Abstract
Background:
Intracranial chondromas are rare benign neoplasms. We report a patient incidentally diagnosed with an intracranial chondroma during her second trimester.
Case Description:
A 22-year-old Caucasian was diagnosed with an incidental parafalcine lesion found during admission due to a motor vehicle accident. Prior to the admission, the patient did not present with any neurological symptom. Magnetic resonance spectroscopy (MRS) suggested this intracranial lesion to be benign. A decision was made to delay the tumor excision until after delivery. Special anesthesia considerations were made to maintain stable blood pressure and euvolemia during the Cesarean section. The patient underwent a successful gross total removal of the intracranial tumor two months postpartum without any post-operative deficit.
Conclusion:
This is the first case report of an intracranial parafalcine chondroma in pregnancy. This report highlights the disease course of this rare type of tumor during pregnancy. This case illustrates relevant aspects of the management of a neurologically asymptomatic patient with an incidentally discovered intracranial tumor of which MRS suggested a benign nature.
Keywords: Falx, intracranial chondroma, pregnancy, spectroscopy
INTRODUCTION
An intracranial chondroma (IC) is a benign cartilaginous tumor first reported by Hirschfeld in 1851.[9,26] They are extremely rare brain tumors, comprising of approximately 0.3-0.5% of primary cerebral tumors.[14,27,32] They usually arise from cartilage found in the basilar synchondroses the base of the skull and can also occur rarely from the convexity dura and falx.[12,26] To our knowledge, there has been only 24 cases of IC originating from the falx and dural convexity in the literature.[9,10,26,27] Interestingly, there are only two cases of IC presenting in pregnancy ever reported.[17,35]
We report a case of a parafalcine chondroma in a pregnant patient. The use of magnetic resonance spectroscopy (MRS) in this case provided a unique radiographic tool to optimize the timing of surgical resection.
CASE REPORT
A 22-year-old Caucasian female in her 24th week of pregnancy was evaluated for a mild headache after being in a motor vehicle accident as a restrained passenger. A non-contrast computed tomography (CT) scan was performed and revealed a hypodense lesion with hyperdense margins in the right frontal lobe [Figure 1a]. It had a predominantly cystic appearance. Magnetic resonance imaging (MRI) without gadolinium contrast showed a mass measuring 4.2 cm in greatest anterior-posterior × 3.2 cm in greatest transverse × 3.1 cm in cephalocaudal dimension, located in the right frontal lobe.(MRI with contrast was performed only after the delivery of the baby, see Figure 1b). No significant surrounding vasogenic edema was observed using T2-weighted and diffusion weighted imaging [Figure 1c and d]. MRS showed significant N-acetylaspartate (NAA) peak in the lesion along with a choline peak (Ch) at 1804 of amplitude and creatine (Cr) peaking at 1122 [Figure 2a]. The Ch/NAA ratio was 0.752. There was no marked elevation of lipid lactate peak, suggesting the lesion to be a low-grade neoplasm. Gadolinium enhanced MRI study was avoided during pregnancy due to risks to the fetus.[30]
Figure 1.
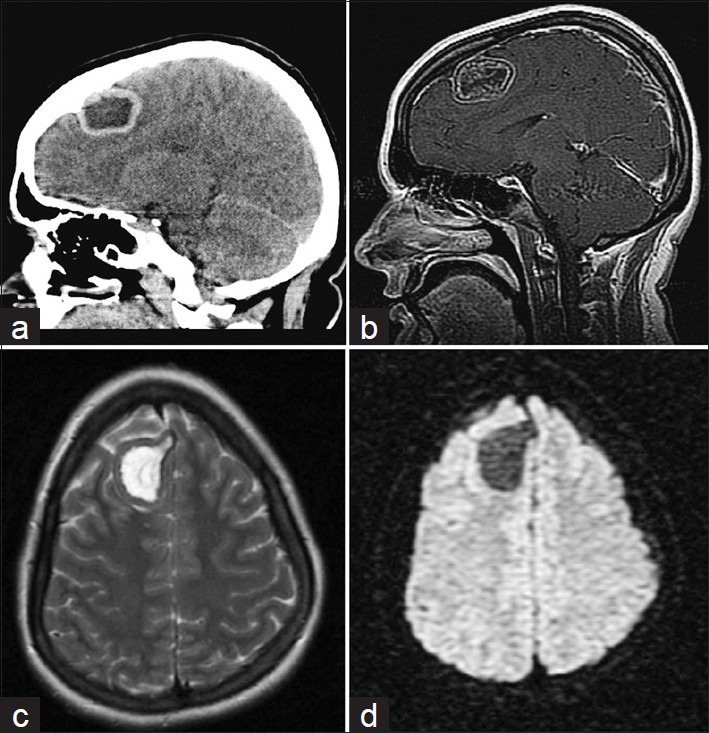
(a) Sagittal head CT without contrast demonstrating predominantly cystic lesion in the right frontal lobe at time of trauma. (b) Pre-operative sagittal T1-weighted MRI with gadolinium contrast. (c) Axial T2-weighted MRI showing minimal edema. (d) Axial Diffusion-weighted imaging also showing lack of vasogenic edema
Figure 2.
