Abstract
The calcium-activated potassium channel KCa3.1 regulates membrane potential and calcium signaling in erythrocytes, activated T and B cells, macrophages, microglia, vascular endothelium, epithelia, and proliferating vascular smooth muscle cells and fibroblasts. KCa3.1 has therefore been suggested as a potential therapeutic target for diseases such as sickle cell anemia, asthma, coronary restenosis after angioplasty, atherosclerosis, kidney fibrosis and autoimmunity, where activation and excessive proliferation of one or more of these cell types is involved in the pathology. This article will review KCa3.1’s physiology and pharmacology and critically examine the available preclinical and clinical data validating KCa3.1 as a therapeutic target.
Keywords: KCa3.1: intermediate-conductance calcium-activated K+ channels (also known as IK1, SK4 or KCNN4); Sickle-cell disease: is a life-long blood disorder characterized by red blood cells that assume an abnormal, rigid, sickle shape because of a mutation in the hemoglobin gene. “Sickling” decreases the flexibility of the erythrocytes and results in a risk of various complications such as baseline anemia, vaso-occlusive events and stroke.; ICA-17043: 4-fluoro-α-(4-fluorophenyl)-α-phenyl-benzeneacetamide, KCa3.1 blocker that entered clinical trials as Senicapoc®; TRAM-34: 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole, commonly used experimental KCa3.1 blocker; Atherosclerosis: is a disease of large and medium-sized muscular arteries and is characterized by endothelial dysfunction, vascular inflammation, and the buildup of lipids, cholesterol, calcium, and cellular debris within the intima of the vessel wall.; Restenosis: means the reoccurrence of stenosis, a narrowing of a blood vessel, leading to restricted blood flow. It typically occurs following angioplasty and is caused by the proliferation of intimal vascular smooth muscle cells (neointimal hyperplasia).; Fibrosis: is the formation or development of excess fibrous connective tissue in an organ or tissue.; Asthma: common chronic disorder of the airways characterized by variable and recurring symptoms, airflow obstruction, smooth muscle hypertrophy, bronchial hyperresponsiveness (bronchospasm), and an underlying inflammation.
Expert Commentary
Introduction/Background
Calcium-activated K+ (KCa) channels open in response to increases in cytosolic calcium and play important roles in modulating calcium-signaling and membrane potential in both excitable and non-excitable cells. The human genome contains eight KCa channels, which can be divided into two groups depending on their genetic relationship, their single channel conductance and the molecular mechanism of their calcium-“sensing” [1]. The first group encompasses KCa1.1, KCa4.1, KCa4.2 and KCa5.1, which were initially grouped together based on structural similarity but later found to differ in their activation mode. While the founding member of the group, KCa1.1 (BK), is indeed a “true” K channel and is activated by Ca2+ Ca binding to a negatively charged segment in the C-terminus, KCa4 ad KCa5 channels are activated by sodium, chloride or alkalization [1]. The channels were initially grouped together based on their structural similarity The second group consists of the three small-conductance channels KCa2.1 (SK1), KCa2.2 (SK2), and KCa2.3 (SK3), and the intermediate-conductance channel KCa3.1 (IK1, SK4), which is the focus of this review (Figure 1A). KCa3.1 was cloned by three groups in 1997 [2-4] and found to show about 42-44% sequence identity to the KCa2 channels. Like the KCa2 channels, KCa3.1 is voltage-independent and is activated with reported EC50s of 95 to 350 nM by intracellular calcium binding to the EF-hands of calmodulin (Figure 1B), which is constitutively associated with the C-terminus of the channel and serves as its calcium-sensing β-subunit [5,6]. At the gene level, KCa3.1 transcription can be repressed by the repressor element-1 silencing transcription factor (REST) [7] and increased through the transcription factors AP-1 (activation protein-1) and Ikaros-2 [8]. At the protein level, KCa3.1 function is increased by protein kinase A (PKA) [9] and nucleoside diphoshate kinase B (NDPK-B) [10] and inhibited by the histidine phosphatase PHPT1 [11]. NDPK-B and PHPT1 directly phosphorylate/dephosphorylate KCa3.1 on histidine 358 in the C-terminus (Figure 1B) and KCa3.1 modulation thus constitutes one of the rare examples of a histidine kinase/phosphatase regulating a biological process in mammals.
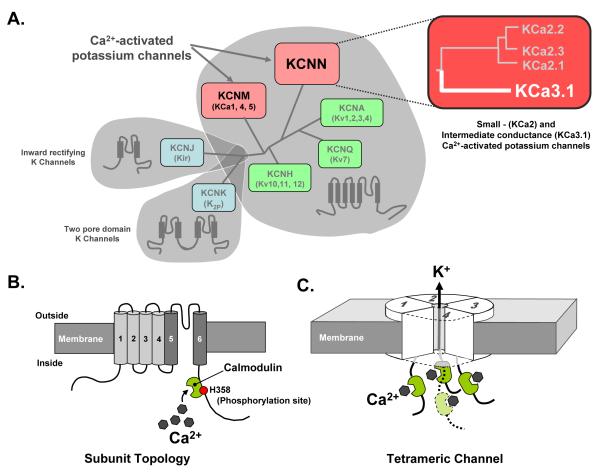
Figure 1.
Properties of KCa3.1 channels. A. Simplified phylogenetic tree of genes for human potassium channel subunits, highlighting in red the two gene families that comprise Ca2+-activated potassium channels and further highlighting the KCNN family which contains the gene for KCa3.1 (KCNN4). B. Illustration of KCa3.1 subunit topology showing the six transmembrane domain signature of this class of channels, along with calmodulin attached to a calmodulin binding domain on the C terminus. The location of the histidine phosphorylation site (H358) known to affect channel activation is also shown. C. Illustration of the homo-tetrameric nature of functional KCa3.1 channels, showing the presence of four calmodulin calcium sensors which accounts for the channel’s steep, highly cooperative sensitivity to changes in intracellular calcium concentration.
