Summary
Purpose
To examine baseline and prospective (2-year) changes in third, fourth, and lateral ventricle volumes in children with new-onset idiopathic epilepsies and controls (age 8–18 years).
Methods
Structural magnetic resonance imaging (MRI) were collected from children with idiopathic generalized epilepsy (IGE, n = 29), idiopathic localization-related epilepsy (ILRE, n = 30), and healthy controls (HCs, n = 49). Volumes of the third, fourth, and lateral ventricles were derived and compared across groups, followed by shape analyses, to identify specific regions of ventricular abnormality. Of the initial cohort, a consecutive sample of 71 children returned 2 years later for reimaging and determination of progressive changes in the ventricular system.
Key Findings
At baseline, children with new-onset IGE had significantly larger lateral and third ventricle volumes relative to the HC group. In addition, lateral ventricle enlargement in IGE was significantly greater compared to new-onset ILRE. Shape analysis of the lateral ventricles revealed that volume expansion in IGE was selective for the anterior horn, a region surrounded by the lateral and medial frontal lobes as well as basal ganglia. These abnormalities did not progress over a 2-year interval.
Significance
Abnormalities in brain development prior to onset and diagnosis of epilepsy are evident and reflected in expansion of the ventricular system, especially among children with IGE. These abnormalities appear to represent an antecedent and possibly static finding given the lack of progressive ventricular expansion over the 2-year interval following diagnosis and treatment.
Keywords: Epilepsy/Seizures, Volumetric MRI use in epilepsy, Pediatric epilepsy
Abnormal expansion of the ventricular system has been observed in many neurologic and psychiatric disorders, including dementia (Carmichael et al., 2007), multiple sclerosis (Dalton et al., 2006) and autism spectrum disorder (Palmen et al., 2005). The natural history of ventricular enlargement and its consequences have been increasingly investigated, and in some disorders such as schizophrenia, enlargement is present at the onset of the disorder (Sowell et al., 2000; Vita et al., 2006) and progresses over time (Kempton et al., 2010). In the literature on aging, age-accelerated ventricular enlargement may herald the cognitive decline associated with the onset and progression of Alzheimer’s disease (Nestor et al., 2008).
In 1917, Thom was the first to note ventricle enlargement in individuals with epilepsy; he reported lateral ventricle dilation following postmortem examinations of patients with idiopathic epilepsy (Thom, 1917). But over the decades, interest in the ventricular system has never matched that seen in other disorders. Recently, Kalnin et al. (2008) used a qualitative magnetic resonance imaging (MRI) scoring system to identify brain abnormalities in children with new-onset epilepsies. Interestingly, enlarged ventricles were the most common finding among those with imaging abnormalities (seen in 51% of abnormal scans), a potentially clinically significant finding as mild generalized cognitive impairment is present in new-onset pediatric epilepsy (Oostrom et al., 2003; Hermann et al., 2006; Fastenau et al., 2009).
Herein we examine volumes of the third, fourth, and lateral ventricles in children with new/recent-onset idiopathic generalized (IGE) and idiopathic localization-related epilepsy (ILRE) and healthy controls (HCs). Shape analysis was subsequently used to determine whether identified volumetric abnormalities were diffuse or localized. A consecutive subset of children were seen 2 years later to facilitate examination of whether ventricle enlargement was progressive in nature or reflected a static neurodevelopmental abnormality.
Methods
Participants
Study participants were age 8–18 years of age and included HCs (n = 49, 27 female) and participants with ILRE (n = 30, 14 female) or IGE (n = 29, 12 female). Criteria for the patient groups included a diagnosis of epilepsy within the preceding 12 months, no other developmental disabilities or neurologic disorders, normal neurologic examinations, and normal clinical MRIs. The clinical charts for all patients were reviewed by a board-certified pediatric neurologist to confirm that all patients had idiopathic epilepsy, that their syndromes were appropriately classified, and that clinically obtained MRIs were normal by conventional visual inspection. Specific syndromes were identified using the modified diagnostic criteria published by the International League Against Epilepsy (ILAE) Task Force on Classification and Terminology (Engel and International League Against Epilepsy, 2001). Table 1 presents the distribution of specific epilepsy syndromes within both ILRE and IGE groups.
Table 1.
