Abstract
Context:
Smoot muscle (SM) is a muscle tissue that contracts without conscious control, made up of spindle-shaped, untreated cells with single nuclei and found in the walls of the internal organs, such us the stomach, intestine, bladder, and blood vessels, excluding the heart and in the (arrector pili) muscle in the skin.
Case Report:
A 59 Caucasian woman was evaluated for pruritic rash and violaceous plaques mostly in the upper extremities with some with ulcers in her mouth. Biopsies for hematoxylin and eosin and immunohistochemistry examination, as well as for direct immunofluorescence analysis were performed. The hematoxylin and eosin staining demonstrated mild epidermal atrophy with focal follicular plugging. A mild interface infiltrate of lymphocytes and histiocytes and a superficial and deep, perivascular and periadnexal dermal infiltrate of lymphocytes, histiocytes and plasma cells was observed. Was difficult to diagnose as either lichen planus or lupus erythematosus. The histological studies from two places showed features of both lupus erythematosus and lichen planus. The direct immunofluorescence revealed focal deposits of immunoglobulins IgG, present at the basement membrane junction of the skin as well as in the sweat glands of IgM, fibrinogen and complement/C3. In addition, deposits of IgE surrounding the superficial dermal blood vessels and ecrine glands. Antibodies to piloerector muscle using several immunoglobulins, corroborated by immunohistochemistry stains.
Conclusions:
This is the first case reporting autoantibodies to the piloerector muscle in a patient having mixed clinical and histopathological diagnoses of lupus/lichen planus overlapping syndrome with strong IgE immune response.
Keywords: Piloerector muscle, smooth muscle antibodies, lupus, lichen planus, autoantibody
Introduction
The gastrointestinal, cardiovascular, genitourinary, and respiratory systems are composed generally of hollow organs, which transport and/or stockpile fluids (either gases or liquids) within the body[1]. The walls of these organs contain smooth muscle take place in a coordinated fashion because the cells are electrically coupled by intercellular connections[1–5]. The piloerector (arrector pili) muscles, which cause skin hair to stand up (goose pumps); and the irises, which control the diameter of the pupils in the eyes, are example of smooth muscle structures[1–5].
Many directly acting biochemical agents affect its contraction, but most smooth muscle is also beneath the control of the autonomic nervous system; in some sites it is influenced only by the sympathetic component and at others by dual, and sometimes opposite, effects of sympathetic and parasympathetic nerves[1–5]. The smooth muscle at different sites is much more heterogeneous than skeletal or cardiac muscle. The functioning of each type of smooth muscle is intimately tied up with the organ or system of which it is a part[1–5].
Case Report
A 59 Caucasian woman was evaluated for a generalized extremely pruritic rash and excoriation in most of her skin and with ulcers in her mouth of few days of eruption. At physical examination, the dermatologist found multiple well-defined flat papulo-escamous scaly violaceous plaques, mostly in the forearms, in the anterior neck, scalp, ears, shoulders, elbows, ears, buttocks, hands and fingers and under the chin. Inside the mouth ulcerated plaques on the palate and the patient were seen and the patient refers a strong burning sensation. There was no history of joint pains, fever, photosensitivity, neuropsychiatric disturbances, cough, and dyspnea. Routine blood tests were within normal limits and no nail changes were seen. A skin biopsy for hematoxylin and eosin staining (H & E), in formalin 10% bufferized and other for direct immunofluorescence (DIF) on Mitchell Medium were taken. She was put on topical triamcinolone steroid cream, 3 times at day, and a prednisone 40 milligrams taper as well as topical steroid and mouth washes with a good response.
Hematoxylin and eosin staining (H & E): Routine H&E staining was performed, and examination of the tissue sections revealed an unremarkable epidermis. A mild focal interface infiltrate of lymphocytes and histiocytes was noticed. Within the dermis, a superficial and deep, perivascular and periadnexal infiltrate of lymphocytes, histiocytes, and plasma cells was observed. Neutrophils and eosinophils were rare. Increased dermal mucin was not found; No evidence of infectious or a paraneoplastic process were seen. The PAS stain show mild reinforcement of the basal membrane zone of the dermal epidermal junctions. Was difficult to diagnose as either lichen planus (LP) or lupus erythematosus (LE). The histopathology suggested a mixed conditions such us lupus/lichen planus overlapping syndrome.
