Abstract
Background
The diagnosis of acute lung injury (ALI) is based on a consensus clinical definition. Despite the simplicity of this definition, ALI remains underdiagnosed and undertreated. Severe trauma is a well-described cause of ALI that represents a relatively homogeneous subset of ALI patients. The goals of this study were to develop a panel of plasma biomarkers to facilitate diagnosis of trauma-induced ALI and to enhance our understanding of the pathogenesis of human ALI.
Methods
A retrospective nested case control of 192 patients admitted to the trauma intensive care unit (ICU) at a university hospital between 2002 and 2006. We compared 107 patients with ALI to 85 patients without ALI. Plasma was collected within 72 h of ICU admission. Twenty-one plasma biomarkers were measured in duplicate in each plasma sample.
Results
Patients with ALI had higher severity of illness scores, more days of mechanical ventilation, longer hospital stays and higher mortality versus controls. Seven biomarkers (RAGE, PCPIII, BNP, ANG2, IL10, TNF-α, and IL8) had a high diagnostic accuracy as reflected by the area under the receiver operating characteristic curve of 0.86 (95% CI 0.82 – 0.92) in differentiating ALI from controls.
Conclusions
A model utilizing seven plasma biomarkers had a high diagnostic accuracy in differentiating patients with trauma-induced ALI from trauma patients without ALI. In addition, use of a panel of biomarkers provides insight into the likely importance of alveolar epithelial injury in the pathogenesis of early acute lung injury.
Keywords: Acute respiratory distress syndrome, acute pulmonary edema, pulmonary contusion, alveolar epithelium
Introduction
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) are common complications in patients with traumatic injuries 1. Trauma-associated ALI/ARDS has a lower mortality than ALI/ARDS associated with sepsis or other clinical risk factors such as pneumonia, pancreatitis or aspiration, possibly implying a different mechanism or pathological basis for the disease 1–7. Despite the evidence that the pathophysiology of ALI/ARDS may differ depending on the underlying clinical disorder, the current clinical standard for diagnosis of ALI/ARDS is the 1994 American-European Consensus Criteria, which does not take into account the underlying cause of ALI/ARDS 8. The Consensus definition includes: 1) the acute onset of bilateral infiltrates on chest radiograph, 2) a low ratio of partial pressure of arterial oxygen to the fraction of inhaled oxygen, and 3) the absence of clinical evidence of left atrial hypertension 8. Because these are all clinical criteria, the definition does not account for the underlying biological and pathological mechanisms. Furthermore, despite the simplicity of the Consensus Criteria, ALI and ARDS are underdiagnosed and undertreated 9,10.
Recent studies evaluating biomarkers in patients with ALI/ARDS have identified a number of markers that are predictive of clinical outcomes; these studies have also provided insight into the underlying pathogenetic mechanisms of ALI/ARDS 1,11–15. Biomarkers that are specific to inflammation, endothelial activation and injury, lung epithelial injury, and disordered coagulation and fibrinolysis have all been previously shown to help differentiate patients with ALI/ARDS 11–17. In a recent study, Calfee and colleagues 1 compared a panel of 8 biomarkers in patients with traumatic ALI/ARDS to patients with nontraumatic ALI/ARDS. They found significant differences in 6 of the 8 biomarkers that were evaluated. This study, however, did not examine the role that biomarkers might play in the diagnosis of ALI/ARDS.
In the current study, the primary objective was to develop and test a panel of biomarkers that could assist in the diagnosis of acute lung injury, thus facilitating early and appropriate treatment. Given the several biologic pathways involved in the development of ALI/ARDS 7,18, we hypothesized that a panel of multiple biomarkers reflecting inflammation, lung epithelial and endothelial injury, fibrosis and dysregulated coagulation and fibrinolysis would have better sensitivity and specificity for diagnosis of ALI/ARDS than any single biomarker. Further, use of a panel of biomarkers makes it possible to assess biochemical markers of injury for their pathogenetic value in the early phase of ALI/ARDS. In order to limit biologic variability and clinical heterogeneity, we focused on patients with a single risk factor for acute lung injury, severe trauma.
Materials and Methods
Patients
We conducted a retrospective nested case control study using a prospectively collected database of 1020 critically injured patients admitted to the trauma intensive care units from June 2002 to May 2006. Both the data within the database and the matching plasma samples were collected as part of a prospective observational study evaluating the role of gender in the incidence and outcome of ICU infection rates 19–22. All critically injured patients greater than 18 years of age that had an ICU stay of greater than 48 hours were entered into the original cohort. Patients within the original cohort who had chest radiographs and a study blood draw obtained within the first three ICU days were entered into the current study.
Selection of Cases and Controls
Chest radiographs and clinical data were reviewed by two of the authors (R.F, L.W.) for all 1020 patients enrolled in the parent study. Control patients included patients with clear chest radiographs during the first 7 days in the hospital (n = 74) and patients with clinical and radiographic evidence of cardiogenic or hydrostatic pulmonary edema on the chest radiograph that corresponded with the available plasma sample (n = 11). Distinction of cardiogenic pulmonary edema from ALI/ARDS was based on published algorithms 23 and review of the medical record and included radiographic findings of increased cardiothoracic ratio and widened vascular pedicle width 24,25 as well as clinical data such as invasive hemodynamic measurements, echocardiography or evidence of myocardial ischemia. Case (ALI/ARDS) patients had the acute onset of bilateral radiographic infiltrates and met the Consensus definition for ALI/ARDS8 within 72 hours of admission to the ICU (n = 107), with PaO2/FiO2 less than or equal to 300 for ALI and less than or equal to 200 for ARDS and no clinical or radiographic evidence for a cardiogenic cause of acute pulmonary edema. Patients with unilateral infiltrates or contusions were not included in the current study. Arterial blood gases obtained within the 24 hours prior to and the 24 hours after the study blood draw were used to ascertain whether patients met the oxygenation criteria for ALI/ARDS.
