Abstract
Background:
Virchow's triad in cardiovascular disease comprises blood viscosity, plasma D-dimer and homocysteine as indices of three associated but separate vascular phenomena.
Aims:
This work investigates prevalence of hyperviscosity in hyperhomocysteinaemia and positive D-dimer; and differences or similarities in stasis status among sub-populations of hyperhomocysteinaemia vs. normohomocysteinaemia and negative vs. positive D-dimer.
Patients and Methods:
10-years de-identified archived clinical pathology data for the period of January 1999 to December 2008 were audited. All cases tested for D-dimer (n=6845) and homocysteine (n=1665), which were concomitantly tested for haematocrit and total proteins, were extracted.
Results:
The results show a very low prevalence of hyperviscosity associated with a positive D-dimer sub-population (1.48%), which is not statistically different in comparison with the negative D-dimer sub-population. The prevalence of hyperviscosity associated with hyperhomocysteinaemia (5.04%) was statistically significantly higher in comparison to the normohomocysteinaemia sub-population (p = 0.05). The prevalence of low viscosity is significantly higher in the positive D-dimer sub-population relative to the negative D-dimer sub-population (p < 0.00001), but not different between hyperhomocysteinaemia vs. normohomocysteinaemia. Normoviscosity is statistically significantly commoner in normoviscosity relative to hyper-homocysteinaemia as well as in negative compared with positive D-dimer (p < 0.00001).
Conclusion:
The findings reported here suggest putting into perspective the specificity of whole blood viscosity relative to stasis, not necessarily sensitivity to disease conditions where it is implicated.
Keywords: Cardiovascular complications, clinical laboratory evaluation, D-dimer, homocysteine, stasis, Virchow's triad, whole blood viscosity
Introduction
Whole blood viscosity (WBV) is one of Virchow's triad, which has been an established concept of three phenomena that ultimately lead to, and/or result from cardiovascular complications[1,2]. The other two phenomena are atherothrombosis and endothelial dysfunction[3–5]. Each phenomenon represents a subclinical vascular process, which in turn is indicated by a pathology index and clinical features. Specifically, D-dimer, homocysteine and WBV are indices for atherothrombosis, endothelial dysfunction and stasis respectively.
Several factors militate against utility of WBV as a laboratory tool, of which one is availability. With arithmetic and extrapolation method[6,7], this may no longer be a problem. There are also issues of sensitivity and specificity. While it is not in dispute that WBV is an indicator for flow rate or stasis, there is still a bit of argument and doubt whether Virchow's triad is useful[8].
Thus, the main question for this, and the next issue on this series, is whether increased WBV vis-à-vis hyperviscosity is significantly prevalent in any other cardiovascular risk condition that has another specific laboratory index. That is, the issue that needs to be addressed is whether WBV is unduly sensitive to other components of Virchow's triad to warrant arguing its specificity and querying its usefulness.
In the physiological scheme of feedback and feed forward responses, atherothrombosis and endothelial dysfunction can arise from a increased blood viscosity (Fig. 1)[9,10]. Hyperhomocysteinaemia potentially increases oxidative stress[11,12], which in turn increases blood viscosity[13]. Homocysteine could also induce enhanced cross-linking of the fibrin network and viscosity of the fluid phase, which is thrombogenesis and blood viscosity respectively[14,15]. It is noteworthy that clinical utility of Batroxobin includes as a thrombolytic agent, of which one of the mechanisms of action is by reduction of blood viscosity and fibrinogen levels[16]. Indeed, haemostatic, oxidative stress and rheological variables are associated with cardiovascular diseases[17].
Fig. 1.