Although so far no human diseases involving KCa3.1 mutations have been described, KCa3.1 constitutes a very attractive and (in some cases) relatively well-validated drug target for diseases or conditions ranging from sickle cell disease, restenosis and atherosclerosis to asthma and traumatic brain injury. In this article we will briefly summarize the physiological and pathophysiological role of KCa3.1, review the existing pharmacological tool compounds and drug candidates, and then discuss the future perspectives of KCa3.1 as a therapeutic target.
Physiological role of KCa3.1
In mammals, KCa3.1 channels are widely expressed throughout the body but are absent from excitable tissues such as cardiac myocytes, skeletal muscle and the nervous system [2-4]. The only exceptions seem to be certain enteric neurons, where KCa3.1 has been suggested to underlie part of the apamin-insensitive slow after hyperpolarization [12]. KCa3.1 channels are primarily found in hematopoietic derived cells (i.e erythrocytes, platelets, lymphocytes, mast cells, monocytes/macrophages); epithelial tissues in the gastrointestinal tract, lung and endo- and exocrine glands; as well as vascular endothelial cells, fibroblasts and proliferating neointimal vascular smooth muscle cells [3,4,8,13-17].
Our initial understanding of the physiological role of KCa3.1 came from studies in the late 1950’s when Gárdos demonstrated that potassium efflux in erythrocytes was activated by calcium entry [18]. Later studies confirmed that the underlying potassium conductance (called the “Gárdos channel”) is KCa3.1 [19,20] and that efflux of potassium ions through this channel is accompanied by a loss of cellular water leading to dehydration and erythrocyte shrinkage. Potassium efflux through KCa3.1 can indeed be quite large as application of calcium ionophores can result in the efflux of greater than 50% of the cell’s intracellular potassium content. In some situations this cell shrinkage is associated with programmed cell death (apoptosis) [21]. In addition to erythrocytes, KCa3.1 has also been reported to be involved in lymphocyte apoptosis by mediating cell shrinkage in calcium-induced apoptosis or P2X7-receptor stimulated cell death in lymphocytes [22,23]. However, it is currently not clear whether the recently reported KCa3.1 expression in the inner mitochondrial membrane is involved in these processes [24]. A similar involvement in volume regulation of other hematopoeitic cell types has also been demonstrated. For example, in transgenic mice where KCa3.1 is functionally absent, both T-lymphocytes and mast cells exhibit attenuated responses to environmental osmotic changes [19,20].
Perhaps the most well defined role of KCa3.1 channels is to regulate calcium entry into cells and thereby modulate calcium-signaling processes. The channel performs this duty by helping to control membrane potential (Figure 2). Entry of positively charged calcium depolarizes the membrane, which limits its own ability to enter the cell through inward-rectifier-type calcium channels like CRAC (calcium release activated calcium channel) or some transient receptor potential channels, which are closed at more positive membrane potentials. Activation of KCa3.1 by elevated intracellular calcium maintains a negative membrane potential, which helps to sustain calcium entry into the cell. KCa3.1-mediated elevation of intracellular calcium is necessary for the production of inflammatory chemokines and cytokines by T cells, macrophages and mast cells [8,25]. It is also a prerequisite for the proliferation of many cell types. Indeed, following stimulation of cells with growth factors (in the case of fibroblast or vascular endothelial and smooth muscle cells) or antigens or mitogens (in the case of T and B cells) proliferation is accompanied by transcriptional up-regulation of functional KCa3.1 expression and can be inhibited by KCa3.1 blockers [8,15,26,27].
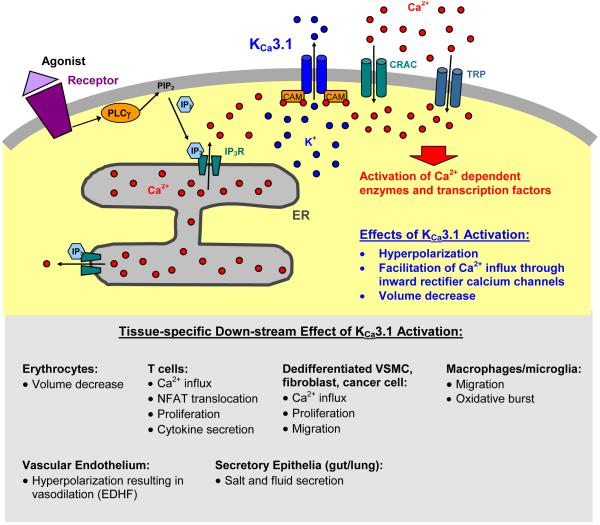
Figure 2.
Cartoon of the physiological role of KCa3.1. The channel is activated by increases in intracellular calcium following calcium release from the ER (endoplasmatic reticulum), and/or calcium influx through inward-rectifier calcium channels like CRAC (calcium release activated calcium channel) or TRP (transcient receptor potential) channels. PLC; phospholipase C; IP3 inositol-triphosphate; CAM; calmodulin.
KCa3.1 mediated control of calcium entry has further been shown to be involved in the migration of macrophages [28], microglia [29], vascular smooth muscle cells [30,31], and mast cells [25]. In microglia KCa3.1 also seems to play a role in the oxidative burst, nitric oxide production and microglia-mediated neuronal killing [32,33]. There is also evidence that activation of KCa3.1 may improve in platelet coagulation [34].
Epithelial cells are the major conduit for electrolyte and fluid transport in tissues and organs throughout the body. KCa3.1 channel protein is expressed in the epithelium lining the gastrointestinal tract, in lung epithelia, in ducts of fluid secreting glands (i.e. salivary, lacrimal, pancreas, prostate) as well as in stratified epithelia, including skin, the cornea, oral mucosa and urothelium [35-38]. In addition to serving a protective role in cells undergoing osmotic challenge, KCa3.1 channels, which are typically expressed on the basolateral membrane, provide a polarized pathway for potassium flux (alone or in partnership with KV7.1 channels), which helps to facilitate chloride secretion and consequently water transport across the epithelia [35,37].