Biomarker plasma measurements
A panel of 21 biomarkers was measured in duplicate in banked plasma samples on each patient. Cytokines and markers of inflammation including interleukin-1 beta (IL-1β), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-12 p70 (IL-12p70), interferon gamma (IFN-λ), granulocyte macrophage colony stimulating factor (GM-CSF) and tumor necrosis factor alpha (TNF-α) were measured by bead-based cytokine array (LINCO Research, St. Charles, MO). The remaining biomarkers were measured using commercially available singleplex ELISA kits including surfactant protein-D (SP-D) (Yamasa Corporation, Tokyo, Japan), Clara cell secretory protein (CC16) (BioVendor, Candler, NC), receptor for advanced glycation end-products (RAGE) (R & D Systems, Minneapolis MN), angiopoietin-2 (ANG-2) (R & D Systems, Minneapolis MN), von-Willebrand factor antigen (VWF) (Diagnostica Stago,Parsippany, NJ), myeloperoxidase (MPO) (ALPCO, Salem, NH), soluble intracellular adhesion molecule-1 (sICAM1) (R & D Systems, Minneapolis MN), plasminogen activator inhibitor-1 (PAI-1) (American Diagnostica, Stamford CT), brain natriuretic peptide (BNP) (Peninsula Laboratories, San Carlos CA). Procollagen peptide III (PCP III) was measured by radioimmunoassay (Peninsula Laboratories, San Carlos CA).
Clinical Data
Clinical data were obtained on each patient by prospective chart review by dedicated research personnel as part of the parent study. We included pulmonary artery catheter data when available as well as severity of illness scores: acute physiology and chronic health evaluation II (APACHE II) 26, injury severity score (ISS) 27 and trauma related injury severity score (TRISS) 28. Classification of patients as having hydrostatic pulmonary edema was based on echocardiography data (when available), pulmonary artery catheter data (if available), vascular pedicle width (VPW) 29, clinical history of a myocardial infarction or treating physicians’ documentation of cardiogenic pulmonary edema on either progress notes or discharge summaries.
Statistical Analysis
Baseline demographic and clinical variables for all patients were assessed using Wilcoxon rank sum tests for continuous variables and Fisher exact tests for categorical variables. All values for biomarkers underwent logarithmic transformation to stabilize variance. Values below the detection limit were imputed at half the lower limit of detection for each biomarker.
Because there were 21 potential biomarker variables, it was necessary to reduce the number of candidate variables before multivariable logistic regression models could be considered. Due to inadequate plasma sample volume, there was one patient who was missing data on all 21 biomarker variables, and this patient was removed from the subsequent analysis. Four additional patients were missing data on the 11 biomarker variables measured on the bead-based cytokine array. Additionally, two patients were missing data on IL-5, and one on PCPIII, respectively. These 7 (3.7%) patients were removed from the variable-selection process described below; removal of such a small proportion of the data would not be expected to affect the conclusion significantly 30. The strength of the marginal relationship (Spearman’s rank correlation coefficient) to the response variable was used to eliminate variables that showed only a very weak relation. With the reduced number of candidate variables, we used a backward elimination model building strategy on 500 bootstrapped data to choose variables for further consideration. For every bootstrap sample, a full model with all the variables in consideration was constructed. Then, the variable with the largest P value (Wald test) was dropped, and a new model was fitted with one fewer variables. This backward elimination process was repeated until only one variable was retained. The predictors were then ranked from most significant (the last one to remain in the model) to least significant (the first one eliminated). The average rank was used to select the variables for further consideration. Pair-wise Spearman’s rank correlations among the candidate covariates were also considered in selecting these variables.
Using the variables selected in the previous steps, a multivariable logistic regression model was constructed. The final model included all variables selected in the previous step; no reduction of the model was attempted to avoid overfitting. From each model, we computed the predicted probability of ALI for each individual and computed Receiver Operating Characteristics (ROC) curves and their areas under the curve (AUC), which is reported with a 95% bootstrap confidence interval 31. All analyses were carried out with R version 2.7.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 192 patients enrolled in this study, 107 were diagnosed with ALI/ARDS, 74 patients had clear chest radiographs and no evidence of acute lung injury, and 11 patients had bilateral radiographic infiltrates due to hydrostatic edema. For the primary analysis, patients with clear radiographs (n=74) and patients with bilateral infiltrates due to hydrostatic edema (n=11) were considered to be controls (total of 85), and patients with ALI/ARDS were considered to be cases. Table 1 shows the clinical characteristics of the cases and controls. Both cases and controls were profoundly hypoxemic with median lowest PaO2/FiO2 ratios on the day of enrollment of less than 200 indicating that oxygenation alone was not sufficient to distinguish between patients with ALI/ARDS and patients with clear chest radiographs or hydrostatic pulmonary edema. Among the patients with ALI/ARDS, all patients had a PaO2/FiO2 ratio less than 200 (maximum 199) indicating that all met diagnostic criteria for ARDS8. Patients in the ALI/ARDS group were more severely ill based on the APACHE II, ISS and TRISS scores than the patients without ALI. Patients with ALI had more days of mechanical ventilation, more days in the ICU and higher mortality than patients without ALI. Although measurements were available only in a subgroup, control patients with hydrostatic pulmonary edema had higher CVP compared to case patients with ALI/ARDS or to control patients with clear radiographs. The median highest CVP on enrollment day in the hydrostatic edema group was 17 mmHg (IQR 16 – 23 mmHg, n = 4) compared to 14 (IQR 11 – 18 mmHg, n = 52) in the ALI/ARDS group and 12 mmHg (IQR 9 – 16 mmHg, n = 23) in the clear group, p = 0.04. Patients in the hydrostatic group had a higher median PAOP compared to patients with ALI/ARDS, although again the number of patients with measurements was small and the difference did not quite reach statistical significance. The median highest PAOP on enrollment day in the hydrostatic edema group was 19 mmHg (IQR 18 – 26 mmHg, n = 4) compared to 16 mmHg (IQR 13 – 21 mmHg, n = 44) in the ALI/ARDS group and 13 mmHg (IQR 10 -18 mmHg, n = 19) in the clear group, p = 0.10.