Specific epilepsy syndromes represented within the ILRE and IGE groups
| Group | Syndrome | N |
|---|---|---|
| ILRE | BECTS | 10 |
| TLE | 4 | |
| Focal-NOS | 10 | |
| Frontal | 6 | |
| IGE | CAE | 3 |
| JAE | 4 | |
| JME | 22 |
Number (N) of participants with specific syndromes based on the modified International League Against Epilepsy (ILAE) Task Force on Classification and Terminology (Engel and International League Against Epilepsy, 2001) diagnostic criteria.
ILRE, Idiopathic localization-related epilepsies; IGE, idiopathic generalized epilepsies; BECTS, benign childhood epilepsy with centrotemporal spikes; TLE, temporal lobe epilepsy; NOS, not otherwise specified; CAE, childhood absence epilepsy; JAE, juvenile absence epilepsy; JME, juvenile myoclonic epilepsy.
Control participants were first-degree cousins who were age and gender matched with no history of seizures, early initial precipitating injuries (e.g., febrile convulsions), other developmental or neurologic disease, or loss of consciousness >5 min. Participants in both groups attended regular school. Further details regarding subject selection criteria have been published previously by our group (Hermann et al., 2006). Relevant demographic and clinical characteristics of the sample are provided in Table 2.
Table 2.
Demographic and clinical characteristics for each group
| Group
|
Comparisonsc | |||
|---|---|---|---|---|
| HC (n = 49) | ILRE (n = 30)a | IGE (n = 29)b | ||
| Aged | 161 (37.36) | 136.30 (32.70) | 175.72 (38.19) | 0.004: HC > ILRE 0.094: HC ≈ IGE < 0.0001: IGE > ILRE |
| Female | 27 (55.1%) | 14 (46.7%) | 12 (41.4%) | |
| IQe | 107.55 (12.47) | 101.53 (13.28) | 103.21 (12.55) | 0.046: HC > ILRE 0.142: HC ≈ IGE 0.621: IGE ≈ ILRE |
| Durationf | – | 6.31 (3.71) | 5.9 (3.6) | 0.757: ILRE ≈ IGE |
| AEDg | – | 76.6 | 100 | 0.009: ILRE > IGE |
Idiopathic localization-related epilepsies.
Idiopathic generalized epilepy.
Probability of a reliable difference.
Age in months.
Full-Scale Wechsler Abbreviated Scale of Intelligence, scaled for age.
Duration of epilepsy as a proportion of participant age calculated as (duration of epilepsy/age of diagnosis).
Percentage of participants taking an antiepileptic drug at time of study.
Standard protocol approvals registrations and patient consents
Approval to conduct this study was granted by the University of Wisconsin School of Medicine and Public Health, Health Sciences Institutional Review Board. Written informed consent was obtained from all legal guardians of the children and adolescents participating in the study; written informed consent was obtained from research participants age 18, and written informed assent was obtained from research participants age 8–17.
Imaging protocol and analyses
Images were obtained on a 1.5 T GE Signa MRI scanner (GE Healthcare, Waukesha, WI, U.S.A.). Sequences acquired for each participant included the following: (1) T1-weighted, three-dimensional (3D) spoiled gradient recall (SPGR) acquired with the following parameters: TE = 5 ms, TR = 24 ms, flip angle = 40 degrees, NEX = 1, slice thickness = 1.5 mm, slices = 124, plane = coronal, field of view (FOV) = 200 mm, matrix = 256 × 256; (2) proton density (PD); and (3) T2-weighted images acquired with the following parameters: echo time (TE) = 36 ms (for PD) or 96 ms (for T2), repetition time (TR) = 3,000 ms, number of excitations (NEX) = 1, slice thickness = 3.0 mm, slices = 64, slice plane = coronal, FOV = 200 mm, matrix = 256 × 256. Images were transferred to a Mac OSX computer for processing with the FreeSurfer image analysis suite (Apple, Cupertino, CA, U.S.A.), a set of software tools for the study of cortical and subcortical anatomy that is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu). A brief technical presentation of these procedures as used in our laboratory is presented in Dabbs et al., 2009.
Shape analysis of the lateral ventricles was performed using FIRST (FSL, http://www.fmrib.ox.ac.uk/fsl). A multivariate Gaussian model of vertex location and intensity variation was employed to automatically generate the most probable surface mesh for the lateral ventricles of each subject. The same number and labeling of vertices was used to enable point-to-point comparisons across all subjects. Following the generation of surface meshes for each subject, corresponding vertex points were aligned to the mean surface of the template lateral ventricle in Montreal Neurological Institute 152 space. This analysis generates a deformable mesh of vertices and the relative position of each corresponding vertex was compared between IGE and HC as well as between ILRE and HC, to define specific regions of ventricular alterations. F-statistics were then carried out in order to compare shape differences (Patenaude et al., 1989; Zarei et al., 2010).