Together with the small-conductance KCa2.3 channel, KCa3.1 initiates the so-called endothelium derived hyperpolarization factor (EDHF) response in vascular endothelium, which causes subsequent relaxation of the underlying vascular smooth muscle cell layer through hyperpolarization mediated via closure of voltage-gated calcium channels [39-41]. As expected, genetic deficiency of both KCa3.1 and KCa2.3 largely abolish EDHF-type dilator responses and increases mean blood pressure in double transgenic animals [42]. However, the single transgenics revealed that the two endothelial KCa channels have distinct stimulus dependent functions. While KCa3.1 deficiency severely impairs acetylcholine-mediated vasodilations [43], KCa2.3 deficiency significantly reduces sheer-stress stimulated vasodilations [42]. These distinct functions seem to be related to a spatial separation of the two KCa channels within the vascular endothelium. KCa2.3 is found at endothelial cell junctions [44], whereas KCa3.1 is localized to the endothelial projections through the holes in the elastic lamina, which are also the sites of the myoendothelial gap junctions that electrically couple endothelial cells to the underlying vascular smooth muscle cells. In these projections, KCa3.1 is seen in close proximity to the endoplasmic reticulum and has been proposed to be preferentially activated by acetylcholine-triggered “calcium pulsars” through calcium release from the endoplasmic reticulum [45].
It is also worth noting here that despite the wide expression of KCa3.1, the functional absence of this channel in transgenic knockout mice has not been associated with gross physiological changes. Animals constitutively lacking KCa3.1 are of normal appearance; reproduce normally and have no abnormalities of any major organs. Apart from impairments in cell volume regulation in erythrocytes and T lymphocytes [20], subtle erythrocyte macrocytosis and progressive splenomegaly during aging [46], a mild 7 mmHg increase in mean arterial blood pressure associated with the reduced EDHF response [42,43], KCa3.1−/− mice exhibit relatively normal phenotypes. A recent study on KCa3.1−/− mice has further reported that their pancreatic beta-cells exhibited increased calcium action potential frequencies and accordingly secreted more insulin at lower glucose concentrations [47]. The mice were not hypoglycemic but showed improved glucose tolerance, which raises the possibility that KCa3.1 blockers might constitute novel insulinotropic drugs [47]. It should of course be noted that all these findings are of limited significance because of the possibility of developmental compensations in constitutive knock-out mice.
Available Drugs and Tool compounds
In contrast to many other potassium channels, KCa3.1 has a relatively well-developed pharmacology, which was recently reviewed by us in great detail and put into its historical perspective [48]. We will therefore here only briefly describe the most commonly used KCa3.1 modulators and then concentrate our discussion on available animal model or clinical trial data.
Traditionally KCa3.1 had been distinguished from the apamin-sensitive KCa2 channels by its sensitivity to the scorpion toxin charybdotoxin (ChTX). However, ChTX (IC50 5 nM) was never an ideal KCa3.1 blocker because it also inhibits KV1.3 and KCa1.1, which made it problematic to use on any cell type that expresses these channels. Another scorpion toxin that potently blocks KCa3.1 is maurotoxin (MTX, IC50 1 nM). However, MTX has an even higher affinity (IC50 100 pM) for KV1.2 and attempts by scientists at Icagen in collaboration with the laboratory of Jean-Marc Sabatier to design KCa3.1-selective derivatives remained unsuccessful [49,50]. Similar efforts by the group of George Chandy produced the ChTX analog ChTX-Glu32, whose negatively charged Glu32 is repelled by negatively charged residues in the outer vestibule of KV1.3, resulting in 30-fold selectivity for KCa3.1 over KV1.3 [51]. However, this modification did not increase selectivity over KCa1.1.
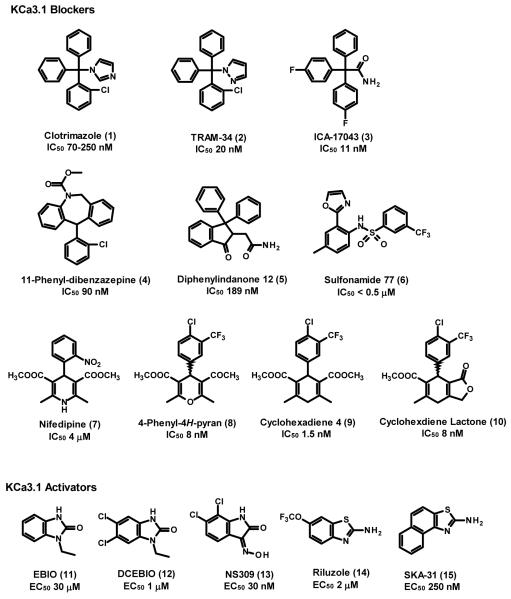
In addition to peptide toxins, KCa3.1 is inhibited by a number of “old drugs” including the antimalarial quinine (IC 2+ 50 100 μM), the vasodilator cetiedil (IC50 25 μM), the L-type Ca channel blockers nifedipine (IC50 4 μM) and nitrendipine (IC50 1 μM), and the antimycotic clotrimazole (1, IC50 70-250 nM) [48]. Interestingly, clotrimazole (1) was initially postulated to affect KCa3.1 through a P450-like heme protein, when it was first reported to block calcium-activated potassium efflux in erythrocytes by Alvares et al. in 1992 [52]. This hypothesis was later proven to be incorrect when Brugnara and coworkers showed that clotrimazole’s metabolite (2-chlorophenyl)diphenylmethanol, which lacks the imidazole ring required for P450 inhibition, also blocked the Gárdos channel [53]. Brugnara and his coworkers at the Children’s Hospital at Harvard University also quickly recognized the therapeutic implications of Alvarez’ observation and used clotrimazole (1) as a tool to demonstrate that pharmacological KCa3.1 blockade reduced erythrocyte dehydration in both a mouse model of sickle-cell anemia [54] and a small number of patients with the disease [55]. However, clotrimazole (1) itself was not suitable for long-term use in patients or experimental animals because of its acute inhibition and chronic induction of human cytochrome P450-dependent enzymes [56,57] leading to liver damage and changes in cortisol levels. Three groups therefore used clotrimazole (1) as a template for the design of triarylmethane based KCa3.1 blockers, which are free of cytochrome P450 inhibition [48]. Brugnara and colleagues synthesized a number of triarylmethanes and patented them for sickle cell disease and abnormal cell proliferation [201]. By replacing clotrimazole’s imidazole ring systematically with other heteroaromatic ring systems or various functional groups, Wulff et al. identified TRAM-34 (2), a compound that inhibits KCa3.1 with an IC50 of 20 nM, has no effect on the P450 enzyme