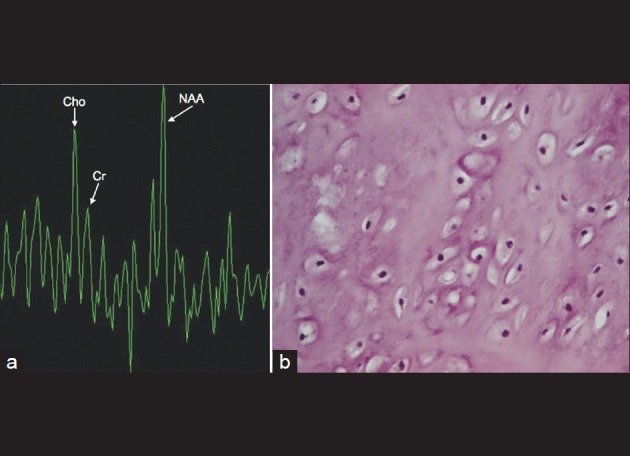
(a) MRS of the brain lesion demonstrating choline and creatinine peaks, and a significant NAA peak. (b) Histology of excised tumor, (H and E, ×400). The nuclei of the chondrocytes are small, have minimal atypia and no mitotic figures are seen
The patient's medical history was significant for two episodes of seizure at 4 and 15 years of age. No tumor was detected at that time as demonstrated by CT imaging used during the second episode. A discussion with the patient's obstetrician ensued. Given the fact that the patient's MRS suggested a benign nature of the brain lesions, and considering that she had no prior symptoms, and the T2 weighted images showed no edema, it was determined that there was no need for acute intervention. Stereotactic biopsy was considered as an option, but it was thought that the risk of intracranial hemorrhage outweighed the benefit of the invasive procedure in the otherwise asymptomatic patient. The patient, her obstetrician, and her primary care doctor were instructed to be aware of neurological symptoms, such as headache, dizziness, nausea, weakness, and numbness. Neurosurgical follow-up was offered to the patient, but she wished to continue the rest of her monitoring with her obstetrician and primary care doctor.
The baby was carried to full term and delivered via Cesarean section under spinal anesthesia. Epidural anesthesia was an optimal choice because it allowed the maintenance of stable blood pressure and neurological assessment of the patient during the section.[3,7] The choice of Cesarean Section was partly due to her previous history of having had the same procedure. Also, since brain surgery may induce labor, Cesarean section could be performed at any time with low morbidity/mortality and low blood loss in the case of worsening neurological status indicating progressing of the intracranial mass.[19] General endotracheal anesthesia was readily available. The patient was admitted prepartum on the morning of her Cesarean section. A lower transverse Cesarean section was performed under special instructions to the anesthesia service for careful monitoring of her blood pressure during the procedure. The patient was given adequate fluids to ensure that she was euvolemic. Otherwise, the Cesarean section was uncomplicated. If the patient were to deteriorate neurologically, the plan would be to place the patient on dexamethasone to reduce the underlying edema, perform emergent CT or MRI, and bring the patient to the operating room for tumor resection. Postpartum management of the patient involved regular checks with her obstetrician and primary care physician, who were educated on signs and symptoms of tumor progression in this case.
Two months after the patient delivered her baby and recovered from the Cesarean section, she underwent a right frontal craniotomy with the use of Neuronavigation to remove the tumor. The patient was placed in a supine position with her head rotated to the left. She was typed and crossed with blood available on call. The patient was given 50 g of mannitol and 10 mg of dexamethasone. The anesthesia service was instructed to maintain the patient's pCo2 at approximately 30 mmHg. The gross tumor was dissected from the falx. Parts of the falx that were adherent to the tumor were resected. The dura overlying the tumor was also resected. Immediately following the surgery, the patient was kept on dexamethasone and was assessed neurologically every hour.
