites could not, to date, compete on price with the use of either anticoccidial drugs or live parasite vaccines. Nevertheless, a single immunization of chickens with DC-derived exosomes induced protective immunity against E. tenella, E. maxima, and E. acervulina infection. Thereby, immunization with exosomes is effective against a combined infection with these eimerian species. Immunization with exosomes could be an alternative strategy to the conventional disease control methods in case the regulation of anticoccidial drugs is tightened. Vaccines consisting of exosomes will both preserve all the positive aspects of live parasite vaccines and avoid their inherent risks. In addition to activating both arms of the immune system, exosome vaccines will never be able to cause infection, because they lack live parasite. Furthermore, recent investigations have proved that exosomes are a useful tool in the development of vaccines against other pathogens (1, 4, 10, 38). Therefore, one may assume that development of polyvalent vaccines consisting of exosomes loaded with Ags from a variety of pathogens such as viruses, bacteria, and parasites may be feasible.
ACKNOWLEDGMENT
This work was supported in part by grant A46 from the Research Council of Aragón, Spain.
Footnotes
Published ahead of print 21 February 2012
REFERENCES
- 1. Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. 2004. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 72:4127–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Natoura MQ, Suleimana MM, Abo-Shehadab MN. 2002. Flock-level prevalence of Eimeria species among broiler chicks in northern Jordan. Prev. Vet. Med. 53:305–310 [DOI] [PubMed] [Google Scholar]
- 3. Augustine PC. 2001. Cell:sporozoite interactions and invasion by apicomplexan parasites of the genus Eimeria. Int. J. Parasitol. 31:1–8 [DOI] [PubMed] [Google Scholar]
- 4. Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. 2007. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes Infect. 9:1614–1622 [DOI] [PubMed] [Google Scholar]
- 5. Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. 2007. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110:3234–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bozza S, et al. 2004. Dendritic cell-based vaccination against opportunistic fungi. Vaccine 22:857–864 [DOI] [PubMed] [Google Scholar]
- 7. Breed DGJ, et al. 1999. Vaccination against Eimeria tenella infection using a fraction of E. tenella sporozoites selected by the capacity to activate T cells. Int. J. Parasitol. 29:1231–1240 [DOI] [PubMed] [Google Scholar]
- 8. Carrión J, Folgueira C, Alonso C. 2008. Immunization strategies against visceral leishmaniosis with the nucleosomal histones of Leishmania infantum encoded in DNA vaccine or pulsed in dendritic cells. Vaccine 26:2537–2544 [DOI] [PubMed] [Google Scholar]
- 9. Chapman HD, Roberts B, Shirley MW, Williams RB. 2005. Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chicken and turkeys. Avian Pathol. 34:279–290 [DOI] [PubMed] [Google Scholar]
- 10. Colino J, Snapper CM. 2006. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J. Immunol. 177:3757–3762 [DOI] [PubMed] [Google Scholar]
- 11. Cornelissen JBWJ, Swinkels WJC, Boersma WA, Rebel JMJ. 2009. Host response to simultaneous infections with Eimeria acervulina, maxima and tenella: a cumulation of single responses. Vet. Parasitol. 162:58–66 [DOI] [PubMed] [Google Scholar]
- 12. Dalloul RA, Lillehoj HS. 2006. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev. Vaccines 5:143–163 [DOI] [PubMed] [Google Scholar]
- 13. De Bie JJ, et al. 2002. Exogenous interleukin-16 inhibits antigen-induced airway hyper-reactivity, eosinophilia and Th2-type cytokine production in mice. Clin. Exp. Allergy 32:1651–1658 [DOI] [PubMed] [Google Scholar]
- 14. del Cacho E, et al. 2011. Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine 29:3818–3825 [DOI] [PubMed] [Google Scholar]
- 15. del Cacho E, Gallego M, Lillehoj HS, López-Bernard F, Sánchez-Acedo C. 2009. Avian follicular and interdigitating dendritic cells: isolation and morphologic, phenotypic, and functional analyses. Vet. Immunol. Immunopathol. 129:66–75 [DOI] [PubMed] [Google Scholar]
- 16. del Cacho E, Gallego M, López-Bernard F, Sánchez-Acedo C, Lillehoj HS. 2008. Isolation of chicken follicular dendritic cells. J. Immunol. Methods 334:59–69 [DOI] [PubMed] [Google Scholar]
- 17. del Cacho E, Gallego M, Sanz A, Zapata A. 1993. Characterization of distal lymphoid nodules in the chicken caecum. Anat. Rec. 237:512–517 [DOI] [PubMed] [Google Scholar]
- 18. Delcayre A, Le Pecq JB. 2006. Exosomes as novel therapeutic nanodevices. Curr. Opin. Mol. Ther. 8:31–38 [PubMed] [Google Scholar]
- 19. Ehigiator HN, McNair N, Mead JR. 2007. Cryptosporidium parvum: the contribution of Th1-inducing pathways to the resolution of infection in mice. Exp. Parasitol. 115:107–113 [DOI] [PubMed] [Google Scholar]
- 20. Garg R, Banerjee DP, Gupta SK. 1999. Immune responses in chickens against Eimeria tenella sporozoite antigen. Vet. Parasitol. 81:1–10 [DOI] [PubMed] [Google Scholar]