CYP3A4, and exhibits 200-500 fold selectivity over KV channels and 1000-fold selectivity over KCa1.1 and KCa2 channels [58]. Through site-directed mutagenesis it was later demonstrated that TRAM-34 (2) and clotrimazole (1) interact with Thr250 in the pore loop and Val275 in S6 and therefore constitute “inner pore” blockers [59]. Independently of the two other groups, scientists at Icagen Inc. explored fluorinated triphenyl acetamides [60]. The compound with the best pharmacokinetic properties from this series is ICA-17043 (3, Senicapoc®), which inhibits KCa3.1 with an IC50 of 11 nM and is orally bioavailable in humans with a half-life of 12.8 days [61]. ICA-17043’s greatly increased metabolic stability in comparison to clotrimazole (1) and TRAM-34 (2), which has a half-life ~2 hours in rhesus macaques and is acid sensitive and not orally available despite attempts at microencapsulation [62], is probably due to the presence of the two fluorine substituents in para position on two of the phenyl rings, which seem to greatly reduce oxidative metabolism [60]. More recently reported “triarylmethane-like” KCa3.1 blockers are 11-phenyl-diazepines exemplified by compound 4 (IC50 for Gárdos channel blockade ~90 nM) and diphenylindanones exemplified by 5 (IC50 189 nM), which were patented by Brugnara and co-workers [202,203]. Aromatically substituted sulfonamides, exemplified by 6 (N-(4-methyl-2-oxazol-2yl-phenyl)-3-trifluoromethyl-benzenesulfonamide) are a more structurally distantly related class of KCa3.1 blockers, described in an Icagen Inc. patent [204], which inhibit 86Rb flux through Gárdos channels in human erythrocytes with IC50s lower than 500 nM.
Another class of KCa3.1 blockers, which was first reported on in literature in 2003, are the 4-phenyl-4H-pyrans [63] and the related cyclohexadienes [64]. While initial attempts by chemists at Neurosearch A/S to use the dihydropyridine nifedipine (7) as a template for the design of a potent KCa3.1 blocker were unsuccessful, Urbahns et al. at Bayer reasoned that isoelectronic replacement of the NH required for voltage-gated calcium channel blockade with an O might lead to more potent and specific KCa3.1 blockers [63]. This assumption turned out to be accurate since even a simple dimethyl ester substituted 4-phenyl pyran blocked KCa3.1 with an affinity of 160 nM. This activity was then further enhanced through additional derivatization on the phenyl ring and finally resulted in a 4-Cl, 3-CF3 substituted phenyl-4H-pyran (8) that blocked KCa3.1 with an IC50 of 8 nM. A subsequent exchange of the NH with a CH2 group through a Carba-Hantzsch reaction resulted in the cyclohexadienes [64] exemplified by compound 9 (IC50 of 1.5 nM). However, the cyclohexadienes showed a tendency to 3,6/3,5 double bond isomerization and the authors therefore also synthesized cyclohexadiene lactones, in which the second ring prevents isomerization. The exemplary cylcohexadiene lactone 10 inhibits KCa3.1 with an IC50 of 8 nM and exhibits a good selectivity over other potassium, calcium and sodium channels as well as transporters and receptors in binding assays [65]. Tissue distribution studies with 9 further showed that the compound is 10-fold enriched in brain tissue [64] suggesting that it might be ideal for studying the role of KCa3.1 in indications that require penetration into the brain. Interestingly, the title of the German patent [205] claiming the initial compounds in 1997 was incorrectly translated into English as “Preparation of dimethyl-substituted cyclohexadiene-derivative calcium channel modulators” which might explain why many companies outside of Germany were initially not aware of the existence of these compounds.
In addition to the above-described blockers, scientists studying KCa3.1 also have the possibility to pharmacologically increase KCa3.1 activity with a number of small molecule activators, which increase the single channel open probability in a calcium-dependent manner resulting in an apparent leftward shift of the calcium activation curve by an order of magnitude [66-68]. However, care should be taken not to term these compounds “openers” because they are ineffective in the absence of calcium and therefore do not “open” the channel per se. The “classical” KCa3.1 activator is ethylbenzimidazolone (EBIO, 11), which was first described by Devor et al. in 1996 [36] and later confirmed to activate cloned KCa3.1 channels with an EC50 of 30 μM. More potent KCa3.1 activators include 5,6-dichloro-EBIO (12, DCEBIO, EC50 1 μM) [69], the Neurosearch AS compound NS309 (13, EC50 30 nM) [70], the neuroprotectant riluzole (14, EC50 2 μM) and its recently identified more selective derivative SKA-31 (15, EC50 250 nM) [71]. However, all these compounds are not perfectly selective for KCa3.1 and only display a 3 to 5-fold selectivity for KCa3.1 over the KCa2 channels. DC-EBIO and NS309 also block L-type calcium channels [72] and in the case of NS309 the cardiac hERG (Kv11.1) channel and are therefore not suitable for human use. The only KCa3.1 activator that has been demonstrated so far to be useful in vivo is SKA-31 (12), which has surprisingly good pharmacokinetics with low plasma protein binding and a plasma half-life of 12 hours in rats [71]. SKA-31 (15) has recently been shown to potentiate EDHF-type dilations in murine carotid arteries and to lower mean arterial blood pressure in both normotensive and hypertensive mice in a KCa3.1-dependent fashion, suggesting KCa3.1 channel activation as a potential new strategy for the treatment of hypertension [42,71]. However, compared with the body of knowledge discussed below for KCa3.1 blockers, this indication for KCa3.1 activators needs to be validated further since it is currently not clear whether KCa3.1 activators will be effective in more long-term experiments. It further remains to be seen what the side-effect profile of KCa3.1 activators will be. Based on the expression of KCa3.1 in various prostate, breast and pancreatic cancer cell lines [73] KCa3.1 activators could potentially induce neoplastic changes.