Statistical analyses
FreeSurfer segmentation yields volumes for the third and fourth ventricles that were used to compare groups. The outputs for the lateral and inferior lateral ventricles were generated separately for the left and right hemispheres. For these ventricular components, the lateral and inferior lateral volumes were summed across hemispheres for each subject. Choroid plexus volume is additionally included in the measure of total lateral ventricle, referred to as full lateral ventricle (Fig. 1). The segmentation process also yields a measure of intracranial volume (ICV) that captures variability in head size (i.e., due to growth, age and sex) across subjects. We computed a ratio representing the proportion of ventricular component for each individual’s ICV. Therefore, our primary analyses included three ventricular system component ratio measures for each subject representing the third, fourth, and full lateral ventricles (see Data S1 for further imaging processing details; Figs. S1 and S2).
Figure 1.
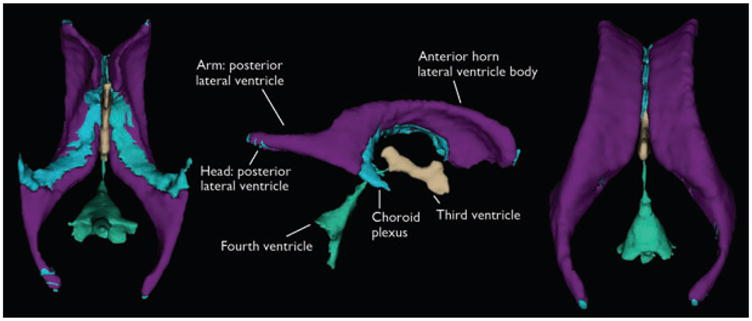
Visualization of ventricular component segmentation produced from FreeSurfer for an individual participant. The views are from the ventral aspect with the anterior margin at the top (left view), lateral aspect with the anterior margin to the right (middle view), and dorsal aspect with the anterior margin at the top (right view).
Epilepsia © ILAE
Before we conducted our analyses, we specifically examined the nature of ventricular volume distributions across participant groups for each ventricular component. Examination of each ventricular index by group using the Shapiro-Wilk test revealed that these distributions were significantly skewed for both HC and IGE patients. ([HC third ventricle: W (49) = 0.944, p = 0.021; fourth ventricle: W (49) = 0.950, p = 0.036; full lateral ventricle: W (49) = 0.853, p < 0.001.] [ILRE third ventricle: W (30) = 0.965, p = ns; fourth ventricle: W (30) = 0.958, p = ns; full lateral ventricle: W (30) = 0.965, p < ns.] [IGE third ventricle: W (29) = 0.811, p < 0.001; fourth ventricle: W (29) = 0.959, p = ns; full lateral ventricle: W (29) = 0.861, p = 0.001.]) It is important to note that ICV was not skewed for any of the participant groups. Because the indices of ventricular component volumes were not normally distributed, analyses used nonparametric methods that included Kruskal-Wallis one-way analysis of variance (ANOVA) with pair-wise group differences examined by Mann-Whitney U tests. Within-group time 1 to time 2 comparisons were analyzed using related-samples Wilcoxon signed-rank test.
Results
Figure 2 presents standardized rank volumes for each of the ventricular components by group, and Table 3 contains raw means and standard deviations for each component.
Figure 2.