Table 1.
Clinical characteristics of 192 critically ill patients with severe trauma
| Clinical Characteristics |
N | Cases ALI/ARDS (n=107) |
Controls Clear (n=74)/Hydrostatic Edema (n=11) n = 85 |
P Value |
|---|---|---|---|---|
| Age | 192 | 39 (26 – 53) | 33 (23 – 47) | 0.19 |
| Male | 192 | 66% | 71% | 0.64 |
| Race | 192 | 0.036 | ||
| White | 82% | 84% | ||
| Black | 11% | 14% | ||
| Other | 7% | 2% | ||
| Body Mass Index | 166 | 26 (24 – 29) | 27 (23 – 31) | 0.87 |
| PaO2/FiO21 | 187 | 72 (38 – 143) | 123 (89 – 153) | < 0.001 |
| TRISS score | 136 | 0.80 (0.35 – 0.93) | 0.93 (0.78 – 0.98) | 0.001 |
| ISS score | 179 | 34 (25 –41) | 29 (17 – 35) | 0.002 |
| APACHE II score | 191 | 17 (14 – 21) | 13 (11 – 17) | < 0.001 |
| Hospital Mortality | 192 | 14% | 5% | 0.049 |
| Ventilator Days | 192 | 9 (5 – 14) | 4 (3 – 7) | < 0.001 |
| ICU Days | 192 | 10 (6 – 16) | 6 (4 – 8) | < 0.001 |
TRISS, Trauma and Injury Severity Score; ISS, Injury Severity Score; APACHE, Acute Physiology and Chronic Health Evaluation
Data as median (interquartile range) or percent of patients; N is the number of non-missing values. Wilcoxon tests (continuous variables) and Fisher’s exact tests (categorical variables) were used.
Lowest PaO2/FiO2 ratio recorded on the day of enrollment
Plasma biomarker levels in cases and controls are shown in Table 2. Overall, 10 of 21 biomarkers measured were highly significantly different between the two groups (p < 0.01). Seven biomarkers were included in the final model to discriminate cases (ALI/ARDS) from controls. The seven markers were RAGE, PCPIII, BNP, ANG2, IL10, TNF-α, and IL8. The final model is described in Table 3. Receiver operator characteristic curve analysis was used to evaluate model performance. Using these 7 biomarkers, the area under the curve was 0.86 (95% CI 0.82 – 0.92) (Figure 1) for the diagnosis of ALI/ARDS. By contrast, the area under the curve for diagnosis of ALI/ARDS based on the ISS score was only 0.63 (95% CI 0.55 – 0.71) and using the TRISS score was only 0.66 (95% CI 0.56 – 0.75). The area under the curve for diagnosis using the single biomarker that is already in clinical use for diagnosis of heart failure (BNP) was also poor, 0.64 (95% CI 0.57 – 0.72).
Table 2.
Comparison of Plasma Levels of Biomarkers Between ALI/ARDS Cases and Controls
| Biomarker | N | Cases ALI/ARDS (n=107) |
Controls Clear/Hydrostatic Edema (n=85) |
P Value1 |
|---|---|---|---|---|
| RAGE (pg/ml) | 191 | 2508 (1780 – 5050) | 1415 (1022 – 2022) | < 0.001 |
| PCP (ng/ml) | 191 | 10.3 (8.1 – 15.5) | 7.8 (6.8 – 10.2) | < 0.001 |
| IL-8 (pg/ml) | 187 | 42 (29 – 84) | 25 (12 – 39) | < 0.001 |
| Ang2 (ng/ml) | 191 | 8.6 (5.7 – 14.0) | 5.7 (3.8 – 8.5) | < 0.001 |
| MPO (ng/ml) | 191 | 46 (29 – 70) | 31 (20 – 45) | < 0.001 |
| BNP (ng/ml) | 191 | 0.23 (0.16 – 0.39) | 0.33 (0.19 – 0.58) | < 0.001 |
| VWF (% control) | 191 | 321 (232 – 494) | 256 (189 – 351) | 0.001 |
| IL-10 (pg/ml) | 187 | 77 (31 – 169) | 32 (19 – 118) | 0.002 |
| IL-6 (pg/ml) | 187 | 240 (139 – 498) | 197 (79 – 340) | 0.005 |
| PAI-1 (ng/ml) | 191 | 87 (49 –191) | 60 (28 – 119) | 0.008 |
| SP-D (ng/ml) | 191 | 79 (47 – 127) | 68 (38 – 115) | 0.027 |
| IL-12 (pg/ml) | 187 | 2.7 (2.7 – 5.8) | 3.2 (2.7 – 7.2) | 0.033 |
| IL-4 (pg/ml) | 187 | 105 (22 – 418) | 49 (14 – 166) | 0.072 |
| TNF-α (pg/ml) | 187 | 7.5 (3.8 – 13.4) | 6.3 (2.9 – 11.0) | 0.14 |
| GM-CSF (pg/ml) | 187 | 4.3 (2.7 – 9.4) | 4.3 (2.7 – 6.8) | 0.38 |
| Interferon-γ (pg/ml) | 187 | 3.8 (2.7 – 10.1) | 4.7 (2.7 – 13.5) | 0.40 |
| ICAM-1 (ng/ml) | 191 | 667 (430 – 877) | 629 (439 – 814) | 0.47 |
| IL-1 (pg/ml) | 187 | 2.7 (2.7 – 3.4) | 2.7 (2.7 – 4.0) | 0.51 |
| IL-5 (pg/ml) | 185 | 2.7 (2.7 – 4.2) | 2.7 (2.7 – 2.8) | 0.57 |
| CC16 (ng/ml) | 191 | 12.5 (7.8 – 20.5) | 13.0 (9.3 – 16.9) | 0.68 |
| IL-2 (pg/ml) | 187 | 2.9 (2.7 – 6.5) | 2.9 (2.7 – 7.3) | 0.98 |
Data as median (interquartile range), cases compared to controls by Wilcoxon test
Table 3.