Pathophysiological role of KCa3.1
In developing an understanding of the physiological role of KCa3.1, it has become evident that this channel may also play an important role in various pathophysiological disorders. First, aberrant dehydration of red blood cells in people with hereditary sickle cell disease can precipitate polymerization of mutated hemoglobin S resulting in the sickling and eventual destruction of erythrocytes. The contribution of KCa3.1 to the etiology of this disease is supported by the finding that inhibiting calcium dependent flux of potassium with the KCa3.1 inhibitors clotrimazole (1) and Senicapoc® (3) results in a reduction in hemolysis as well as in reduced numbers of dense erythrocytes [54,74,75], which are a precursor to sickled cells. Second, while the cellular processes underlying immune responses to infection and/or tissue damage are typically well orchestrated, under certain conditions they can become unbalanced leading to a variety of autoimmune and inflammatory disorders. For example, in many inflammatory disorders, immune responses are over-amplified or lack a resolution process. As such, it is not uncommon for migration, activation and proliferation of immunologically active cells like lymphocytes, macrophages and mast cells to become uncontrolled leading to further tissue damage and disease progression. Activation of KCa3.1 is believed to contribute to a number of these cellular events. For example, KCa3.1 expression is up-regulated in activated naïve and central memory T-cells and IgD+ B-cells [27,76] and the channel has therefore been proposed as a target for the treatment of autoimmune diseases and transplant rejection [77]. In animal models, the KCa3.1 inhibitors TRAM-34 (2) or Senicapoc® (3) have been reported to prevent experimental autoimmune encephalomyelitis (EAE) induced by immunization with MOG peptide in mice [78] or to inhibit/attenuate inflammatory responses in mice with collagen antibody induced rheumatoid arthritis [79]. Indeed it is worth noting that the archetypal KCa3.1 inhibitor clotrimazole (1) was reported to improve the symptoms of rheumatoid arthritis in a small clinical trial in the 1980s [80]. The combination of TRAM-34 (2) with the KV1.3 blocking peptide ShK was further recently shown to reduce T cell and macrophage infiltration in the early stages of chronic kidney transplant rejection in rats [81], suggesting that KCa3.1 blockers should be further investigated in chronic transplant rejection models. The group of Edward Skolnik also recently demonstrated that TRAM-34 (2) significantly reduced the severity of TNBS induced colitis in mice, a model of human inflammatory bowl disease [82]. This finding was supported by the observation that adoptive transfer of T lymphocytes from KCa3.1−/− mice failed to induce colitis in rag2−/− mice in contrast to wild type T cells.
KCa3.1 channels have also been reported to play various roles in mast cell function. Early studies by Bradding and colleagues demonstrated functional expression of KCa3.1 channels in human lung mast cells and showed that inhibition of this channel with agents like TRAM-34 (2) affected both degranulation and migration responses [25]. Furthermore, KCa3.1 appears to contribute to the modulation of mast cell function through its regulation by a variety of receptors (i.e. beta adrenoreceptor, adenosine and prostaglandin) known to play a role in asthma and other inflammatory airway disorders [83]. A potential role of KCa3.1 in the pathophysiology of airway inflammatory diseases [84] is supported by the observation that the KCa3.1 inhibitor Senicapoc® (3) reduces allergen challenge induced increases in airway resistance and hyper-reactivity in a sheep model of asthma [85]. KCa3.1 may also participate in neuroinflammation. In cultured microglia KCa3.1 is involved in the respiratory burst [32], iNOS induction, nitric oxide production [33], migration [29] and in microglia-mediated neuronal killing [33]. It should of course be noted that cultured microglia are not necessarily a good model of microglia in vivo. Intraocular injection of the KCa3.1 inhibitor TRAM-34 (2) reduces retinal ganglion cell degeneration after optic nerve transection in rats [33]. Interestingly, KCa3.1 blockade does not prevent microglia from phagocytosing damaged neurons but increases the number of surviving retinal ganglion cells, presumably by reducing the production and/or secretion of neurotoxic molecules in the retina [33]. Additional evidence for a neuroprotective role for KCa3.1 blockers comes from the observation that both cylcohexadiene lactone and triaryl methane – type KCa3.1 inhibitors reduce brain edema and infarct volume caused by traumatic brain injury in rats [65].
The contribution of KCa3.1 to pathophysiological inflammatory and proliferative responses can be further highlighted by the role of the channel in vascular diseases, particularly in restenosis, atherosclerosis and kidney fibrosis. TRAM-34 (2) has been demonstrated to significantly reduce balloon angioplasty induced neointimal smooth muscle hyperplasia (restenosis) in the carotid artery in rats [15]. Similar observations have been made in a porcine coronary overstretch injury model, which very closely mimics post-angioplasty injury seen in humans. TRAM-34 (2) administered directly to the coronary vessel wall at the time of the balloon catheter angioplasty was found to significantly reduce the magnitude of restenosis observed 28 days post surgery [86]. Again using TRAM-34 (2), the group of Hiroto Miura showed that KCa3.1 blockade significantly reduced atherosclerosis development in ApoE−/− mice by reducing smooth muscle cell proliferation and lymphocyte infiltration into atherosclerotic plaques [87]. However, in blood vessels KCa3.1 does not only drive the proliferation of dedifferentiated smooth muscle cells but also the proliferation of microvascular and macrovascular endothelial cells and KCa3.1 blockade therefore also potently suppresses angiogenesis in the mouse matrigel plug assay [17].