Direct immunofluorescence (DIF): Skin cryosections were prepared, and incubated with multiple fluorescein isothiocyanate (FITC)-conjugated secondary antibodies. The secondary antibodies were of rabbit origin and included: a) anti-human IgG (γ chain), b) anti-human IgA (α chains), c) anti-human IgM (μ-chain), d) anti-human fibrinogen, and e) anti-human albumin (all used at 1:20 to 1:60 dilutions and obtained from Dako (Carpinteria, California, USA). We also utilized secondary antibodies of goat origin, including: a) anti-human IgE antiserum (Vector Laboratories, Bridgeport, New Jersey, USA) and b) anti-human C1q (Southern Biotech, Birmingham, Alabama, USA). Finally, monoclonal anti-human IgG4 FITC conjugated antibody was used to test for possible intercellular staining between keratinocytes (Sigma-Aldrich Saint Louis, Missouri, USA). The slides were then counterstained with 4’,6-diamidino-2-phenylindole (Dapi) (Pierce, Rockford, Illinois, USA) or TO-PRO® -3/DNA. (Invitrogen Corporation, Carlsbad, California USA). Slides were then washed, coverslipped, and dried overnight at 4°C.
In Figures 1, 2 and 3, we demonstrate some of the most prominent H&E and DIF patterns observed, as well as the improvement of the quality and power of DIF staining by the use of simultaneous multicolor fluorescence and counterstaining of cell nuclei. The DIF results were classified as follows: (0, negative; +, weak positive; ++ and +++, positive; and ++++, maximum positivity). We found 1) deposits of IgG, in a granular pattern at the basement membrane zone (BMZ) of the dermal/epidermal junction, as well as at the BMZ of the sebaceous glands (+++); 2) deposits of IgA surrounding the superficial dermal blood vessels (++); 3) deposits of IgM in a granular pattern at the BMZ of the skin (+++); 4) deposits of complement C3 in a granular pattern at the BMZ of the skin and of the sebaceous glands (+++) and 5) deposits of fibrinogen, in a granular pattern at the BMZ of the skin (++).
Fig 1.
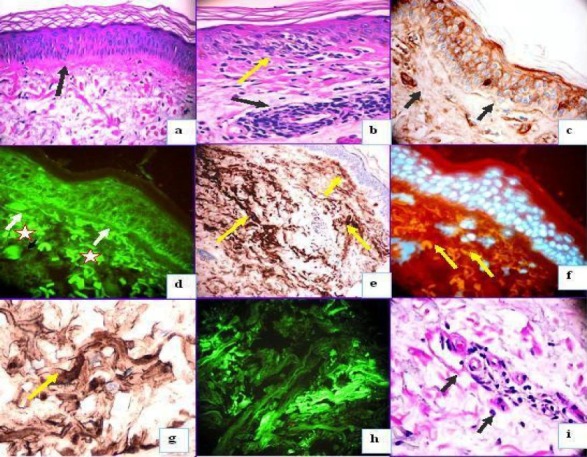
Mixture findings of lupus/lichen planus overlapping syndrome. 1a. PAS stain showing in an essential normal epidermis with some reinforcement of extracellular matrix material under the base membrane zone (BMZ) (black arrow). 1b. H & E stain showing some focal liquefaction of the BMZ (yellow arrow) and some lymphohistiocytic superficial infiltrate around the vessels (black arrow). 1c. IHC staining with anti-human-IgG showing some linear deposit at the BMZ (black arrows) and in the upper vessels. Please notice some intra-cytoplasmatic stain inside the keratinocytes. These findings were corroborated by using DIF with anti-human IgG FITC conjugated (1d). Please also notice the asterisk in red showing several colloid bodies in the upper dermis. In 1e, we use again IHC and corroborated the deposits of fibrinogen and alteration of the extracellular matrix material (brown stain) in both the papillary and the reticular dermis (yellow arrows). In 3f, we corroborated alterations showed on 1e, using DIF (see the multiple cytoid bodies) (yellow arrows, orange stain). 1g. Higher magnification to 1e. 1h DIF using antihuman antibodies to fibrinogen-FITC conjugated corroborating the data obtained previously by IHC about several deposits of fibrinogen in the dermis. 1i. PAS showing some thickening of some smooth muscle around dermal vessels with some lymphohisticitic cells around (black arrows).