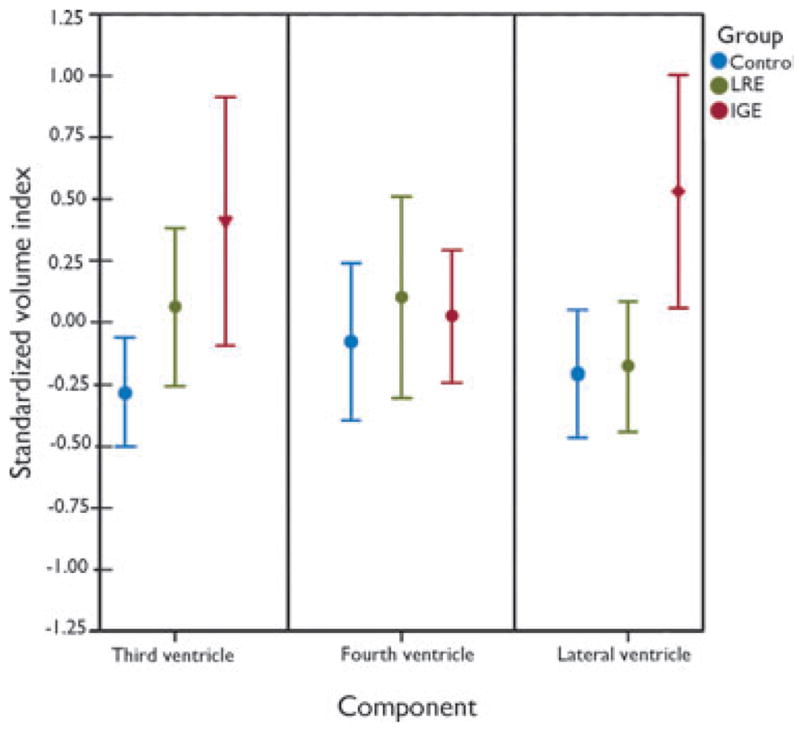
Standardized (z-scores) volume index means (±95% confidence interval, CI) as a function of ventricular component. HC, healthy controls; ILRE, localization-related epilepsy; IGE, ideographic-generalized epilepsy. Volume indices were standardized within component across groups. The inverted triangle marker indicates the mean volume rank of IGE participants was significantly greater than for HC participants. The diamond marker indicates the mean volume rank of the IGE participants was reliably greater than both ILRE and HC participants. See the Results section for details on the analyses. There were no other significant within-component pair-wise effects.
Epilepsia © ILAE
Table 3.
Means (standard deviations) of the segmented volumes for each ventricular component for each group
| Ventricular components and indices | Group (sample size)
|
||
|---|---|---|---|
| Control (n = 49) | LRE (n = 30) | IGE (n = 29) | |
| ICV (mm3) | 1493104.92 (126137.55) | 1496942.99 (134491.42) | 1509495.44 (157310.09) |
| Third ventricle (mm3) | 666.31 (138.67) | 729.90 (156.87) | 803.55 (264.10) |
| Third ventricle index | 0.0004 (0.00009) | 0.0005 (0.00010) | 0.0005 (0.00016) |
| Fourth ventricle (mm3) | 1803.31 (632.87) | 1892.40 (581.05) | 1884.02 (468.25) |
| Fourth ventricle index | 0.0012 (0.00041) | 0.0013 (0.00040) | 0.0012 (0.00026) |
| Lateral ventricle (mm3) | 10887.53 (5481.07) | 10782.63 (3389.95) | 14790.97 (7107.78) |
| Full lateral ventricle index | 0.0072 (0.00307) | 0.0073 (0.00240) | 0.0097 (0.00426) |
The component indices are calculated as the quotient of the component and the intracranial volume (ICV) for each participant.
ILRE, idiopathic localization-related epilepsy; IGE, idiopathic generalized epilepsy.
Fourth ventricle
There was no significant group difference in fourth ventricle volume, χ2 (2, N = 108) = 1.078, p = 0.583.
Third ventricle
There was a significant group difference in third ventricle volume, χ2; (2, N = 108) = 6.495, p = 0.039, driven by larger volumes in the IGE group (mean rank 47.59) compared to HC (mean rank 34.71) (Mann-Whitney U = 476, z = −2.425, p = 0.015). There were no differences between either ILRE (mean rank 38.37) and HC (mean rank 42.67) participants (z = −0.808, p = 0.419) or ILRE (mean rank 28.53) and IGE (mean rank 31.52) participants (z = −0.667, p = 0.505). Therefore, the volume of the third ventricle differentiated IGE from HC participants only.
Lateral ventricles
There was a significant group difference in lateral ventricle volume, chi-square (2, n = 108) = 9.833, p = 0.007. Volumes were similar between the ILRE (mean rank 41.77) and HC (mean rank 38.92) participants (z = −0.535, p = 0.592). However, volumes were larger for IGE (mean rank 49.38) compared to HC (mean rank 33.65) participants (Mann-Whitney U = 424, z = −2.962, p = 0.003) and IGE (mean rank 35.55) compared to ILRE (mean rank 24.63) participants (Mann-Whitney U = 274, z = −2.441, p = 0.015). Therefore, lateral ventricle volume differentiated IGE from both ILRE and HC participants, with no difference between the latter two groups. Furthermore, there were no asymmetry effects when comparing left versus right lateral ventricle indices across all groups.