Summary of the final diagnostic model using seven biomarkers to diagnose ALI/ARDS
| Biomarker | Quartiles (25th – 75th) |
Odds Ratio for ALI/ARDS1 |
95% CI | P-value |
|---|---|---|---|---|
| RAGE | 1216 – 3310 | 3.33 | 1.85 – 5.99 | <0.001 |
| PCP III | 7.3 – 13.1 | 2.90 | 1.61 – 5.23 | <0.001 |
| BNP | 0.17 – 0.45 | 0.45 | 0.26 – 0.77 | 0.004 |
| ANG2 | 4659 – 12078 | 2.20 | 1.19 – 4.05 | 0.01 |
| IL8 | 16.8 – 64.6 | 1.81 | 1.03 – 3.17 | 0.04 |
| TNF-α | 3.6 – 13.1 | 0.51 | 0.27 – 0.98 | 0.04 |
| IL10 | 21.4 – 151.2 | 2.02 | 0.96 – 4.25 | 0.06 |
Odds ratios represent change from the lowest quartile to the highest quartile
Figure 1.
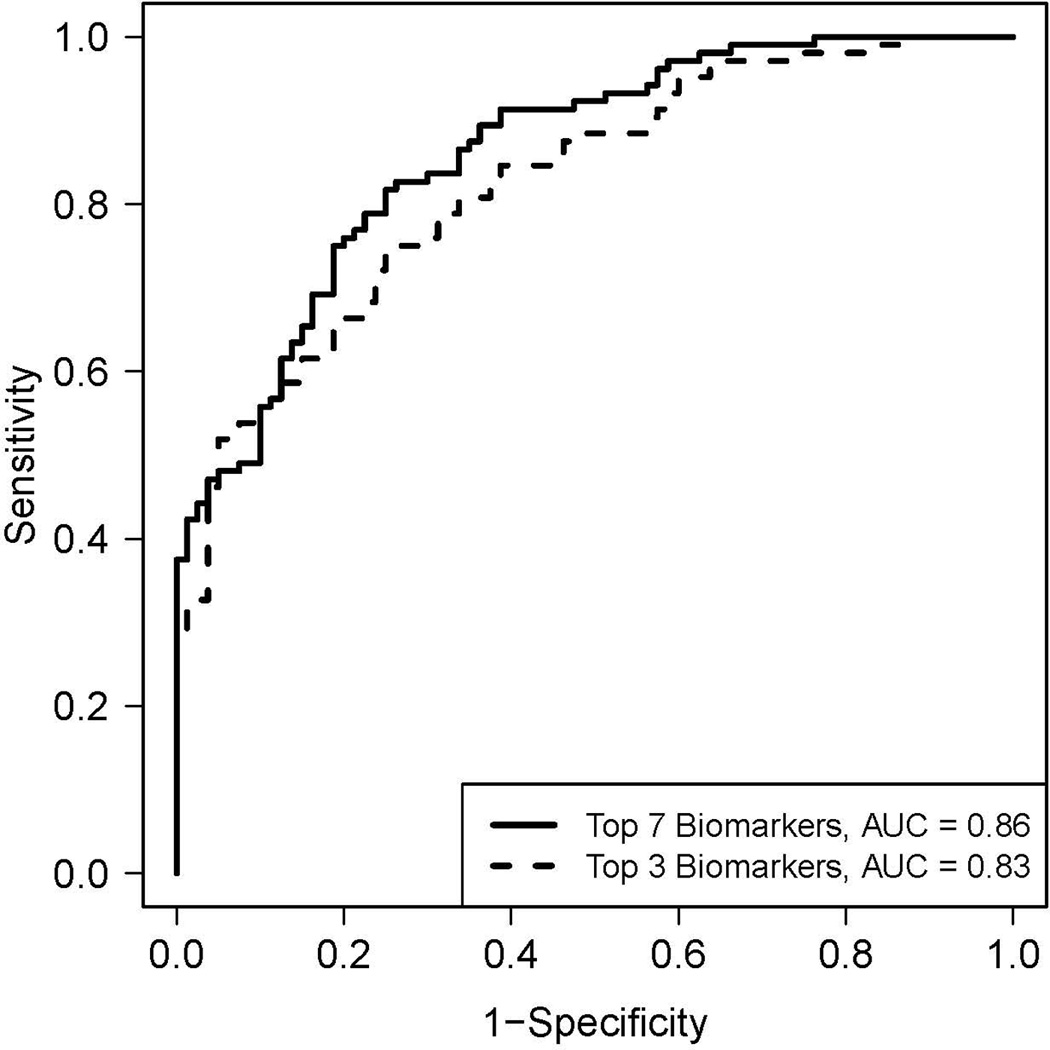
Receiver operator characteristic (ROC) curve analysis of the plasma biomarker panels for differentiating ALI/ARDS (cases) from controls. Predicted probability of recurrence of ALI/ARDS for each subject was computed from a logistic regression model that includes the 7 biomarkers (RAGE, PCPIII, BNP, ANG2, IL10, TNF-α, and IL8). Specificity and sensitivity were computed at each possible cutoff of the predicted probability. The area under the curve (AUC) is 0.86 (95% CI 0.87 – 0.95). The dashed line shows the ROC analysis using only the 3 most discriminatory biomarkers (RAGE, PCPIII and BNP). The area under the curve for this model is 0.83 (95% CI 0.84 – 0.94).