Fig 2.
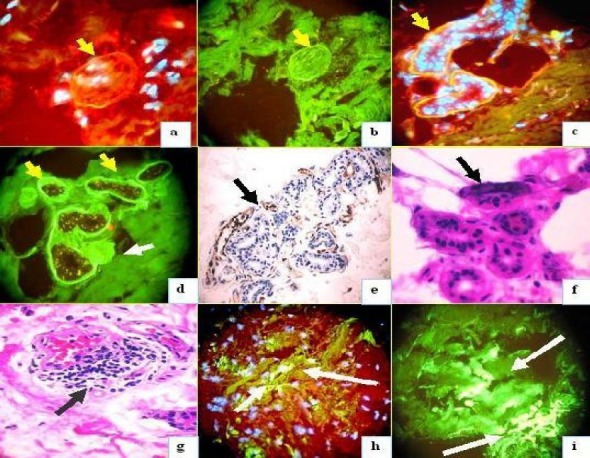
Positive stains to some vessels, nerve, and sweat glands. 2a. Positive anti-human IgE Texas red conjugated antibody (yellow arrow) (round structure). 2b. positive anti-human IgE FITCI conjugated antibody (yellow arrow). 2c. Positive anti-human IgE Texas red conjugated antibody (yellow arrow). 2d. Positive stain of the sweat glands with anti-human albumin (yellow arrows) and the adjacent nerve is also positive (white arrow). 2e. IHC positive IgE to the sweat glands (brown stain) (black arrow). 2f. H & E shows some necrosis of the sweat glands and damage in their structure. 3g. H & E shows inflammatory infiltrated around some vessels (black arrow). 3h. Shows positive stain with anti-human fibrinogen (green-yellowish stain) to the same vessels by DIF (white arrows), in this case with nuclei counterstained with Dapi. 3i. Similar to 3h but without nuclear counterstained. 2a and 2h also with nuclear counterstained in white-blue with Dapi.
Fig 3.
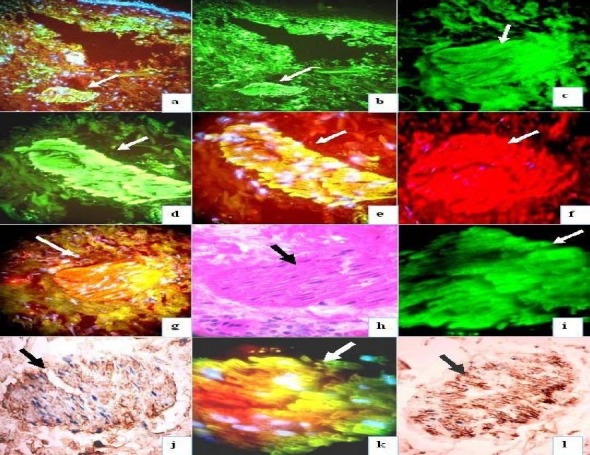
Shows autoantibodies to the smooth muscle arrector pili using DIF and IHC. (white or black arrows). In 3a, with anti-human-albumin FITCI conjugated, in 3b, with anti-human-fibrinogen (both lower magnification), in 3c with anti-IgE, in 3d, e, g, i and k with anti-human IgG at several magnifications 3j. IHC positive stain using anti-human fibrinogen. 3h, PAS positive of the muscle arrector pili. In 3l with anti-human albumin. In 3a, e, f, g, and k the nuclei were couterstained with Dapi (white-blue stain). In 3g and k, the antibodies colocalized with antibodies to smooth muscle (orange-reddish stain) with Texas red. In 3f, positive stain with smooth muscle antibody conjugated with Texas red.