- 21. Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP. 2006. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 114:209–223 [DOI] [PubMed] [Google Scholar]
- 22. Johnson J, Reid WM. 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 28:30–36 [DOI] [PubMed] [Google Scholar]
- 23. Joyner LP, Norton CC. 1983. Eimeria mitis in mixed infections with E. acervulina and E. brunetti in the fowl. Parasitology 86:381–390 [DOI] [PubMed] [Google Scholar]
- 24. Lee SH, et al. 2007. Influence of Pediococcus based probiotic on coccidiosis in broiler chickens. Poult. Sci. 86:63–66 [DOI] [PubMed] [Google Scholar]
- 25. Lee SH, et al. 2011. Development and characterization of mouse monoclonal antibodies reactive with chicken CD80. Comp. Immunol. Microbiol. Infect. Dis. 34:273–279 [DOI] [PubMed] [Google Scholar]
- 26. Li XB, Zhang ZR, Schluesener HJ, Xu SQ. 2006. Role of exosomes in immune regulation. J. Cell. Mol. Med. 10:364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. López CB, Fernandez-Sesma A, Czelusniak SM, Schulman JL, Moran TM. 2000. A mouse model for immunization with ex vivo virus infected dendritic cells. Cell. Immunol. 206:107–115 [DOI] [PubMed] [Google Scholar]
- 28. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 29. Lu H, Zhong G. 1999. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live Chlamydia trachomatis infection. Infect. Immun. 67:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lynch EA, Heijens CAW, Horst NF, Center DM, Cruikshank WW. 2003. Cutting edge: IL-16/CD4 preferentially induces Th1 cell migration: requirement of CCR5. J. Immunol. 171:4965–4968 [DOI] [PubMed] [Google Scholar]
- 31. McDonald V. 2000. Host cell-mediated responses to infection with Cryptosporidium. Parasite Immunol. 22:597–604 [DOI] [PubMed] [Google Scholar]
- 32. Murk JL, Stoorvogel W, Kleijmeer MJ, Geuze HJ. 2002. The plasticity of multivesicular bodies and the regulation of antigen presentation. Semin. Cell Dev. Biol. 13:303–311 [DOI] [PubMed] [Google Scholar]
- 33. Ogedengbe JD, Hunter DB, Barta JR. 2011. Molecular identification of Eimeria species infecting market-age meat chickens in commercial flocks in Ontario. Vet. Parasitol. 178:350–354 [DOI] [PubMed] [Google Scholar]
- 34. Petry F, Jakobi V, Tessema TS. 2010. Host immune response to Cryptosporidium parvum infection. Exp. Parasitol. 126:304–309 [DOI] [PubMed] [Google Scholar]
- 35. Raether W, Hofmann J, Uphoff M, Eckert HS. 1995. In vitro cultivation of avian Eimeria species: Eimeria tenella, p 79–84 In Eckert J, Braun R, Shirley MW, Coudert P. (ed), Biotechnology. Guidelines on techniques in coccidiosis research. European Commission, Brussels, Belgium [Google Scholar]
- 36. Remer KA, Apetrei C, Schwarz T, Linden C, Moll H. 2007. Vaccination with plasmacytoid dendritic cells induces protection against infection with Leishmania major in mice. Eur. J. Immunol. 37:2463–2473 [DOI] [PubMed] [Google Scholar]
- 37. Rose ME. 1987. Immunity to Eimeria infections. Vet. Immunol. Immunopathol. 17:333–343 [DOI] [PubMed] [Google Scholar]
- 38. Schnitzer JK, Berzel S, Fajardo-Moser M, Remer KA, Moll H. 2010. Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine 28:5785–5793 [DOI] [PubMed] [Google Scholar]
- 39. Shirley MW. 1995. Eimeria and Isospora, p 4–7 In Eckert J, Braun R, Shirley MW, Coudert P. (ed), Biotechnology. Guidelines on techniques in coccidiosis research. European Commission, Brussels, Belgium [Google Scholar]
- 40. Shirley MW, Bedrnfk P. 1997. Live attenuated vaccines against avian coccidiosis: success with precocious and egg-adapted lines of Eimeria. Parasitol. Today 13:481–484 [DOI] [PubMed] [Google Scholar]
- 41. Simhadri VR, et al. 2008. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One 10:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steinman RM. 2007. Dendritic cells: understanding immunogenicity. Eur. J. Immunol. 37:S53–S60 [DOI] [PubMed] [Google Scholar]
- 43. Stumbles PA, et al. 1998. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 188:2019–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Talebi A, Mulcahy G. 2006. Eimeria tenella:B-cell epitope mapping following primary and secondary infections. Exp. Parasitol. 113:235–238 [DOI] [PubMed] [Google Scholar]
- 45. Tan A, De La Peña H, Seifalian AM. 2010. The application of exosomes as a nanoscale cancer vaccine. Int. J. Nanomed. 5:889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tessema TS, et al. 2009. Dynamics of gut mucosal and systemic Th1/Th2 cytokine responses in interferon-γ and interleukin-12p40 knock out mice during primary and challenge Cryptosporidium parvum infection. Immunobiology 214:454–466 [DOI] [PubMed] [Google Scholar]
- 47. Thery C, et al. 2002. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 3:1156–1162 [DOI] [PubMed] [Google Scholar]
- 48. Yun CH, Lillehoj HS, Choi KD. 2000. Chicken IFN-γ monoclonal antibodies and their application in enzyme-linked immunosorbent assay. Vet. Immunol. Immunopathol. 73:297–308 [DOI] [PubMed] [Google Scholar]
- 49. Yun CH, Lillehoj HS, Lillehoj EP. 2000. Intestinal Immune responses to coccidiosis. Dev. Comp. Immunol. 24:303–324 [DOI] [PubMed] [Google Scholar]