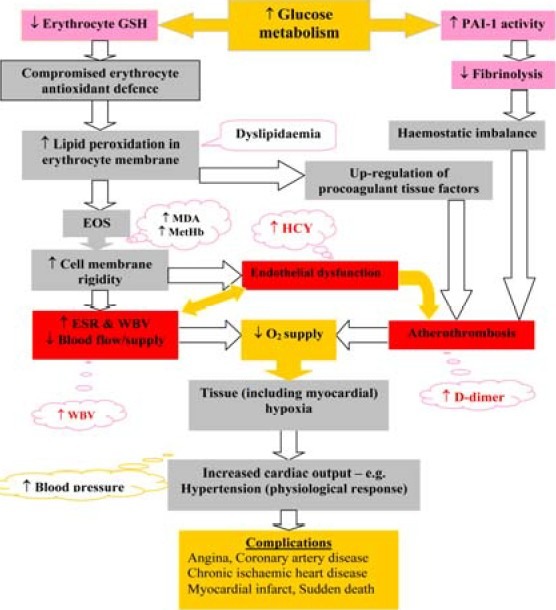
Illustration of multiple factors that have capacity to impact on whole blood viscosity. Keys: EOS = erythrocyte oxidative stress, HCY = homocysteine, PAI-1 = plasminogen activator inhibitor-1, WBV = whole blood viscosity, ↓ = decreased level, ↑ = increased level; red boxes = Virchow's triad components, grey boxes = other processes, light orange boxes & call out = pathophysiological effects, pink bars & call outs = key laboratory indicators
Atherothrombosis, endothelial dysfunction and oxidative stress are separately indicated by plasma D-dimer, homocysteine and erythrocyte oxidative stress (EOS) indices respectively. The prevalence of positive EOS indices, especially reduced glutathione (GSH), has been determined to be highly prevalent in high WBV. The suggestion was to re-define the criteria for use of WBV on the basis of sensitivity to underlying oxidative stress, instead of specificity to a disease condition.
Considering the fact that more sensitive tests are often less specific and vice versa[18–20], it will be interesting to determine whether WBV is unduly highly sensitive and less specific. The hypothesis here is that if WBV is highly sensitive and less specific, hyperviscosity would be highly prevelant in cases that present positive for D-dimer and hyperhomocysteinaemia compared to those that present with negative results.
The objective of this work is to investigate the prevalence of (i) hyperviscosity in hyperhomocysteinaemia or positive D-dimer and (ii) whether hyperviscosity is significantly more prevalent in hyperhomocysteinaemia or positive D-dimer when compared to the prevalence in normohomocysteinaemia or negative D-dimer. The findings from this study will lend credence to whether WBV test result showing increased level should be considered in the light of ‘multifactorial’ and discountenanced, or a clinically specific complication worth managing.
Patients and Methods
This work was part of Translational Biomedical Science Research initiative of the author. It was supported materially by the Albury South West Pathology – a unit of Western Pathology Cluster of NSW Health Australia. The Ethics Committee of the Area Health Service granted request through the Operations Manager for the use of de-identified data.
Ten years de-identified archived clinical pathology data for the period of January 1999 to December 2008 were audited. All cases tested for D-dimer (n=6845) and homocysteine (n=1665), which were concomitantly tested from one phlebotomy collection point for haematocrit and total proteins, were extracted. In the ten years period, only eight cases were concomitantly tested for D-dimer, homocysteine, haematocrit and total proteins. The prevalence of hyperviscosity associated with positive result of homocysteine and plasma D-dimer were evaluated. Prevalence of low and normal viscosity was also noted.
Homocysteine was measured by quantitative analysis and reported in mmol/L. Normal values are ≤15mmol/L and any result >15mmol/L is considered to indicate probable hyperhomocysteinaemia. In this study, the data (n=1665) were sorted by homocysteine results and dichotomized into homocysteine negative or positive as representative of normohomocysteinaemia or hyperhomocysteinaemia respectively, using 15mmol/L as cut off point. Plasma D-dimer tests were performed by either qualitative or semi-quantitative methods. The qualitative tests were resulted as negative or positive; and dichotomized in this study as such. Semi quantitative D-dimer tests were reported by indicating the values obtained and the normal reference levels (<0.2, <0.3, or <0.5mg/L in pregnancy) in bracket. In this study, the data were also separately sorted by D-dimer results and dichotomized into negative or positive using the applicable (<0.2, <0.3, or <0.5mg/L in pregnancy) reference ranges.
To determine prevalence of high, low or normal viscosity, WBV at high shear stress was determined from haematocrit and total proteins as previously published[6]. Results of WBV were categorized into levels of ≤15.00, 15.01-19.01 and ≥19.02 within the continuum as indicative of low, normal and high WBV levels respectively. The prevalence of the different levels of WBV was determined in the dichotomized D-dimer and homocysteine sub-populations. Statistical analysis was performed by simple Student's t-test using Microsoft Excel tool as well as by analysis of variance (ANOVA) using S-Plus version 6.1.
Results
The demographics of the dichotomized sub-populations are presented in table (Table 1).
Table 1.