Shape analysis of lateral ventricles
Shape analysis revealed local expansion of surface anatomy of the lateral ventricles in IGE, when compared to HC (Fig. 3). Furthermore, the ventricular expansions were localized to the anterior horn. On the right side, ventricular enlargement was selective for brain regions surrounded by the lateral frontal lobe as well as medial frontal lobe and basal ganglia, whereas on the left side, the ventricular expansion focused more in regions surrounded by the medial frontal lobe and basal ganglia. No significant shape alteration was noted between ILRE and HC groups.
Figure 3.
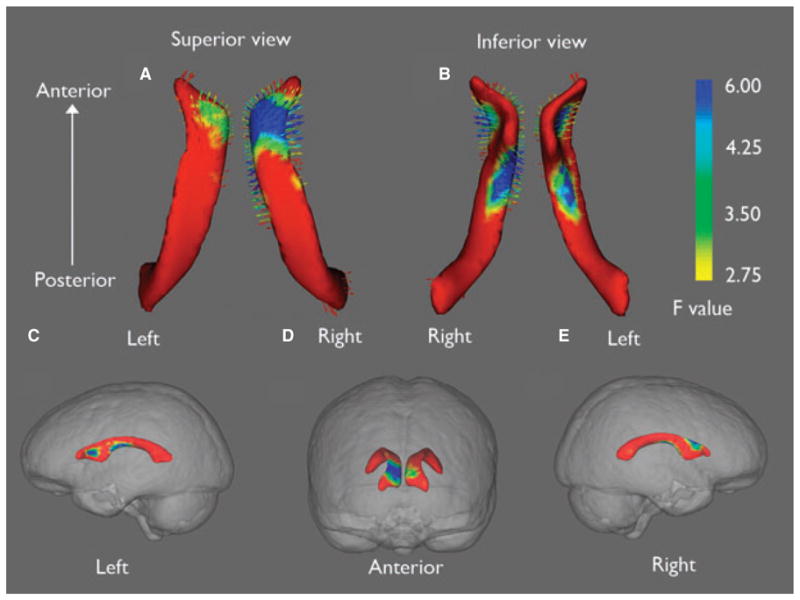
Selective expansion of lateral ventricles in IGE. Shape analysis showed expansion (arrows pointing outward) of lateral ventricles is selective mainly for bilateral superior horns (A, B). Lateral ventricle shape differences are superimposed on glass brains to illustrate approximate brain regions surrounding areas of ventricular expansion (C, D, E). On the right side, ventricular enlargement is located in brain regions surrounding lateral and medial frontal lobe as well as basal ganglia (C, D). On the left side, the ventricular expansion is more localized to regions surrounding the medial frontal region and basal ganglia (D, E). Color bar indicates the statistic for the shape analysis, F (3,74) with associated probabilities of p = 0.12 (red), p = 0.02 (green), and p = 0.003 (blue).
Epilepsia © ILAE
Longitudinal data
To date, 71 consecutive participants (19 IGE, 21 ILRE, 31 HCs) have completed a second MRI assessment 2 years after baseline data were collected. Interval volume change was examined to identify any group differences in ventricular volume changes over time. No significant effects were found for third, fourth, or lateral ventricles in either patients or controls. Therefore, the baseline abnormalities appear stable over time.
Discussion
Structural brain abnormalities may play an important role in the cognitive and behavioral comorbidities that are commonly reported among children with epilepsy. Most quantitative neuroimaging studies to date have focused on gray and white matter volumes and/or cortical thickness measures (but see Kalnin et al., 2008). Of importance, this literature has focused almost exclusively on children with established and chronic epilepsy; the natural history of the identified abnormalities remain uncertain—a question that could be addressed by examining children with new-onset epilepsies at baseline and following them prospectively. In addition, very little attention has been paid to ventricular anomalies—abnormalities that have been carefully studied in a variety of pediatric and adult neurologic and psychiatric disorders, as reviewed earlier. In the current study, we show that children with new-onset IGEs exhibit significantly larger lateral and third ventricle volumes relative to healthy controls. In addition, the lateral ventricle enlargement associated with IGE was significantly greater than that associated with new-onset idiopathic localization-related epilepsies, representing a distinguishing characteristic of new-onset IGE as compared to new-onset ILRE. These findings support and extend the observations of Kalnin et al. (2008) in which ventricular enlargement was the most common brain abnormality in children with new-onset epilepsy.