IHC: To study correlation with our vimentin DIF data, we performed IHC utilizing a Dako dual endogenous peroxidase blockage system, with the addition of an Envision dual link. We utilized Dako technical instructions for staining. We applied 3, 3 diaminobenzidine as an IHC chromogen, and counterstained with hematoxylin. We tested for anti-human IgG, IgA, IgM, IgD, IgE, C1q, C3c, C3d, IgE, albumin, fibrinogen, kappa, lambda, mast cell tryptase (MCT), (all from Dako). The intensity of immunoreactivity was measured in units of staining ranging from four (strongest) to one (weakest). All measurements were performed by the same observer. Figures 1, 2 and 3 highlight our results, including the mixed features of lichen planus and lupus erythematosus, as well as the autoreactivity to the sweat glands, vessels and the reactivity to the arrector pili. The DI and IHC findings correlated with each other and the deposits immunoglobulins, complement and other immunosurfactants (such as fibrinogen) were similar.
Discussion
Discoid lupus erythematosus (LE) and lichen planus (LP) are considered as two different entities with characteristic clinical, histological and immunological features[6]. Few reports there have been described of overlapping features of both disorders. The overlap syndrome has been properly characterized by clinical and histological criteria[7]. It consists of discolored bluish red (violaceous) patches or plaques affecting the acral areas of the extremities that show a hypocellular or hypercellular lichenoid pattern in papillary dermis. Pruritus and photosensitivity are usually absent[7]. This differs from our case report, however this is the first time of someone reporting a combine overlapping syndrome of lupus and lichen with the presence of autoantibodies to the erector pili muscle. In some cases, long term follow up may show a progression to SLE in some cases but persistence of skin lesions in others[8–14].
Often, even histological features can be non-specific and suggestive of more than one clinical entity. One such condition described and documented is the occurrence of lupus erythematosus (LE) and lichen planus (LP) together as an overlapping syndrome (LE/LP). The overlapping syndrome occurs so infrequently as to limit clinical cases for study. Clinicians, dermatopathologist and immunodermatopathologist face a heterogeneous group of patients who had clinical, histological and immuno-pathological characteristics of both diseases at the same time raises the question whether this is an autonomous disorder, or a variant of discoid lupus erythematosus with features that resemble lichen planopilaris, or a case lichen planopilaris with concurrent lupus erythematosus[8–14]. To understand the clinical features of lupus erythematosus (skin disease in which there are red scaly patches, especially over the nose and cheeks)-lichen planus overlap syndrome, one must understand the individualistic features of lichen planus (a primary disorder of the skin resulting in violaceous, polygonal, flat skin lesions) as well as lupus erythematosus. Lichen planus is a disorder of the skin and mucous membranes resulting in inflammation, itching, and distinctive skin lesions. The exact cause is unknown, but the disorder is likely to be related to an allergic or immune reaction. Lupus erythematosus (LE) is a chronic skin condition of sores with inflammation and scarring favoring the face, ears, and scalp and at times on other body areas. These lesions develop as a red, inflamed patch with a scaling and crusty appearance. The center of the lesions may appear lighter in color with a darker rim. When lesions occur in the scalp, permanent scarring and hair loss can occur[8–14]. Some cases of lupus/lichen planus overlap syndrome shown cicatricial alopecia[15], but in this case this feature was not seen. This overlapping syndrome affects peripheral parts such as limbs, fingers, and ears. Warts or wart like projections or ringed red – violet color plaques are the typical of the condition. In this case we see mostly the flatten violaceous plaques. In this patient we see mixed histopathological and DIF features of lupus/lichen planus overlap syndrome. As a direct consequence of a fundamental dearth of critical information with respect to etiological, clinical and pathological features, the condition is still incompletely defined[16].