KCa3.1 has further long been known to play a role in fibroblast proliferation [13] but the relationship of this action to pathophysiology was not clearly evident until a recent study reported that genetic disruption of KCa3.1 or pharmacological blockade of KCa3.1 with TRAM-34 (2) significantly reduces renal fibrosis in mice and rats following unilateral ureteral obstruction [88]. The contribution of KCa3.1 to fibrosis in other tissue remains to be determined.
Given the extensive evidence that KCa3.1 plays an important role in the proliferative responses of many cell types, it is perhaps not surprising that this channel has also been reported to contribute to the development of various forms of cancer. KCa3.1 is involved in the proliferation of human LNCaP and PC-3 prostate cancer cells, leukemic HL-60 and glioblastoma GL-15 cells, MCF-7 breast cancer cells, BxPC-3 and MiaPaCa-2 pancreatic cancer cells, and HEC-1A and KLE endometrial cancer cells [see [73] for review]. Two in vivo studies provide some evidence for the hypothesis that KCa3.1 could constitute a target for cancer treatment. Benzaquen et al. showed in 1995 that clotrimazole (1) reduced the number of lung metastases in SCID mice that were inoculated with human melanoma cells [89], while Zhang et al. demonstrated in 2007 that both clotrimazole (1) and TRAM-34 (2) slowed the growth of tumors in nude mice injected with human endometrial cancer cells [90]. Furthermore, due to the inhibition of angiogenesis by KCa3.1 inhibitors, a role as an adjuvant in inhibition of tumor angiogenesis could be pursued [17].
The role of KCa3.1 in epithelial tissue dysfunction remains to be fully explored. However, there are reports suggesting that KCa3.1 channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease [91]. Furthermore, an abolition of Ca2+-mediated intestinal anion secretion and increased fecal dehydration has been observed in transgenic mice lacking functional KCa3.1 channels [92]. The observations are in line with reports from 1997 by Rufo et al. that clotrimazole (1) reduces chloride secretion in vitro by T84 cell monolayers and in vivo in mice that were challenged with cholera toxin to induce secretory diarrhea [93].
Clinical experience with KCa3.1 blockers
Given its broad expression profile, as well as the extensive evidence supporting a role for KCa3.1 in various pathophysiological mechanisms, development of modulators of this channel would appear to be an obvious strategy for pharmaceutical companies interested in developing treatments for one or more of diseases/disorders described above. The first reported drug development program focused on KCa3.1 was for the treatment of sickle cell disease. The evidence for a role of KCa3.1 in erythrocyte water homeostasis and viability in sickle cell disease is compelling [54,74]. An early attempt to target the disease using the weakly selective KCa3.1 inhibitor clotrimazole (1) showed promise in a small clinical trial, although toxicity issues prevented further development [55]. Icagen Inc. followed these studies and targeted sickle cell disease for the clinical development of its more potent and selective KCa3.1 blocker Senicapoc® (see [94] for a review). Senicapoc® proved to be very efficacious in an animal model of sickle cell disease [75], particularly reversing hemolysis and reducing the number of dense red blood cells, the precursor to sickled cells. In a randomized, double-blind, placebo-controlled phase I trial in healthy volunteers, Senicapoc® (3) was found to be safe and well tolerated at doses that effectively blocked a large percentage of Gárdos channels in the test subjects [61]. A 12-week, multi-center, randomized double-blind Phase II clinical trial showed that Senicapoc® (3) was able to reduce hemolysis and increase hemoglobin levels in sickle cell patients [95]. A subsequent Phase III study was designed to compare the rate of acute vaso-occlusive pain crises occurring in sickle cell disease patients receiving 10 mg of Senicapoc® (3) daily for 48 weeks versus placebo. While Senicapoc® (3) reduced hemolysis and increased hemoglobin levels as seen in the Phase II trial [96], the study was terminated early due to a low probability of achieving a reduction in the primary clinical end point, the rate of vaso-occlusive pain crisis. Thus, despite achieving the desired biological endpoints, treatment did not reduce clinical painful crises.
While the results from the sickle cell disease clinical trials were disappointing, it was clear from these studies that Senicapoc® (3) was a safe and well tolerated drug candidate in humans, and that it was biologically active at the doses given. Therefore, Icagen Inc. explored other therapeutic opportunities for this drug candidate. Consistent with its ability to reduce the increase in airway resistance and hyper-reactivity in a sheep allergen challenge model of asthma [85] Senicapoc® (3) demonstrated encouraging results in a small phase II allergen challenge study in patients with allergic asthma. In this trial, following two weeks of Senicapoc® treatment at 40 mg/day there was a reduction in the late allergen mediated increase in airway resistance along with a reduction in the inflammatory marker exhaled nitric oxide. In contrast, in a second proof-of-concept Phase II trial, in which Senicapoc®’s effect on exercise induced asthma was examined, no improvement in lung function was observed following 4 weeks of treatment (40 mg/day p.o.) [101].
Five-Year View
Initial clinical trials with the selective KCa3.1 inhibitor Senicapoc® (3) have provided evidence that blocking KCa3.1 produces biological effects in humans and is well tolerated. While the clinical benefits of KCa3.1 inhibition have not been established to date, there is sufficient scientific rationale to expect that this channel will continue to be pursued as a drug development target by pharmaceutical companies. KCa3.1 also constitutes an attractive target for autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease as well as for conditions like kidney fibrosis, post-angioplasty restenosis and atherosclerosis, which are associated with the proliferation of fibroblasts and dedifferentiated vascular smooth muscle cells. Of particular interest are atherosclerosis and restenosis given that 1) existing studies demonstrate that KCa3.1 blockers work in multiple species (mice, rats, and pigs), 2) the channel is strongly expressed in the intimal and medial layers of blood vessels from patients with coronary artery disease [87], and 3) both conditions have an inflammatory component. Whether as oral agents or in conjunction with devices such as drug eluting stents, KCa3.1 blockers may be particularly helpful in these important cardiovascular diseases. Recent studies on the beneficial effects of both genetic deletion and pharmacological KCa3.1 blockade in inflammatory bowel disease [82] and kidney fibrosis [88] make these indications of interest as well. We may expect to see groups seeking to further validate such an approach given the need for new therapies for these disorders as well as for liver and lung fibrosis, the later of which plays a significant role in disease mediated airway remodeling [84].