The intra-op fresh frozen sample of the brain lesion demonstrated cartilaginous tissue, which was later determined to be a parafalcine chondroma [Figure 2b]. No immunohistochemistry was performed as the cartilaginous nature of the tumor provided the definite pathological diagnosis. Post-surgical MRI with gadolinium was performed every 6 months and there was no evidence of any recurrence or residual of the neoplasm up until 1.5 years after surgical resection of the tumor. [Figure 3] The patient had no neurological deficits post-resection.
Figure 3.
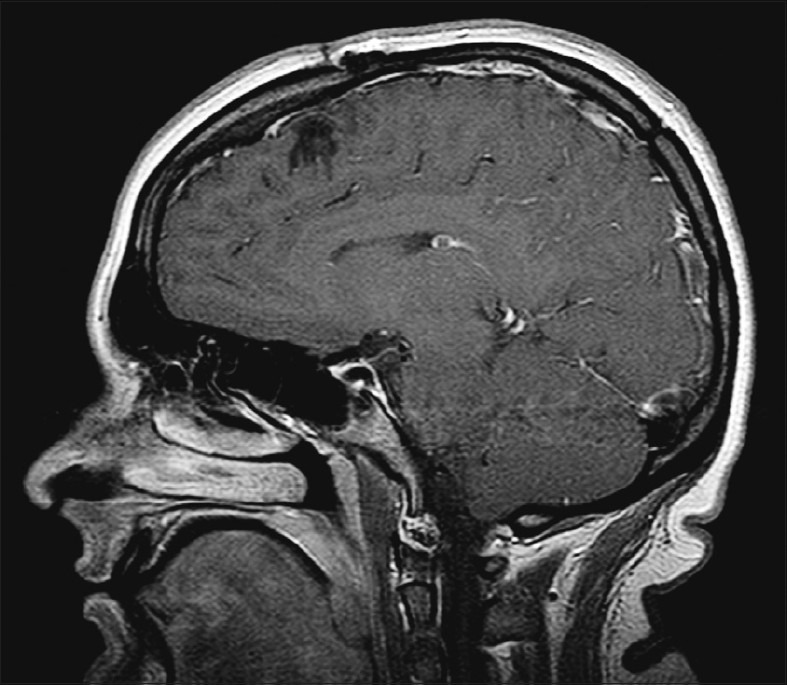
Post-operative (1.5 year) sagittal T1-weighted MRI with gadolinium contrast
DISCUSSION
Intracranial chondromas (ICs) are rare, benign lesions of which the pathogenesis is still unknown. It is theorized that chondromas may arise from heterotopic chondrocytes, metaplastic meningeal fibroblasts, perivascular mesenchymal tissue, or aberrant activation of fibroblasts as a result of trauma.[1,2,5,8,11,29] Bruner and Tien also suggested possible development of falcine chondromas from abnormal multipotential mesenchymal dural cells or their differentiated cellular descendants.[6] They can occur as part of Ollier disease (multiple enchondromatosis) or Mafucci Syndrome (multiple enchondromatosis with hemangiomas).[31]
ICs have been documented in patients from the age of 15 months to 60 years.[9,12,23] To the authors’ knowledge, there is no account of the natural history of intracranial falcine chondromas in the absence of treatment and no description of their evolution especially with MRI follow-up. Chondromas have an indolent growth pattern such that they may have long clinically silent periods.[15,16,24,35] The general malignant potential of an intracranial chondroma is still unclear. However, it is known that chondromas that originate as part of Mafucci Syndrome have a potential for malignant transformation into chondrosarcomas.[20] Immunohistochemistry that was performed on such tumors as in the present case, were positive for S-100 and vimentin.[36] Hematoxylin and eosin staining should also show calcium deposits.[36]
There have only been two reported cases of ICs involving pregnant patients. Wu and Lapi first described in 1970 a patient with fainting spells, increasingly severe speech difficulty, and weakness in her right arm in the third trimester.[35] A second case reported by Honan and Shieff in 1987 reported a patient with multiple chondromatosis presenting with rapidly evolving cranial nerve palsies after 5 weeks of amenorrhea.[17] The pregnancy was terminated and a craniotomy was performed to reveal a benign IC with cellular enlargement and a swollen matrix. Sex hormones released during pregnancy, especially progesterone, have been implicated in the worsening of neurological systems from intracranial neoplasms.[21] This is especially true in meningiomas, in which approximately 80% of cases stained positive for progesterone receptors.[4,18] Although the presence of sex hormone receptors in chondromas has not been tested, this provides a possible mechanism by which pregnancy can accelerate the growth of an intracranial tumor. Some have theorized that water retention, increased fluid retention within the tumor, and increased intracellular fluid within the tumor cells, leading to overall tumor enlargement, may be correlated with symptomatic presentations during the late stages of pregnancy.[25,34]