This finding of enlarged ventricles at or near the time of seizure onset is intriguing in that mild diffuse cognitive impairment in new-onset epilepsy has been reported by several groups (e.g., Oostrom et al., 2003; Fastenau et al., 2009), a finding that is apparent even among children with “epilepsy only,” that is, children with normal intelligence who are attending regular schools and who have normal neurologic evaluations and normal clinical MRIs. In addition, several groups have reported that academic difficulties, psychiatric comorbidities such as depression and attention-deficit/hyperactivity disorder (ADHD), and general emotional–behavioral problems clearly antedate the first recognized seizure, and diagnosis and treatment of epilepsy in a substantial proportion of children with new-onset epilepsies (Austin et al., 2001; Berg et al., 2005; Hermann et al., 2006; Jones et al., 2007). Although reliably reported by several groups, it has proven difficult to identify the factors underlying this apparent abnormal early neurodevelopmental course. Perhaps ventricular enlargement at onset may serve as an easily derived imaging biomarker of abnormal antecedent neurodevelopment that may be related to the risk of preepilepsy neurobehavioral and cognitive comorbidities. Furthermore, the degree to which baseline ventricular enlargement is associated with anomalies in subsequent (postdiagnosis and treatment) brain development, cognition, and behavior is worthy of investigation, especially given that this approach has proven useful in other disorders. We plan to examine prospective cognitive development across epilepsy subtypes, in order to identify syndome- and subsyndrome-specific cognitive abnormalities and assess their relation to ventricular enlargement in IGE and ILRE participants.
Differences in the specific nature and pattern of ventricular abnormalities (including shape variations) at baseline, and the time course of changes in ventricle volume and shape, appear to be valuable indices with which to characterize disease-specific factors in both children and adults (Styner et al., 2005; Qui et al., 2009); we examined these relationship in detail here. In the current study, shape analysis demonstrated that the ventricular volume expansion in IGE was selective for the anterior horn. This region of the lateral ventricle is surrounded by lateral and medial frontal lobes as well as basal ganglia. Because total intracranial volume remains relatively constant throughout the life span (Courchesne et al., 2000), the expansion of the anterior horn might suggest concomitant decrease in frontal lobe and basal ganglia brain volume. Further evaluation of the frontal lobe and basal ganglia is required to validate these hypotheses. The ventricular enlargement may be a function of syndrome-specific anatomic abnormalities underlying IGE versus ILRE. These results are consistent with the notion that IGE likely involves a more distributed anatomic network than ILRE. Our most robust finding was the enlargement of lateral ventricles, which distinguished IGE from HC as well as IRLE. Furthermore, the ventricular expansion was most evident in the bilateral frontal horns, regions that are surrounded by medial frontal lobe and basal ganglia. Indeed, these two regions have been consistently implicated in the anatomic network of chronic IGE (Keller et al., 2011; Luo et al., 2011; O’Muircheartaigh et al., 2011; Vollmar et al., 2011). Therefore, the findings of the current study not only provide additional support for the critical role of frontostriatal network in IGE but provide a new understanding that such network disruption occurs early in the course of epilepsy and appears to be static over 2 years. Such involvement would also help to explain some of the cognitive deficits such as executive dysfunction commonly observed in IGE but not ILRE (Pulsipher et al., 2009).
It is notable that we did not find evidence for progressive abnormality in ventricle volume for the epilepsy groups over the 2-year follow-up interval. The ventricular abnormalities in the ILRE and IGE groups thus appear to be nonprogressive in nature. That said, we appreciate the possibility that more sophisticated neuroimaging approaches (e.g., shape analyses) might detect further progressive alterations. As mentioned previously, evidence of enlarged ventricles at or shortly after the time of diagnosis implies a result from antecedent neurodevelopmental abnormalities that—at least for the subsequent 2-year period following diagnosis—do not worsen or progress in a major fashion. However, a limitation of the current study is that we cannot rule out the possibility that there may be progressive changes in subsequent years, as our follow-up 2-year evaluation represents a relatively short developmental window. We are now in the process of reexamining these children and adolescents 5–6 years following their baseline evaluation to further address longer-term prospective ventricular enlargement.