Summary demographics of D-dimer and homocysteine data
In the 10-years period evaluated, an average of 1.48% prevalence of high WBV is observed among people who tested positive for plasma D-dimer (Table 2). Analysis of variance (ANOVA) shows statistically significant differences between categories of WBV in the cumulative D-dimer negative sub-population (p < 0.00001). No statistically significant difference was observed in the cumulative D-dimer positive sub-population, though a critical evaluation shows a very significantly lowest prevalence of high WBV.
Table 2.
Prevalence (%) of different levels of blood viscosity associated with negative and positive plasma D-dimer results
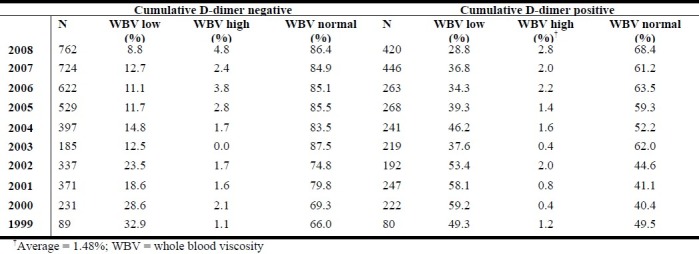
The result further demonstrates the following
Normal blood viscosity is more prevalent in the subgroup with negative plasma D-dimer results compared to those where deep vein thrombosis or pulmonary embolism is indicated (p < 0.00001)
Hyperviscosity indicated by high WBV level is not more prevalent in normal results compared to where deep vein thrombosis or pulmonary embolism is indicated (p > 0.16).
Instead, prevalence of low WBV level is observed to be more where deep vein thrombosis or pulmonary embolism is indicated compared to where plasma D-dimer results is negative (p < 0.00001).
In the same 10-years period, an average of 5.04% prevalence of high WBV was observed among people who tested positive for hyperhomocysteinaemia (Table 3)
Table 3.
Prevalence (%) of different levels of blood viscosity associated with negative and positive homocysteine results
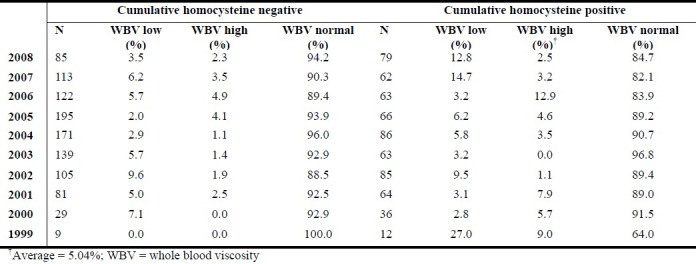
Analysis of variance (ANOVA) shows statistically significant difference between categories of WBV in both the cumulative normohomocysteinaemia and hyperhomocysteinaemia sub-populations (p < 0.00001). The results further demonstrate normal blood viscosity is more prevalent in the subgroup with normohomocysteinaemia results compared to those where endothelial dysfunction is indicated (p < 0.04). Further, while hyperviscosity indicated by high WBV level is considerably less prevalent in normohomocysteinaemia (p = 0.05), prevalence of low WBV level is observed to be not statistically significantly different in normohomocysteinaemia compared to the hyperhomocysteinaemia sub-population.
The distribution of results of the eight cases that has all tests under evaluation is presented (Fig. 2). It is remarkable that any of the cases that were positive for D-dimer and/or homocysteine did not present with hyperviscosity. This affirms the observations of low prevalence of hyperviscosity, which is an indication that WBV is not highly sensitive to endothelial dysfunction or thrombus formation dynamics.
Fig. 2.
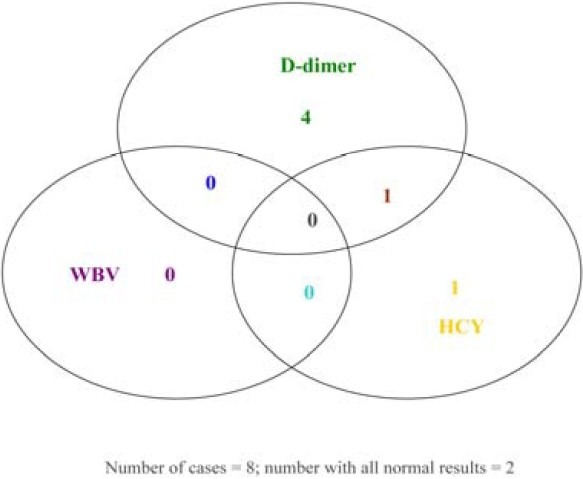
Vein diagram showing abnormal presentations among the cases (n = 8) that were concomitantly tested for D-dimer, homocysteine, haematocrit and total proteins – 6 of 8 were positive for D-dimer and/or homocysteine; 2 of 8 presented normal results in all the tests; none is positive for WBV.