Our patient was asymptomatic with no neurological deficits at presentation. This was a key determinant in this patient's management when the benefits of tumor removal and the risks of surgery during pregnancy were weighed. Prior to the MRS, the radiographic characteristics of the tumor and the indolent clinical presentation suggested a benign process. The most likely differential diagnoses at that point were meningioma, hemangiopericytoma, or old calcified hematoma. Other possibilities included oligodendroglioma, glioblastoma multiforme, hemangioblastoma, chondrosarcoma, vascular malformation, cerebral abscess, and fungal infection. The lack of neurological symptoms favored conservative management to avoid surgical risks during pregnancy due to elevated blood pressure and the increased rate of intracranial hemorrhage.[33]
Poptani et al., showed that pyogenic abscesses display strong resonances for lactate, alanine, and acetate with absence of NAA, Choline (Cho), and creatine (Cr) peaks.[28] Meningioma should have a distinctive elevation in the resonance peak for alanine, which was not the case for this patient.[22] The presence of a significant NAA peak in the current lesion is evidence against the diagnosis of a meningioma or an abscess. The ratio of Cho/NAA ratio was more suggestive of low-grade growth. Furthermore, lipid elevation at 1.3 ppm, characteristic of glioblastoma multiforme and metastasis, was absent in this case.[28] A recent small-sized study suggests that MRS can differentiate chondroma from other rare intracranial neoplasms.[13]
In this case the combination of 3 features supported our decision to delay surgical intervention until after delivery: 1) the lack of neurological symptoms, 2) well-circumscribed finding on MRI, and 3) an MRS study that suggested a benign process. Stereotactic biopsy under local anesthesia was considered and discussed with the patient. Given the benign nature of the tumor as suggested by MRS, it was thought that the risk of intracranial hemorrhage in an otherwise neurologically asymptomatic patient outweighed the benefits obtained from an intracranial biopsy.
The patient was followed by her obstetrician for the rest of her pregnancy as a normal pregnancy. She was neurologically asymptomatic and, therefore, no interval imaging was performed during the rest of her pregnancy. The patient underwent a successful gross total removal of the intracranial tumor two months postpartum. MRS was key in providing guidance in the management of the intracranial neoplasm in this pregnant patient. The clinical course of intracranial parafalcine chondroma in this case allowed enough time for a complete course of pregnancy to occur.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/44/94930
Contributor Information
Jacky T. Yeung, Email: yeungtao@msu.edu.
Terry S. Krznarich, Email: Dr.Krznarich@hurleymc.com.
Edilberto A. Moreno, Email: emoreno1@hurleymc.com.
AppaRao Mukkamala, Email: AMukkam1@hurleymc.com.
Aftab S. Karim, Email: aftabskarim@yahoo.com.