In a sensitivity analysis, we reanalyzed the association between the biomarkers and the diagnosis of ALI/ARDS comparing only the group with clear chest radiographs to the group with ALI/ARDS, excluding the patients with hydrostatic edema. In this analysis, the same seven biomarkers were included in the final model (RAGE, PCPIII, BNP, ANG2, TNF-α-10, IL-8) indicating that the differences in biomarker levels between cases and controls are not driven primarily by the patients with hydrostatic pulmonary edema.
The performance of a reduced model with only the top three best performing biomarkers was also explored to determine whether the three biomarkers that had the strongest relationship with ALI/ARDS might have significant predictive value. Using only RAGE, PCPIII and BNP the area under the curve was 0.83 (95% CI 0.76 – 0.88) (Figure 1). A formal statistical comparison of these two models was not conducted, because the 3 biomarkers, (RAGE, PCPIII and BNP) were not pre-specified but were selected based on their performance in the original 7-biomarker model. A nomogram that illustrates the potential clinical use of the 7-biomarker panel is illustrated in Figure 2.
Figure 2.
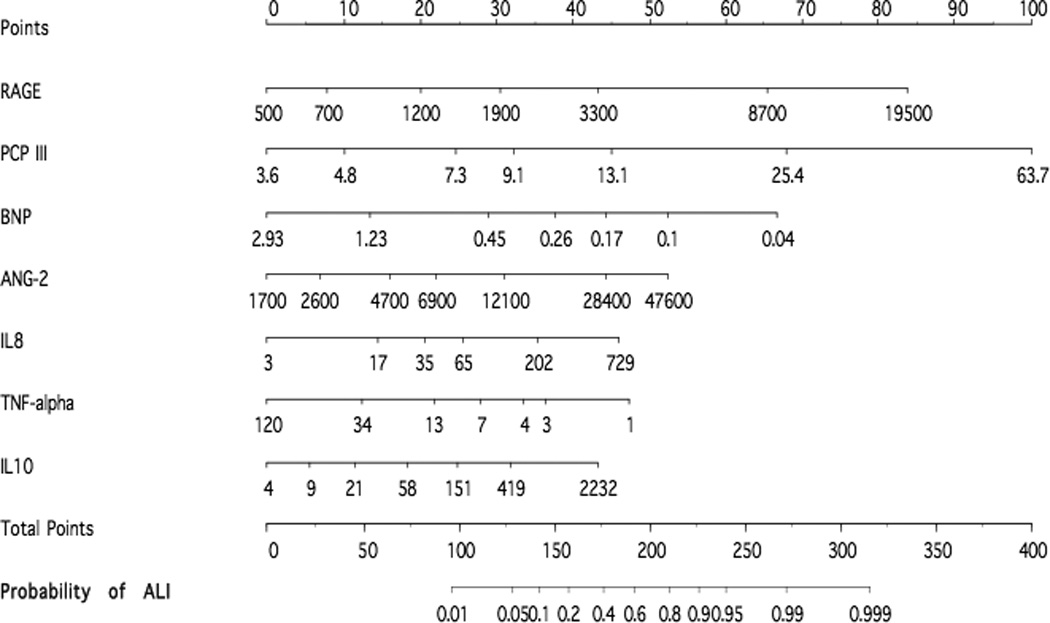
The multi-variable logistic regression model was used to create a prediction model for the probability of ALI. A value in each predictor variable corresponds to a point scale at the top. The sum of the individual predictor variable points corresponds to the total points and the probability of ALI shown at the bottom. For each predictor variable, the shown values are approximately 1st, 5th, 25th, 50th, 75th, 95th, and 99th percentiles.
To illustrate the clinical significance of patient classification using the biomarker model, we compared clinical outcomes including mortality, ventilator days and ICU days between patients classified as high probability of ALI/ARDS (> 80%, n = 56) or low probability of ALI/ARDS (< 20%, n = 36). As shown in Table 4, patients with a high probability of ALI/ARDS had substantially worse clinical outcomes compared to patients with a low probability of ALI/ARDS.
Table 4.
Comparison of clinical outcomes in patients classified as high or low probability of ALI/ARDS using the biomarker model
| High Probability ALI/ARDS (>80%) n = 56 |
Low Probability ALI/ARDS (<20%) n = 36 |
P Value | |
|---|---|---|---|
| Ventilator Days | 10 (5 – 14) | 5 (3 – 9) | 0.002 |
| ICU Days | 10 (6.8 – 17) | 6 (4 – 7) | <0.001 |
| Hospital Mortality | 18% (10) | 6% (2) | 0.11 |
Data as median (interquartile range) or percent of patients
Discussion
The primary goal of this study was to test the hypothesis that a panel of biomarkers that reflects the complex pathophysiology of acute lung injury can be used to diagnose ALI/ARDS among a group of critically ill patients with severe traumatic injuries. From an initial panel of 21 biomarkers of inflammation, lung epithelial and endothelial cell injury, fibrosis and dysregulated coagulation and fibrinolysis, a combination of 7 biomarkers were used to develop a diagnostic model. The final model includes markers of lung epithelial injury (RAGE), collagen deposition (PCPIII), cardiac dysfunction (BNP), endothelial activation and injury (Ang2) and inflammation (IL-10, TNF-α and IL-8)). This diagnostic model had excellent performance for differentiating ALI/ARDS cases from controls with an area under the ROC curve of 0.86. A simplified model using the three top performing biomarkers was also tested (RAGE, PCPIII, BNP); this model also had excellent discriminatory power with an area under the ROC curve of 0.83.