Discussion
The results from this study demonstrate a low prevalence of hyperviscosity among people who have laboratory evidence of possible coagulation/fibrinolysis imbalance, deep vein thrombosis or pulmonary embolism, as indicated by positive plasma D-dimer results. However, the low prevalence of hyperviscosity is not statistically significantly different between those who tested negative compared to those who tested positive for plasma D-dimer. It is inferred that WBV is not unduly sensitive to its multifactorial influences. The result shows that hypoviscosity is more prevalent where plasma D-dimer result is negative. In current practice, negative plasma D-dimer result is interpreted as “not useful”. It is not interpreted to mean that the patient is clinically well. Therefore, the findings reported here is in consonance with current practice.
Nevertheless, plasma D-dimer test does not provide any information on stasis. Hence, a negative D-dimer result does not provide evidence base to continue or discontinue antiplatelet or thrombolytic therapy. The observation of hypoviscosity being more prevalent than hyperviscosity in both negative and positive D-dimer sub-populations implies that beside the majority who fall within the normal range, a considerable number of people do not have stasis requiring antiplatelet or thrombolytic preventive therapy. It further provides information on those individuals who surely have hyperviscosity syndrome and are at risk of developing cardiovascular complications, even though plasma D-dimer test is negative. The opinion here is that concomitant assessment of WBV with plasma D-dimer will compliment the latter to make sense out of an otherwise “not useful” clinical laboratory result.
Case review: In the course of discussing possible explanation to the observation that prevalence of low WBV level is greater where deep vein thrombosis or pulmonary embolism is indicated compared to where plasma D-dimer result is negative, an unpublished family story was told as follows:
A 14-year old Northern Nigerian boy, who was a sickle cell disease patient developed crisis just before bedtime. Usually, the boy is given a tablet of therapeutic aspirin whenever he develops such crises. He was only taken to the hospital if the crisis persisted. On this fateful evening, he was given the usual dose of aspirin and everybody in the family went to bed. By the next morning, the boy was found dead in his pool of blood in which he bled till death. It was not until the brother became a clinician that the family learnt a possible explanation to what happened to the boy[21].
Thrombosis is one of the complications and crises in sickle cell disease[22,23]. Antiplatelet (including aspirin) and anticoagulants are part of the preventive therapies for the management of such complications[24–26]. Paradoxically, the process of thrombus formation utilizes fibrin proteins in the blood as well as platelets[27]. It is known that thrombolytic agents such as batroxobin have the capacity to reduce WBV[16]. While the use up of fibrin proteins could feedforward to effect a reduction in WBV level[1,28,29], the use up of platelets implies that there may actually be no need for a medication that further reduces platelet levels. Thus, the boy may have died due to a manifest antiplatelet bleeding risk.
What this report highlights is that hyperviscosity syndrome or stasis is not always associated with deep vein thrombosis or pulmonary embolism. Therefore, laboratory determination of the level of stasis before antiplatelet and thrombolytic therapy would be good evidence-based clinical practice.
The issue is not exactly the same in homocysteinaemia. In this study, it has been observed that hyperviscosity is significantly infrequent in hyperhomocysteinaemia (approximately 5%), but it is statistically significantly commoner than in normohomocysteinaemia. Also unlike in D-dimer sub-populations, low WBV level is observed to be not statistically significantly different in normohomocysteinaemia relative to the hyperhomocysteinaemia. This is evidence of low sensitivity of WBV to closely related vascular pathology. By default, it is indication of specificity of the parameter. It suggests putting into perspective the specificity of WBV to assess stasis in, not necessarily sensitivity to, any disease condition where cardiovascular risk is implicated.
In current clinical practice, WBV is assessed mainly in the management of critical hyperproteinaemia, polycythemia and retinal occlusion. Considering the implication of stasis in chronic diseases and metabolic diseases, such usage is under-utility. Moreover, many pathology units in the Western world as well as most (if not all) in the developing and underdeveloped countries do not test for plasma D-dimer or homocysteine. This is either due to lack of the capacity to perform the test, or inability of the average population to pay for the service. However, virtually every clinical chemistry laboratory is able to test for serum total proteins as a discrete or selective liver function test. Also, virtually every hematology laboratory performs a routine full blood count that includes haematocrit.