REFERENCES
- 1.Acampora S, Troisi F, Fusco G, DelGaizo S. Voluminous intracranial chondroma. Surg Neurol. 1982;18:254–7. doi: 10.1016/0090-3019(82)90335-4. [DOI] [PubMed] [Google Scholar]
- 2.Ahyai A, Sporri O. Intracerebral chondroma. Surg Neurol. 1979;11:431–3. [PubMed] [Google Scholar]
- 3.Atanassoff PG, Alon E, Weiss BM, Lauper U. Spinal anaesthesia for caesarean section in a patient with brain neoplasm. Can J Anaesth. 1994;41:163–4. doi: 10.1007/BF03009818. [DOI] [PubMed] [Google Scholar]
- 4.Blaauw G, Blankenstein MA, Lamberts SW. Sex steroid receptors in human meningiomas. Acta Neurochir (Wien) 1986;79:42–7. doi: 10.1007/BF01403464. [DOI] [PubMed] [Google Scholar]
- 5.Berkmen YM, Blatt ES. Cranial and intracranial cartilaginous tumours. Clin Radiol. 1968;19:327–33. doi: 10.1016/s0009-9260(68)80019-4. [DOI] [PubMed] [Google Scholar]
- 6.Bruner JM, Tien RD. Secondary tumors. In: Bigner DD, McLendon RE, Bigner DD, editors. Russell and Rubinstein's: Pathology of tumors of the nervous system. 6th ed. Vol. 2. Baltimore: Williams and Wilkins Publishers; 1989. p. 420. [Google Scholar]
- 7.Chang L, Looi-Lyons L, Bartosik L, Tindal S. Anesthesia for cesarean section in two patients with brain tumours. Can J Anaesth. 1999;46:61–5. doi: 10.1007/BF03012517. [DOI] [PubMed] [Google Scholar]
- 8.Chorobski J, Jarzymski J, Ferens E. Intracranial solitary chondroma. Surg Gynecol Obstet. 1939;68:677–86. [Google Scholar]
- 9.Colpan E, Attar A, Erekul S, Arasil E. Convexity dural chondroma: A case report and review of the literature. J Clin Neurosci. 2003;10:106–8. doi: 10.1016/s0967-5868(02)00281-3. [DOI] [PubMed] [Google Scholar]
- 10.Delgado-López PD, Martín-Velasco V, Galacho-Harriero AM, Castilla-Díez JM, Rodríguez-Salazar A, Echevarría-Iturbe C. Large chondroma of the dural convexity in a patient with Noonan's syndrome.Case report and review of the literature. Neurocirugia (Astur) 2007;18:241–6. [PubMed] [Google Scholar]
- 11.Dutton J. Intracranial solitary chondroma. J Neurosurg. 1978;49:460–3. doi: 10.3171/jns.1978.49.3.0460. [DOI] [PubMed] [Google Scholar]
- 12.Erdogan S, Zorludemir S, Erman T, Akgul E, Ergin M, Ildan F, et al. Chondromas of the falx cerebri and dural convexity: Report of two cases and review of the literature. J Neurooncol. 2006;80:21–5. doi: 10.1007/s11060-005-9082-0. [DOI] [PubMed] [Google Scholar]
- 13.Fortuniak J, Jaskólski DJ, Stefańczyk L, Zawirski M, Gajewicz W. Magnetic resonance imaging of rare intracranial neoplasms--role of the in vivo 1h spectroscopy in the radiological differential diagnostics. Cen Eur Neurosurg. 2010;71:181–8. doi: 10.1055/s-0030-1261947. [DOI] [PubMed] [Google Scholar]
- 14.Fountas K, Stamatiou S, Sotiris B, Kourtopoulos H. Intracranial falx chondroma: Literature review and a case report. Clin Neurol Neurosurg. 2008;110:8–13. doi: 10.1016/j.clineuro.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Hadadian K, Abtahii H, Asil ZT, Rakhshan M, Vessal P. Cystic falcine chondroma: Case report and review of the literature. Neurosurgery. 1991;29:909–12. [PubMed] [Google Scholar]
- 16.Hirvonen J, Heikinheimo H. A case of intracerebral chondroma.A case report. Acta Pathol Microbiol Scand. 1969;76:19–24. doi: 10.1111/j.1699-0463.1969.tb03228.x. [DOI] [PubMed] [Google Scholar]
- 17.Honan WH, Shieff C. Skull base chondroma presenting in pregnancy. J Neurol Neurosurg Psychiatry. 1987;50:1078–9. doi: 10.1136/jnnp.50.8.1078-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu DW, Efırd JT, Hedley-Whyte ET. Progesterone and estrogen receptors in meningiomas: Prognostic considerations. Neurosurgery. 1997;86:113–20. doi: 10.3171/jns.1997.86.1.0113. [DOI] [PubMed] [Google Scholar]
- 19.Isla A, Alvarez F, Gonzalez A, García-Grande A, Perez-Alvarez M, García-Blazquez M. Brain tumor and pregnancy. Obstet Gynecol. 1997;89:19–23. doi: 10.1016/s0029-7844(96)00381-x. [DOI] [PubMed] [Google Scholar]