The gold standard for diagnosis of ALI/ARDS in the current study was the American European Consensus definitions 8. Despite widespread use of these definitions for enrollment into clinical trials, ALI and ARDS remain underdiagnosed and undertreated 9,10. Furthermore, these consensus definitions may not be ideal in trauma patients. In one study of patients with trauma induced ARDS 32 defined by standard consensus definitions, there was little uniformity among the patients who met consensus criteria when comparing severity of illness scores, incidence of multi-organ failure and incidence of sepsis. The authors concluded that application of the consensus criteria for the diagnosis of ALI/ARDS in trauma patients captures a widely disparate group with poor specificity for identifying patients at risk for prolonged respiratory failure and associated complications 32. In addition, at least three studies have found that a diagnosis of ALI/ARDS is not an independent predictor of mortality in patients with severe trauma 33–35, in contrast to other etiologies of ALI/ARDS where the diagnosis is associated with increased mortality 1,36. In the current nested case control study, patients with a clinical diagnosis of ALI/ARDS had significantly higher mortality and longer duration of mechanical ventilation and ICU stay compared to patients with clear chest radiographs or hydrostatic pulmonary edema. When the biomarker panel was used to classify patients as high or low probability of ALI/ARDS, substantial differences in important clinical outcomes including duration of mechanical ventilation and duration of ICU stay were seen.
In addition to its diagnostic performance, the multimarker panel provides novel insight into the pathogenesis of trauma-associated ALI/ARDS. Although several of the biomarkers that were studied have been previously studied in early trauma-associated ALI/ARDS 1, the majority of the biomarkers most highly associated with ALI/ARDS in our study have not been previously studied in a large trauma population including CC16, RAGE, PCPIII, Ang2 and BNP. The diagnostic value of RAGE is of particular interest, providing biochemical confirmation of the critical role of alveolar epithelial injury in trauma-associated ALI/ARDS, a concept that has been suggested in several prior studies 16,37–42 and was first emphasized in pathologic specimens by Bachofen and Weibel 38. Although RAGE is expressed in several organs, RAGE is most highly expressed in the lung where it is an alveolar type-1 epithelial cell associated protein localized primarily to the basal membrane of the type 1 cells. RAGE is a multi-ligand binding receptor that binds advanced glycation end products, amyloid beta-peptide, s100 proteins and high-mobility group box-1. RAGE-ligand interaction results in intracellular signaling and NF-kB activation 15. Two previous human studies found RAGE to be elevated in patients with acute lung injury compared to either normal controls 15 or to patients with less severe acute lung injury 39. Since RAGE is a marker of alveolar epithelial injury, its release into the plasma implies damage to the lung epithelium as one important mechanism of early injury in trauma-induced ALI/ARDS. Injury to the alveolar epithelium is associated with impaired alveolar fluid clearance in both experimental and clinical studies 42,43 and in experimental models, hemorrhagic shock-induced lung injury causes impaired alveolar fluid clearance 44–46. Thus, alveolar edema in trauma-induced ALI/ARDS may be accounted for in part by epithelial injury.
The second top performing biomarker in the diagnostic model was PCP-III, a marker of collagen synthesis. PCPIII is cleaved from the precursor procollagen molecule by extracellular proteases during collagen synthesis and reflects fibrogenesis 47. Elevated levels have been described in fibrotic lung diseases 48,49 as well as hepatic cirrhosis 50 and cardiac fibrosis 51. Levels of PCPIII were five-fold higher in the pulmonary edema fluid of patients with ALI/ARDS compared to patients with hydrostatic pulmonary edema 47; among patients with ALI/ARDS, higher levels were associated with increased mortality, a finding that was replicated in a study of bronchoalveolar lavage fluid from patients with ARDS 52. Higher levels in plasma of patients with ALI/ARDS have also been reported in a few small studies 53,54. In one study of 57 patients with severe trauma, elevated PCPIII levels were associated with higher mortality, longer duration of mechanical ventilation and poorer oxygenation 55. The finding of elevated plasma PCPIII levels in trauma patients with ALI/ARDS supports the hypothesis that lung fibrosis is triggered early in the course of ALI/ARDS. However, it should be noted that increased PCPIII levels in trauma patients may also reflect wound healing, fibrosis in other organs and decreased clearance due to hepatic dysfunction 55.
BNP was also a top performing biomarker. BNP is the main clinical marker used to differentiate hydrostatic pulmonary edema from increased permeability pulmonary edema,23 and for this reason it was included in the original panel of biomarkers for testing. BNP is secreted by the cardiac ventricles in response to increased stretch, as occurs in the setting of increased circulating volume or decompensated systolic or diastolic heart failure. We found that BNP levels were significantly lower in the patients with ALI/ARDS compared to those without ALI/ARDS, suggesting a higher prevalence of volume overload and/or cardiac dysfunction in the control group in this study. However, as a single biomarker, BNP performed very poorly for the discrimination of ALI/ARDS with an area under the curve of 0.64. The remaining four biomarkers in the model reflect other aspects of the pathophysiology of ALI/ARDS endothelial injury and activation and inflammation.