The clinical practice implication or issue here is that while many clinicians/laboratories are unable to assess/test any of the Virchow's triad, there is now a method whereby WBV can be easily and readily derived for any patient who has results of haematocrit and total serum proteins[7], and extrapolation chart has been developed to further simplify the issue[6]. The interesting addition by the report is that the test is of low sensitivity to what it is not meant to assess. Therefore, a positive result can be regarded as highly specific for (stasis) cardiovascular risk event.
It has been difficult to establish whether abnormalities of endothelial dysfunction and thrombus formation affect the microcirculation, due to lack of comprehensive data[2]. Determination of the impact of clot formation on stasis syndrome has been suggested[30], but yet to be elusively addressed. This study utilizing a comprehensive data presents evidence that abnormality of microcirculation parameter is not very prevalent in abnormalities of endothelial dysfunction and thromboembolism. The report further presents evidence that the impact of clot formation on stasis syndrome could include hypoviscosity.
Conclusion
There is significantly low prevalence of hyperviscosity associated with hypercoagulation and hyperhomocysteinaemia. Over and above the desire for laboratory parameters that have both high sensitivity and high specificity, the findings suggest putting into perspective the specificity of WBV to stasis.
Acknowledgement
Receipt of an approval from HREC committee of the Greater Southern Area Health Service, NSW Health was obtained, and hereby gratefully appreciated. The operations manager of the South West Pathology Service and Nathan Cann are also appreciated for their contribution in data acquisition and collaboration in the research initiative. Dr Peter Kench and Simon Tawasu are appreciated for their valuable discussions.
References
- 1.Mammen EF. Pathogenesis of venous thrombosis. Chest. 1992;102:640S–644S. doi: 10.1378/chest.102.6_supplement.640s. [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, La Celle PL. Blood viscosity and thrombosis: clinical considerations. Prog Hemost Thromb. 1982;6:179–201. [PubMed] [Google Scholar]
- 3.Bagot CN, Arya R. Virchow's triad: a question of attribution. Br J Haematol. 2008;143:180–190. doi: 10.1111/j.1365-2141.2008.07323.x. [DOI] [PubMed] [Google Scholar]
- 4.Higgins C. Recurrence of venous thromboembolism. The Biomedical Scientist. 2006;50:865–867. [Google Scholar]
- 5.Lowe GD. Virchow's triad revisited: abnormal flow. Pathophysiol Haemost Thromb. 2003;33:455–457. doi: 10.1159/000083845. [DOI] [PubMed] [Google Scholar]
- 6.Nwose EU. Whole blood viscosity assessment issues I: Extrapolation chart and reference values. North Am J Med Sci. 2010;2:165–169. doi: 10.4297/najms.2010.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamariz LJ, Young JH, Pankow JS, Yeh HC, Schmidt MI, Astor B, Brancati FL. Blood viscosity and hematocrit as risk factors for type 2 diabetes mellitus: the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. 2008;168:1153–1160. doi: 10.1093/aje/kwn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malone PC, Agutter PS. Is ‘Virchow's triad’ useful? Br J Haematol. 2009;145:839. doi: 10.1111/j.1365-2141.2009.07685.x. [DOI] [PubMed] [Google Scholar]
- 9.Satti TM, Ullah K, Ahmed P, Raza S, Kamal MK, Chaudhry QU, Akhtar FM. Deep vein thrombosis--a rare post transplant complication. J Pak Med Assoc. 2007;57:515–516. [PubMed] [Google Scholar]
- 10.Nwose EU, Jelinek HF, Richards RS, Kerr PG. Erythrocyte oxidative stress in clinical management of diabetes and its cardiovascular complication. Br J Biomed Sci. 2007;64:35–43. doi: 10.1080/09674845.2007.11732754. [DOI] [PubMed] [Google Scholar]
- 11.Barghash NA, Elewa SM, Hamdi EA, Barghash AA, El Dine R. Role of plasma homocysteine and lipoprotein (a) in coronary artery disease. Br J Biomed Sci. 2004;61:78–83. doi: 10.1080/09674845.2004.11732648. [DOI] [PubMed] [Google Scholar]