It will be important for future studies of ventricular volumes in pediatric epilepsy populations to identify and evaluate both IGE and ILRE participants. The number of participants examined in the current study is modest and larger participant pools would facilitate examination of specific ILRE (e.g., benign childhood epilepsy with centrotemporal spikes) and IGE (e.g., juvenile myoclonic epilepsy) groups in order to more fully characterize these and other structural findings. Furthermore, our age range is broad (8–18 years), and a larger group of participants would allow finer examination of narrower age groupings in order to determine whether there is age dependence of this ventricular expansion.
Despite these limitations, it appears clear that careful quantitative analysis of ventricular volumes in children with epilepsy is useful, as has been shown to be the case in several other pediatric and adult neurologic and psychiatric disorders. Baseline and prospective analysis of new-onset cases clearly provides unique information regarding the neurodevelopmental abnormalities that are antecedent to the onset and diagnosis of pediatric epilepsy and the changes that may be associated with the development of recurrent seizures and their treatment.
Current clinical practice does not call for quantitative analyses of ventricular volume or shape visualized on brain MRI studies except in situations where there are established clinical implications, as for example in the setting of progressive hydrocephalus. If a consistent relation between ventricular expansion and current (and future) neurobehavioral comorbidities can be demonstrated in children with IGE, quantitative assessment of ventricular anatomy may help to identify children at risk of neurobehavioral comorbidities early in their epilepsy course so that interventions may be put into place for them.
Supplementary Material
Figure S1. The choroid plexus within the lateral (body) and fourth ventricles as seen in a coronal section of an individual T1-weighted MRI image.
Figure S2. Visualization of ventricular component segmentation produced from FreeSurfer for an individual participant. The view is from the ventral aspect with the anterior margin at the top and left hemisphere on the right side of the figure.
Acknowledgments
The authors thank Cory A. Burghy and Natalie M. Walker for their numerous contributions to the preparation of the text and figures. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Funding: Supported by NIH (NINDS RO1 44351).
Footnotes
Disclosures
DCJ, WI, and KD, and MS report no disclosures.
JJL receives research support from NIH (NINDS K-23 NS060993).
JEJ and DAH receive research support from NIH [NINDS RO1 44351 (coinvestigators)].
CES has received compensation as a consultant for Questcor Pharmaceuticals, serves on the Scientific Board of The Charlie Foundation, serves as an Associate Editor of Epilepsia and Coeditor of Epilepsy Currents, has received royalties from publication of the books: (1) Epilepsy and the Ketogenic Diet, and (2) Epilepsy: Mechanisms, Models and Translational Perspectives, and received support from NIH [NINDS RO1 44351 (coinvestigator)].
BPH serves as an Associate Editor for Epilepsia and receives research support from the NIH [NINDS 2RO1 NS44351 (PI), RO1 AG027161 (coinvestigator), P50AG3314 (coinvestigator), 1RO1NS064034 (coinvestigator), and RO1AG031790 (coinvestigator)]. DCJ, WI, JJL, and BH drafted and revised the manuscript. DCJ, WI, KD, and JJL performed image and statistical analyses. JEJ, DAH, CES, and MS contributed to interpretation of the data.
References
- Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107:115–122. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- Berg AT, Smith SN, Frobish D, Levy SR, Testa FM, Beckerman B, Shinnar S. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol. 2005;47:749–753. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC, Liu Y, Toga AW, Becker JT. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007;21:14–24. doi: 10.1097/WAD.0b013e318032d2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Dabbs K, Jones J, Seidenberg M, Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav. 2009;15:445–451. doi: 10.1016/j.yebeh.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton CM, Miszkiel KA, O’Connor PW, Plant GT, Rice GPA, Miller DH. Ventricular enlargement in MS: one-year change at various stages of disease. Neurology. 2006;66:693–698. doi: 10.1212/01.wnl.0000201183.87175.9f. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr International League Against Epilepsy. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- Fastenau PS, Johnson CS, Perkins SM, Byars AW, de Grauw TJ, Austin JK, Dunn DW. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009;73:526–534. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- Jones JE, Watson R, Sheth R, Caplan R, Koehn M, Seidenberg M, Hermann B. Psychiatric comorbidity in children with new onset epilepsy. Dev Med Child Neurol. 