This study has some limitations. The diagnostic model was designed to differentiate patients with ALI/ARDS from those without evidence of lung injury (clear chest radiograph) and those with hydrostatic (cardiogenic) pulmonary edema. Differentiation of patients with ALI/ARDS from patients with hydrostatic pulmonary edema can be particularly challenging and may require invasive hemodynamic monitoring 23. However, we were only able to identify 11 patients with hydrostatic pulmonary edema in our cohort; it is difficult to predict whether the model will perform as well in a larger group of patients where hydrostatic edema is more prevalent. Also, it is possible that some of the patients with ALI/ARDS had a combination of both increased permeability and elevated hydrostatic pressure as the cause of their pulmonary edema, as has been documented in a recent large multicenter clinical trial 56. We also did not attempt to distinguish between ALI/ARDS due to pulmonary contusion and ALI/ARDS due to other causes such as aspiration of gastric contents, or severe hemorrhagic shock with multiple blood product transfusions. Pulmonary contusion is histologically similar to ALI/ARDS from other causes 57 and the underlying cause of ALI/ARDS may be difficult to ascertain with confidence even in the relatively homogeneous severe trauma population. Another limitation is the retrospective nature of this study. Although most of the clinical data was collected prospectively as part of the parent study, none of the investigators were involved in clinical care of the patients in this study, and all determinations of clinical diagnosis of ALI/ARDS were made by chart review and review of the chest radiographs. A prospective analysis of this diagnostic biomarker panel in unselected trauma patients will be needed for validation. Finally, the model was developed in patients who developed ALI/ARDS due to severe trauma. Although trauma is an important cause of ALI/ARDS, other clinical disorders including aspiration and infectious causes are also important. Thus, the value of this biomarker panel for diagnosis and pathogenesis of ALI/ARDS in patients with other risk factors for ALI requires further study.
Conclusions
In this retrospective nested case control study, a seven biomarker panel that includes plasma biomarkers of lung epithelial injury, collagen deposition, cardiac dysfunction, endothelial activation and injury and inflammation distinguished patients with trauma-associated ALI/ARDS from patients without ALI/ARDS. A reduced model containing only the top three biomarkers (RAGE, PCPIII, BNP) performed nearly as well as the full model. If validated prospectively, use of this biomarker panel could facilitate the clinical diagnosis of ALI/ARDS. Furthermore, this panel provides valuable insights into the pathological and biological mechanisms that may contribute to the early phase of trauma induced lung injury.
Footnotes
Meeting Presentations: This study was presented as a poster at the American Thoracic Society International Conference in Toronto in May 2008.
Contributor Information
Richard D. Fremont, Email: fremontr@yahoo.com.
Tatsuki Koyama, Email: tatsuki.koyama@vanderbilt.edu.
Carolyn S. Calfee, Email: carolyn.calfee@ucsf.edu.
William Wu, Email: william.wu@vanderbilt.edu.
Lesly A. Dossett, Email: lesly.dossett@vanderbilt.edu.
Fred R. Bossert, Email: frederick.r.bossert@vanderbilt.edu.
Daphne Mitchell, Email: daphne.mitchell@vanderbilt.edu.
Nancy Wickersham, Email: nancy.wickersham@vanderbilt.edu.
Gordon R. Bernard, Email: gordon.bernard@vanderbilt.edu.
Michael A. Matthay, Email: michael.matthay@ucsf.edu.
Addison K. May, Email: addison.may@vanderbilt.edu.
Lorraine B. Ware, Email: lorraine.ware@vanderbilt.edu.
References
- 1.Calfee CS, Eisner MD, Ware LB, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007 Oct;35(10):2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005 Aug;128(2):525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 3.Eisner M, Thompson T, Hudson L, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 4.Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 6.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 7.Ware LB, Matthay MA. Medical progress: The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 8.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 9.Goss CH, Brower RG, Hudson LD, Rubenfeld GD the ARDS Network. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 10.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 11.Ware LB, Eisner MD, Thompson BT, Parsons P, Matthay MA the Acute Respiratory Distress Syndrome Network. Significance of von Willebrand factor in septic and non-septic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 12.McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12(2):R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin DB, Wiener-Kronish JP, Murray JF, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis. J Clin Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 15.Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006 May 1;173(9):1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K the ARDS Network. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhakaran P, Ware L, White K, Cross M, Matthay M, Olman M. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L20–L28. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 18.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005 Oct;33(4):319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dossett LA, Heffernan D, Lightfoot M, et al. Obesity and pulmonary complications in critically injured adults. Chest. 2008 Nov;134(5):974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dossett LA, Swenson BR, Evans HL, Bonatti H, Sawyer RG, May AK. Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surg Infect (Larchmt) 2008 Feb;9(1):41–48. doi: 10.1089/sur.2007.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dossett LA, Swenson BR, Heffernan D, et al. High levels of endogenous estrogens are associated with death in the critically injured adult. J Trauma. 2008 Mar;64(3):580–585. doi: 10.1097/TA.0b013e31816543dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May AK, Dossett LA, Norris PR, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. 2008 Jan;36(1):62–68. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005 Dec 29;353(26):2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Haponik EF. Using the chest radiograph to determine intravascular volume status. The role of vascular pedicle width. Chest. 2002;121:942–950. doi: 10.1378/chest.121.3.942. [DOI] [PubMed] [Google Scholar]