Approximately between 50 to 60 cases of LE/LP overlap syndrome have been reported[8–16]. Previous case reports have described two heterogeneous groups of lesions. One type occurs primarily on the extremities as bluish red, atrophic plaques while another type occurs as verrucous papules and nodules on the upper extremities[8–16]. Cutaneous lesions have also been described in other sites, including the face, trunk, mucosa, and nails. The diagnosis, however, is based primarily on the combined presence of clinical, histologic, and/or immunologic features of both diseases occurring at the same time. Of note, different lesions occurring in the same patient may be consistent with either LP or LE[8–16]. It is important to differentiate between LE and LP in order to make an accurate diagnosis of this overlap syndrome. Both diseases may display similar histologic and immunologic findings of colloid bodies and basement membrane changes. Colloid bodies are generally more numerous and are situated deeper in LP. Basement membrane cleft formation is seen in LP, while areas of basement membrane thickening are more commonly seen in LE. On DIF both LE and LP may reveal deposits of immunoglobulins or linear fibrinogen at the basement membrane zone. Immunoglobulin and complement deposition forms a granular pattern along the basement membrane zone in LE (lupus band), while a linear fibrinogen band is often seen at the basement membrane zone in LP[8–16]. Possible etiologies of LE/LP overlap syndrome include autoimmune, viral, and genetic predisposition. Isoniazid, procainamide, and acebutolol have also been reported as inducing drugs. In this specific case the patient was not taking any medicine or over the counter herbal supplements or vitamins. Conversion of cutaneous LE into systemic LE has been shown to occur more frequently in LE-overlap syndromes and has been reported[8–16]. Like LE, LE/LP overlap syndrome is associated with a chronic course. Treatment options include systemic retinoids as well as cyclosporin, which have been reported to be somehow effective.
In regards of the autoreactivity to the erector pili, as far as we review the literature, the only report describing autoreactivity to this was seen in a large study of the DIF in skin biopsies of 62 patients with discoid lupus erythematosus[17]. In that study, all the specimens were examined to determine the presence of immunoglobulins (IgG, IgM, and IgA) and complement (C3) on the different histological structures of the skin. The presence of immunoglobulins and complement was detected not only in the basement membrane of the lesion but also on the hyaline bodies, collagen fibers, dermal capillaries, basal membrane of hair follicles, dermal inflammatory cells, sweat glands and arrector pili muscles[17]. The significance of these findings remains unknown[17]. In our study, the patient showed a lot of reactivity with anti-human IgE, to the erector pili muscle, sweat glands and nerve around those sweat glands, no history of atopic or allergic disease or drug intake was positive for this patient. We just can speculate that the presence of this immunoglobulin deposit in all these areas may be one of the triggering factors to the autoreactivty to the erector pili muscle. We were also unable to find any indexed literature in regard of this findings and the overlapping syndrome of LE and LP.
We are reporting a male patient with features of both lupus and lichen disease, but with autoantibodies to piloerector muscle for the first time in the literature. Further research, study and documentation are required to increase our understanding of the condition as well as for development of a defined course of effective treatment.
The authors did not have funding for the study.
Footnotes
Conflicts of Interest: The authors did not have funding for the study.