- 12.Stehouwer CD, Jakobs C. Abnormalities of vascular function in hyperhomocysteinaemia: relationship to atherothrombotic disease. Eur J Pediatr. 1998;157:S107–S111. doi: 10.1007/pl00014293. [DOI] [PubMed] [Google Scholar]
- 13.Yang ZC, Xia K, Wang L, Jia SJ, Li D, Zhang Z, Deng S, Zhang XH, Deng HW, Li YJ. Asymmetric dimethylarginine reduced erythrocyte deformability in streptozotocin-induced diabetic rat. Microvasc Res. 2007;73:131–136. doi: 10.1016/j.mvr.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Rojas AM, Kordich L, Lauricella AM. Homocysteine modifies fibrin clot deformability: Another possible explanation of harm. Biorheology. 2009;46:379–387. doi: 10.3233/BIR-2009-0459. [DOI] [PubMed] [Google Scholar]
- 15.Edirisinghe SP. Homocysteine-induced thrombosis. Br J Biomed Sci. 2004;61:40–47. doi: 10.1080/09674845.2004.11732643. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Xu C. Portal vein thrombosis as the first sign of nephrotic syndrome. Nat Clin Pract Neph. 2008;4:342–345. doi: 10.1038/ncpneph0810. [DOI] [PubMed] [Google Scholar]
- 17.Lowe GD. Etiopathogenesis of cardiovascular disease: hemostasis, thrombosis, and vascular medicine. Ann Periodontol. 1998;3:121–126. doi: 10.1902/annals.1998.3.1.121. [DOI] [PubMed] [Google Scholar]
- 18.Anderson DR, Wells PS. D-dimer for the diagnosis of venous thromboembolism. Curr Opin Hematol. 2000;7:296–301. doi: 10.1097/00062752-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Karras DJ, Kane DL. Serum markers in the emergency department diagnosis of acute myocardial infarction. Emerg Med Clin North Am. 2001;19:321–337. doi: 10.1016/s0733-8627(05)70186-3. [DOI] [PubMed] [Google Scholar]
- 20.Palestro CJ, Love C. Nuclear medicine and diabetic foot infections. Semin Nucl Med. 2009;39:52–65. doi: 10.1053/j.semnuclmed.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Tawasu S. Personal Communication (ed) Australia: Nolan House Albury Base Hospital; 2010. A family story of 14-year old sickle cell disease boy who bled to death. [Google Scholar]
- 22.Ataga KI. Hypercoagulability and thrombotic complications in hemolytic anemias. Haematologica. 2009;94:1481–1484. doi: 10.3324/haematol.2009.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin H, Key NS, Benson JM, Lally C, Dowling NF, Whitsett C, Hooper WC. Sickle cell trait and the risk of venous thromboembolism amongblacks. Blood. 2007;110:908–912. doi: 10.1182/blood-2006-11-057604. [DOI] [PubMed] [Google Scholar]
- 24.Hathaway WE. Use of antiplatelet agents in pediatric hypercoagulable states. Am J Dis Child. 1984;138:301–304. doi: 10.1001/archpedi.1984.02140410079023. [DOI] [PubMed] [Google Scholar]
- 25.Mehta SH, Adams RJ. Treatment and prevention of stroke in children with sickle cell disease. Curr Treat Options Neurol. 2006;8:503–512. doi: 10.1007/s11940-006-0040-8. [DOI] [PubMed] [Google Scholar]
- 26.Peverill RE. Warfarin or aspirin: both or others? Med J Aust. 1999;171:321–326. doi: 10.5694/j.1326-5377.1999.tb123671.x. [DOI] [PubMed] [Google Scholar]
- 27.Foulon I, Bachir D, Galacteros F, Maclouf J. Increased in vivo production of thromboxane in patients with sickle cell disease is accompanied by an impairment of platelet functions to the thromboxane A2 agonist U46619. Arterioscler Thromb. 1993;13:421–426. doi: 10.1161/01.atv.13.3.421. [DOI] [PubMed] [Google Scholar]
- 28.Larcan A, Stoltz JF. Blood hyperviscosity syndromes. Classification and physiopathological understanding. Therapeutic deductions. Ann Med Interne (Paris) 1983;134:395–410. [PubMed] [Google Scholar]
- 29.Mori K, Hiratsuka I, Sakai H, Hiwatashi K, Takahashi T. Hemorrhagic diathesis in Weber-Christian disease. Tohoku J Exp Med. 1976;118:227–243. doi: 10.1620/tjem.118.suppl_227. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman JR. Warfarin prophylaxis after total knee arthroplasty. Am J Knee Surg. 1999;12:49–53. [PubMed] [Google Scholar]