- 20.Kasper EM, Hess PE, Silasi M, Lim K, Gray J, Reddy H, et al. A pregnant female with a large intracranial mass: Reviewing the evidence to obtain management guidelines for intracranial meningiomas during pregnancy. Surg Neurol Int. 2010;1:95. doi: 10.4103/2152-7806.74242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis RJ, Ketcham AS. Mafucci's syndrome: Functional and neoplastic significance. Case report and review of the literature. J Bone Joint Surg. 1973;55:1465–79. [PubMed] [Google Scholar]
- 22.Majós C, Alonso J, Aguilera C, Serrallonga M, Pérez-Martín J, Acebes JJ, et al. Proton magnetic resonance spectroscopy ((1)H MRS) of human brain tumours: Assessment of differences between tumour types and its applicability in brain tumour categorization. Eur Radiol. 2003;13:582–91. doi: 10.1007/s00330-002-1547-3. [DOI] [PubMed] [Google Scholar]
- 23.Mapstone TB, Wongmongkolrit T, Roessman U, Ratcheson RA. Intradural chondroma: A case report and review of the literature. Neurosurgery. 1983;12:111–4. doi: 10.1227/00006123-198301000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Matz S, Israeli Y, Shalit MN, Cohen ML. Computed tomography in intracranial supratentorial osteochondroma. J Comput Assist Tomogr. 1981;5:109–15. doi: 10.1097/00004728-198102000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Michelsen JJ, New PF. Brain tumour and pregnancy. J Neurol Neurosurg Psychiatry. 1969;32:305–7. doi: 10.1136/jnnp.32.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama M, Nagayama T, Hirano H, Oyoshi T, Kuratsu J. Giant chondroma arising from the dura mater of the convexity.Case report and review of the literature. J Neurosurg. 2001;94:331–4. doi: 10.3171/jns.2001.94.2.0331. [DOI] [PubMed] [Google Scholar]
- 27.Patel A, Munthali L, Bodi I. Giant cystic intracranial chondroma of the falx with review of literature. Neuropathology. 2009;29:315–7. doi: 10.1111/j.1440-1789.2008.00957.x. [DOI] [PubMed] [Google Scholar]
- 28.Poptani H, Gupta RK, Roy R, Pandey R, Jain VK, Chhabra DK. Characterization of intracranial mass lesions with in vivo proton MR spectroscopy. AJNR Am J Neuroradiol. 1995;16:1593–603. [PMC free article] [PubMed] [Google Scholar]
- 29.Russell DS. Meningeal tumours: A review. J Clin Pathol. 1950;3:191–211. doi: 10.1136/jcp.3.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundgren PC, Leander P. Is administration of gadolinium-based contrast media to pregnant women and small children justified? J Magn Reson Imaging. 2011;34:750–7. doi: 10.1002/jmri.22413. [DOI] [PubMed] [Google Scholar]
- 31.Traflet RF, Babaria AR, Barolat G, Doan HT, Gonzalez C, Mishkin MM. Intracranial chondroma in a patient with Ollier's disease. Case report. J Neurosurg. 1989;70:274–6. doi: 10.3171/jns.1989.70.2.0274. [DOI] [PubMed] [Google Scholar]
- 32.Ustün MO, Paksoy N, Kilicarslan B. Cystic chondroma arising from the falx cerebri: A case study with review of literature. Clin Neuropathol. 1997;16:27–9. [PubMed] [Google Scholar]
- 33.Vougioukas VI, Kyroussis G, Gläsker S, Tatagiba M, Scheufler KM. Neurosurgical interventions during pregnancy and the puerperium: Clinical considerations and management. Acta Neurochir (Wien) 2004;146:1287–91. doi: 10.1007/s00701-004-0354-9. [DOI] [PubMed] [Google Scholar]
- 34.Weyand RD, MacCarty CS, Wilson RB. The effect of pregnancy on intracranial meningiomas occurring about the optic chiasm. Surg Clin North Am. 1951;31:1225–33. doi: 10.1016/s0039-6109(16)33403-x. [DOI] [PubMed] [Google Scholar]
- 35.Wu WQ, Lapi A. Primary non-skeletal intracranial cartilaginous neoplasms: Report of a chondroma and a mesenchymal chondrosarcoma. J Neurol Neurosurg Psychiatry. 1970;33:469–75. doi: 10.1136/jnnp.33.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xin Y, Hao S, Zhang J, Wu Z, Jia G, Tang J, et al. Microsurgical treatment of intracranial chondroma. J Clin Neurosci. 2011;18:1064–71. doi: 10.1016/j.jocn.2010.12.028. [DOI] [PubMed] [Google Scholar]