Another intriguing area is the targeting of KCa3.1 to reduce microglia activity in traumatic brain injury [65] and possibly ischemic stroke and other neurodegenerative disorders with an inflammatory component. Pharmaceutical companies of course tend to shy away from stroke as an indication since the failure of numerous drug development programs including the KCa1.1 channel activator BMS-204352 [97] in Phase III clinical trials for this in indication in 2000 [98]. However, we would like to point out here that microglia activation in the wake of an ischemic stroke or traumatic brain injury develops slowly and has been shown to continue up to the chronic cystic stages months after stroke in human brain [99]. Treatments aimed at neuroinflammation might therefore still be able to demonstrate beneficial effects if started hours or even days after an ischemic stroke or brain injury has occurred by reducing inflammatory damage in the so-called penumbra around the necrotic core area of the infarct. Since the contribution of microglia to the pathology of stroke and Alzheimer’s disease is currently a very active research area, we expect that KCa3.1 modulators will be further evaluated for their ability to reduce detrimental microglia functions and that a clearer picture of their usefulness for CNS indications will emerge in the next 5-10 years.
Although pharmacological blockade of KCa3.1 appears to be safe and well tolerated in animals and humans, the fact that genetic deletion of the channel impairs the EDHF response and raises mean arterial blood pressure in the transgenic animals by ~7 mmHg [42,43], raises concerns that KCa3.1 blockers might increase blood pressure. However, blood pressure increases with KCa3.1 blocker have so far not been observed in mice receiving TRAM-34 [87] or over 500 human volunteers and patients taking Senicapoc® (3) for up to two years [95], presumably because combined blockade of endothelial KCa3.1 and KCa2.3 is necessary to abolish the EDHF response. Taken together, KCa3.1 constitutes an attractive, apparently safe and drugable target for indications ranging from atherosclerosis and fibrosis to inflammatory bowel disease and neuroinflammation. It is anticipated that discovery and development activities around this exciting target will increase in the next 5 years.
Key Issues.
◆ KCa3.1 regulates calcium-influx and membrane potential in erythrocytes, activated T and B cells, macrophages, mast cells, microglia, vascular endothelium, epithelia, and proliferating vascular smooth muscle cells and fibroblasts.
◆ KCa3.1 blockers inhibit immune cell migration and cytokine production and are effective in rodent models of EAE, IBD, rheumatoid arthritis and in an asthma model in sheep
◆ KCa3.1 blockers inhibit vascular smooth muscle cell proliferation and migration and reduce restenosis in rats and pigs
◆ KCa3.1 blockers prevent atherosclerosis development in ApoE−/− mice
◆ KCa3.1 blockers inhibit fibroblast proliferation in vitro and prevent kidney fibrosis in mice and rats
◆ KCa3.1 blockers decrease hemolysis and increase hemoglobin levels in patients with sickle cell disease but have not been shown to reduce the frequency vaso-occlusive crises in the clinic.
◆ The KCa3.1 inhibitor Senicapoc® has been shown to reduce allergen induced increase in airway resistance in allergic asthma patients, but not the asthmatic responses to exercise challenge in patients with mild asthma.
◆ Despite the recent disappointing clinical trial results with KCa3.1 modulators, KCa3.1 remains a promising drug target for a variety of indications including restenosis, atherosclerosis, autoimmune diseases and possibly neuroinflammation.
Figure 3.
Structures of KCa3.1 inhibitors and activators.
Footnotes
Financial and competing interests disclosure Heike Wulff gratefully acknowledges support from the National Institute of Health (RO1 GM076063). She is an inventor on the University of California patents claiming TRAM-34 and related compounds for immunosuppression, restenosis, atherosclerosis and fibrosis. Neil A. Castle is an employee of Icagen Inc., the company that discovered and developed the KCa3.1 inhibitor Senicapoc®.
Contributor Information
Heike Wulff, Department of Pharmacology, University of California, Davis; hwulff@ucdavis.edu.
Neil A. Castle, Icagen Inc., 4222 Emperor Boulevard, Suite 350, Durham, NC 27709; ncastle@icagen.com.
Bibliography
* = of interest
** = of considerable interest
- 1.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol. Rev. 2005;57(4):463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 2.Joiner WJ, Wang LY, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc. Natl. Acad. Sci. USA. 1997;94(20):11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. USA. 1997;94(21):11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logsdon NJ, Kang J, Togo JA, Christian EP, Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J. Biol. Chem. 1997;272:32723–32726. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- 5.Xia XM, Fakler B, Rivard A, et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395(6701):503–507. doi: 10.1038/26758. * First study showing that KCa2 channels have calmodulin bound to their C-terminus.
- 6.Fanger CM, Ghanshani S, Logsdon NJ, et al. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J. Biol. Chem. 1999;274:5746–5754. doi: 10.1074/jbc.274.9.5746. [DOI] [PubMed] [Google Scholar]
- 7.Cheong A, Bingham AJ, Li J, et al. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol. Cell. 2005;20(1):45–52. doi: 10.1016/j.molcel.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Ghanshani S, Wulff H, Miller MJ, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation: Molecular mechanism and functional consequences. J. Biol. Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 9.Gerlach AC, N. GN, Devor DC. Kinase-dependent regulation of the intermediate conductance, calcium-dependent potassium channel, hIK1. J. Biol. Chem. 2000;275:585–598. doi: 10.1074/jbc.275.1.585. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava S, Li Z, Ko K, et al. Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa3.1 and CD4 T cells. Mol. Cell. 2006;24(5):665–675. doi: 10.1016/j.molcel.2006.11.012. * Study demonstrating that KCa3.1 activity is regulated by a histidine kinase, which is a very rare form of regulation in mammals.