References
- 1.Kao CY, Carsten ME. Cellular aspects of smooth muscle function. Cambridge University Press; 1997. [Google Scholar]
- 2.Mendelson JK, Smoller BR, Mendelson B, Horn TD. The microanatomy of the distal arrector pili: possible role for α1β1 and α5β5 integrins in mediating cell–cell adhesion and anchorage to the extracellular matrix. J Cutan Pathol. 2000;27:61–66. doi: 10.1034/j.1600-0560.2000.027002061.x. [DOI] [PubMed] [Google Scholar]
- 3.Poblet E, Ortega F, Jiménez F. The arrector pili muscle and the follicular unit of the scalp: a microscopic anatomy study. Dermatol Surg. 2002;28:800–803. doi: 10.1046/j.1524-4725.2002.02038.x. [DOI] [PubMed] [Google Scholar]
- 4.Poblet E, Jiménez F, Ortega F. The contribution of the arrector pili muscle and sebaceous glands to the follicular unit structure. J Am Acad Dermatol. 2004;51:217–222. doi: 10.1016/j.jaad.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 5.Song WC, Hwang WJ, Shin C, Koh KS. A new model for the morphology of the arrector pili muscle in the follicular unit based on three-dimensional reconstruction. J Anat. 2006;208(5):643–648. doi: 10.1111/j.1469-7580.2006.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black MM. Lichen planus and lichenoid eruptions. In: Rook A, Wilkinson DS, Ebling FJD, Champion RH, Burton JL, editors. Textbook of dermablogy. 4th edn. Oxford: Blackwell Scientific Publications; 1986. pp. 1681–6. [Google Scholar]
- 7.Jamison TH, Cooper NM, Wallace V, Epsteen Lichen planus and discoid lupus erythematosus. Arch Dermatol. 1978:1039–1041. [PubMed] [Google Scholar]
- 8.Camisa C, Neff JC, Olsen RG. Use of indirect immunofluorescence in the lupus erythematosus/lichen planus overlap syndrome: an additional diagnostic clue. J Am Acad Dermatol. 1984;11:1050–1059. doi: 10.1016/s0190-9622(84)70258-1. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed AR, Schreiber P, Abramovits W, Ostreicher M, Lowe NJ. Coexistence of lichen planus and systemic lupus erythematosus. J Am Acad Dermatol. 1982;7:478–483. doi: 10.1016/s0190-9622(82)70129-x. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Pomeranz MK. Related Articles, Links Lupus erythematosus and lichen planus overlap syndrome. J Drugs Dermatol. 2004;3:311–312. [PubMed] [Google Scholar]
- 11.Tursen U, Oz O, Ikizoglu G, Kaya TI, Dusmez D. A case of lichen planus-lupus erythematosus overlap syndrome with eyelid involvement. Eur J Ophthalmol. 2002;12:244–246. doi: 10.1177/112067210201200314. [DOI] [PubMed] [Google Scholar]
- 12.Nieboer C. Lupus erythematosus/lichen planus (LE/LP) overlap syndrome. J Am Acad Dermatol. 1985;13:297. doi: 10.1016/s0190-9622(85)80281-4. [DOI] [PubMed] [Google Scholar]
- 13.Mahler V, Hornstein OP, Meyer S, Albrecht HP, Kiesewetter F. Lupus erythematosus/lichen ruber planus overlap syndrome. 5 cases in a patient sample of the Erlangen University Dermatology Clinic (1894-1995)] Hautarzt. 1998;49:295–302. doi: 10.1007/s001050050744. [DOI] [PubMed] [Google Scholar]
- 14.de Jong EM, van der Vleuten CJ, van Vlijmen-Willems IM. Differences in extracellular matrix proteins, epidermal growth and differentiation in discoid lupus erythematosus, lichen planus and the overlap syndrome. Acta Derm Venereol. 1997;77:356–360. doi: 10.2340/0001555577356360. [DOI] [PubMed] [Google Scholar]
- 15.Inaloz HS, Chowdhury MM, Motley RJ. Lupus erythematosus/lichen planus overlap syndrome with scarring alopecia. J Eur Acad Dermatol Venereol. 2001;15:171–174. doi: 10.1046/j.1468-3083.2001.00223.x. [DOI] [PubMed] [Google Scholar]
- 16.Stary A, Schwarz T, Duschet P, Gschnait F. Lichen ruber planus--lupus erythematosus/overlap syndrome. Z Hautkr. 1987;62:381–394. [PubMed] [Google Scholar]
- 17.Mauro JF Honeyman, Velasco A Robles. Direct immunofluorescence in cutaneous lupus erythematosus. Med Cutan Ibero Lat Am. 1976;4:41–44. [PubMed] [Google Scholar]