- 25.Ely EW, Smith AC, Chiles C, et al. Radiologic determination of intravascular volume status using portable, digital chest radiography: A prospective investigation in 100 patients. Crit Care Med. 2001;29:1502–1512. doi: 10.1097/00003246-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Knaus W, Wagner D, Draper E, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 27.Baker SP, O'Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974 Mar;14(3):187–196. [PubMed] [Google Scholar]
- 28.Boyd CR, Tolson MA, Copes WS. Evaluating trauma care: the TRISS method. Trauma Score and the Injury Severity Score. J Trauma. 1987 Apr;27(4):370–378. [PubMed] [Google Scholar]
- 29.Milne EN, Pistolesi M, Miniati M, Giuntini C. The vascular pedicle of the heart and the vena azygos. Part I: The normal subject. Radiology. 1984 Jul;152(1):1–8. doi: 10.1148/radiology.152.1.6729098. [DOI] [PubMed] [Google Scholar]
- 30.Harrell FEJ. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag New York, Inc; 2001. [Google Scholar]
- 31.Effron B, Tibshirani R. An introduction to the bootstrap. New York: Chapmna & Hall; 1993. [Google Scholar]
- 32.Dicker RA, Morabito DJ, Pittet JF, Campbell AR, Mackersie RC. Acute respiratory distress syndrome criteria in trauma patients: why the definitions do not work. J Trauma. 2004 Sep;57(3):522–526. doi: 10.1097/01.ta.0000135749.64867.06. discussion 526-528. [DOI] [PubMed] [Google Scholar]
- 33.Salim A, Martin M, Constantinou C, et al. Acute respiratory distress syndrome in the trauma intensive care unit: Morbid but not mortal. Arch Surg. 2006 Jul;141(7):655–658. doi: 10.1001/archsurg.141.7.655. [DOI] [PubMed] [Google Scholar]
- 34.Salim A, Martin M, Brown C, et al. The presence of the adult respiratory distress syndrome does not worsen mortality or discharge disability in blunt trauma patients with severe traumatic brain injury. Injury. 2008 Jan;39(1):30–35. doi: 10.1016/j.injury.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Treggiari MM, Hudson LD, Martin DP, Weiss NS, Caldwell E, Rubenfeld G. Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med. 2004;32:327–331. doi: 10.1097/01.CCM.0000108870.09693.42. [DOI] [PubMed] [Google Scholar]
- 36.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006 Feb;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 37.Albertine KH, Soulier MF, Wang Z, et al. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977;116:589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- 39.Calfee CS, Ware LB, Eisner MD, et al. Plasma Receptor for Advanced Glycation End-Products And Clinical Outcomes in Acute Lung Injury. Thorax. 2008;63:1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank J, Gutierrez J, Jones K, Allen L, Dobbs L, Matthay M. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med. 2002;165:242–249. doi: 10.1164/ajrccm.165.2.2108087. [DOI] [PubMed] [Google Scholar]
- 41.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990;142:1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 42.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 43.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 44.Laffon M, Lu LN, Modelska K, Matthay MA, Pittet JF. alpha-adrenergic blockade restores normal fluid transport capacity of alveolar epithelium after hemorrhagic shock. Am J Physiol. 1999 Oct;277(4 Pt 1):L760–L768. doi: 10.1152/ajplung.1999.277.4.L760. [DOI] [PubMed] [Google Scholar]
- 45.Modelska K, Matthay MA, Brown LAS, Deutsch E, Lu L, Pittet JF. Inhibition of beta-adrenergic dependent alveolar epithelial clearance by oxidant mechanisms after hemorrhagic shock in rats. Am J Physiol. 1999;276:L844–L857. doi: 10.1152/ajplung.1999.276.5.L844. [DOI] [PubMed] [Google Scholar]
- 46.Modelska K, Matthay MA, Pittet JF. Prolonged hemorrhagic shock in rats causes an oxidant mediated decrease in alveolar epithelial fluid transport capacity. Am J Respir Crit Care Med. 1998;157(3):A350. [Google Scholar]
- 47.Chesnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. Am J Respir Crit Care Med. 1997;156:840–845. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 48.Low RB, Cutroneo KR, Davis GS, Giancola MS. Lavage type III procollagen N-terminal peptides in human pulmonary fibrosis and sarcoidosis. Lab Invest. 1983 Jun;48(6):755–759. [PubMed] [Google Scholar]
- 49.Low RB, Giancola MS, King TE, Jr, Chapitis J, Vacek P, Davis GS. Serum and bronchoalveolar lavage of N-terminal type III procollagen peptides in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1992 Sep;146(3):701–706. doi: 10.1164/ajrccm/146.3.701. [DOI] [PubMed] [Google Scholar]
- 50.Raedsch R, Stiehl A, Sieg A, Walker S, Kommerell B. Biliary excretion of procollagen type III peptide in healthy humans and in patients with alcoholic cirrhosis of the liver. Gastroenterology. 1983 Dec;85(6):1265–1270. [PubMed] [Google Scholar]
- 51.Weber KT. Monitoring tissue repair and fibrosis from a distance. Circulation. 1997 Oct 21;96(8):2488–2492. [PubMed] [Google Scholar]
- 52.Clark JG, Milberg JA, Steinberg KP, Hudson LD. Type III procollagen peptide in the adult respiratory distress syndrome. Ann Intern Med. 1995;122:17–23. doi: 10.7326/0003-4819-122-1-199501010-00003. [DOI] [PubMed] [Google Scholar]
- 53.Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158:1432–1441. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 54.Marshall R, Bellingan G, Webb S, et al. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162:1783–1788. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 55.Waydhas C, Nast-Kolb D, Trupka A, et al. Increased serum concentrations of procollagen type III peptide in severely injured patients: an indicator of fibrosing activity? Crit Care Med. 1993 Feb;21(2):240–247. doi: 10.1097/00003246-199302000-00016. [DOI] [PubMed] [Google Scholar]
- 56.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006 Jun 15;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 57.Cohn SM. Pulmonary contusion: review of the clinical entity. J Trauma. 1997 May;42(5):973–979. doi: 10.1097/00005373-199705000-00033. [DOI] [PubMed] [Google Scholar]