2007;49:493–497. doi: 10.1111/j.1469-8749.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- Kalnin AJ, Fastenau PS, de Grauw TJ, Musick BS, Perkins SM, Johnson CS, Matthews VP, Egelhoff JC, Dunn DW, Austin JK. Magnetic resonance imaging findings in children with a first recognized seizure. Pediatr Neurol. 2008;39:404–414. doi: 10.1016/j.pediatrneurol.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Ahrens T, Mohammadi S, Moddel G, Kugel H, Bernd Ringelstein E, Deppe M. Microstructural and volumetric abnormalities of the putamen in juvenile myoclonic epilepsy. Epilepsia. 2011;52:1715–1724. doi: 10.1111/j.1528-1167.2011.03117.x. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Stahl D, Williams SCR, De Lisi LE. Progressive lateral ventricular enlargement in schizophrenia: a meta-analysis of longitudinal MRI studies. Schizophr Res. 2010;120:54–62. doi: 10.1016/j.schres.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Luo C, Xia Y, Li Q, Xue K, Lai Y, Gong Q, Zhou D, Yao D. Diffusion and volumetry abnormalities in subcortical nuclei of patients with absence seizures. Epilepsia. 2011;52:1092–1099. doi: 10.1111/j.1528-1167.2011.03045.x. [DOI] [PubMed] [Google Scholar]
- Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R Alzheimer’s Disease Neuroimaging Initiative. Ventricular enlargement as a possible measure of Alzheimer’s disease progression validated using the Alzheimer’s disease neuroimaging initiative database. Brain. 2008;131:2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Muircheartaigh J, Vollmar C, Barker GJ, Kumari V, Symms MR, Thompson P, Duncan JS, Koepp MJ, Richardson MP. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology. 2011;76:34–40. doi: 10.1212/WNL.0b013e318203e93d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostrom KJ, Smeets-Schouten A, Kruitwagen CLJJ, Peters ACB, Jennekens-Schinkel A Dutch Study Group of Epilepsy in Childhood. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only” —a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–1344. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, Hulshoff Pol HE, Kemner C, Schnack HG, Durston S, Lahuis BE, Kahn RS, Van Engeland H. Increased gray-matter volume in medication-naïve high-functioning children with autism spectrum disorder. Psychol Med. 2005;35:561–570. doi: 10.1017/s0033291704003496. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M. FMRIB Technical Report TR07BP1. Oxford: 1989. Bayesian Shape and Appearance Models. [Google Scholar]
- Pulsipher D, Seidenberg M, Guidotti L, Tuchscherer VN, Morton J, Sheth RD, Hermann B. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009;50:1210–1219. doi: 10.1111/j.1528-1167.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui A, Fennema-Notestine C, Dale AM, Miller MI Alzheimer’s Disease Neuroimaging Initiative. Regional shape abnormalities in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2009;45:656–661. doi: 10.1016/j.neuroimage.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Levitt J, Thompson PM, Holmes CJ, Blanton RE, Kornsand DS, Caplan R, McCracken J, Asarnow R, Toga AW. Brain abnormalities in early-onset schizophrenia spectrum disorder observed with statistical parametric mapping of structural magnetic resonance images. Am J Psychiatry. 2000;157:1475–1484. doi: 10.1176/appi.ajp.157.9.1475. [DOI] [PubMed] [Google Scholar]
- Styner M, Lieberman JA, McClure RK, Weinberger DR, Jones DW, Gerig G. Morphometric analysis of lateral ventricles in schizophrenia and healthy controls regarding genetic and disease-specific factors. Proc Natl Acad Sci USA. 2005;102:4872–4877. doi: 10.1073/pnas.0501117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom DA. Dilation of the lateral ventricles as a common brain lesion in epilepsy. J Nerv Ment Dis. 1917;46:355–358. [Google Scholar]
- Vita A, De Peri L, Silenz C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82:75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Vollmar C, O’Muircheartaigh J, Barker GJ, Symms MR, Thompson P, Kumari V, Duncan JS, Dieter J, Richardson MP, Koepp MJ. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain. 2011;134:1710–1719. doi: 10.1093/brain/awr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, Barkhof F, Rombouts SA, Sanz-Arigita E, Jenkinson M. Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer’s disease. Neuroimage. 2010;49:1–8. doi: 10.1016/j.neuroimage.2009.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The choroid plexus within the lateral (body) and fourth ventricles as seen in a coronal section of an individual T1-weighted MRI image.
Figure S2. Visualization of ventricular component segmentation produced from FreeSurfer for an individual participant. The view is from the ventral aspect with the anterior margin at the top and left hemisphere on the right side of the figure.