- 11.Srivastava S, Zhdanova O, Di L, et al. Protein histidine phosphatase 1 negatively regulates CD4 T cells by inhibiting the K+ channel KCa3.1. Proc. Natl. Acad. Sci. USA. 2008;105(38):14442–14446. doi: 10.1073/pnas.0803678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furness JB, Kearney K, Robbins HL, et al. Intermediate conductance potassium (IK) channels occur in human enteric neurons. Auton. Neurosci. 2004;112(1-2):93–97. doi: 10.1016/j.autneu.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Pena TL, Rane SG. The fibroblast intermediate-conductance KCa channel, FIK, as a prototype for the cell growth regulatory function of the IK channel family. J. Membr. Biol. 1999;172:249–257. doi: 10.1007/s002329900601. [DOI] [PubMed] [Google Scholar]
- 14.Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molceular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle: relationship between KCa channel diversity and smooth muscle cell function. Circ. Res. 1999;85:e33–e43. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- 15.Kohler R, Wulff H, Eichler I, et al. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108(9):1119–1125. doi: 10.1161/01.CIR.0000086464.04719.DD. ** Study showing that KCa3.1 constitutes a target for the prevention of restenosis.
- 16.Chen MX, Gorman SA, Benson B, et al. Small and intermediate conductance Ca(2+)-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004;369(6):602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- 17.Grgic I, Eichler I, Heinau P, et al. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler. Thromb. Vasc. Biol. 2005;25(4):704–709. doi: 10.1161/01.ATV.0000156399.12787.5c. [DOI] [PubMed] [Google Scholar]
- 18.Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochem. Biophys. Acta. 1958;30:653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- 19.Vandorpe DH, Shmukler BE, Jiang L, et al. cDNA cloning and functional characterization of the mouse Ca2+-gated K+ channel, mIK1. Roles in regulatory volume decrease and erythroid differentiation. J. Biol. Chem. 1998;273(34):21542–21553. doi: 10.1074/jbc.273.34.21542. [DOI] [PubMed] [Google Scholar]
- 20.Begenisich T, Nakamoto T, Ovitt CE, et al. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel KCNN4. J. Biol. Chem. 2004;279(46):47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 21.Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am. J. Physiol. Cell. Physiol. 2003;285(6):C1553–1560. doi: 10.1152/ajpcell.00186.2003. [DOI] [PubMed] [Google Scholar]
- 22.Elliott JI, Higgins CF. IKCa1 activity is required for cell shrinkage, phosphatidylserine translocation and death in T lymphocyte apoptosis. EMBO Rep. 2003;4(2):189–194. doi: 10.1038/sj.embor.embor722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor SR, Gonzalez-Begne M, Dewhurst S, et al. Sequential shrinkage and swelling underlie P2X7-stimulated lymphocyte phosphatidylserine exposure and death. J. Immunol. 2008;180(1):300–308. doi: 10.4049/jimmunol.180.1.300. [DOI] [PubMed] [Google Scholar]
- 24.De Marchi U, Sassi N, Fioretti B, et al. Intermediate conductance Ca2+-activated potassium channel (KCa3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell Calcium. 2009;45(5):509–516. doi: 10.1016/j.ceca.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Cruse G, Duffy SM, Brightling CE, Bradding P. Functional KCa3.1 K+ channels are required for human lung mast cell migration. Thorax. 2006;61(10):880–885. doi: 10.1136/thx.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pena TL, Chen SH, Konieczny SF, Rane SG. Ras/MEK/ERK Up-regulation of the fibroblast KCa channel FIK is a common mechanism for basic fibroblast growth factor and transforming growth factor-beta suppression of myogenesis. J. Biol. Chem. 2000;275(18):13677–13682. doi: 10.1074/jbc.275.18.13677. [DOI] [PubMed] [Google Scholar]
- 27.Wulff H, Knaus HG, Pennington M, Chandy KG. K+ channel expression during B-cell differentiation: implications for immunomodulation and autoimmunity. J. Immunol. 2004;173:776–786. doi: 10.4049/jimmunol.173.2.776. [DOI] [PubMed] [Google Scholar]
- 28.Chung I, Zelivyanskaya M, Gendelman HE. Mononuclear phagocyte biophysiology influences brain transendothelial and tissue migration: implication for HIV-1-associated dementia. J. Neuroimmunol. 2002;122(1-2):40–54. doi: 10.1016/s0165-5728(01)00462-3. [DOI] [PubMed] [Google Scholar]
- 29.Schilling T, Stock C, Schwab A, Eder C. Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglia migration. Eur. J. Neurosci. 2004;19:1469–1474. doi: 10.1111/j.1460-9568.2004.03265.x. [DOI] [PubMed] [Google Scholar]
- 30.Tharp DL, Casati J, Turk JR, Bowles DK. Functional upregulation of intermediate conductance calcium activated potassium channels is necessary for coronary smooth muscle cell dedifferentiation and migration. FASEB J. 2005;19:A1628. [Google Scholar]
- 31.Tharp DL, Wamhoff BR, Turk JR, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2006;291(5):H2493–2503. doi: 10.1152/ajpheart.01254.2005. [DOI] [PubMed] [Google Scholar]
- 32.Khanna R, Roy L, Zhu X, Schlichter LC. K+ channels and the microglial respiratory burst. Am. J. Physiol. Cell. Physiol. 2001;280(4):C796–806. doi: 10.1152/ajpcell.2001.280.4.C796. [DOI] [PubMed] [Google Scholar]
- 33.Kaushal V, Koeberle PD, Wang Y, Schlichter LC. The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J. Neurosci. 2007;27(1):234–244. doi: 10.1523/JNEUROSCI.3593-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfs JL, Wielders SJ, Comfurius P, et al. Reversible inhibition of the platelet procoagulant response through manipulation of the Gardos channel. Blood. 2006;108(7):2223–2228. doi: 10.1182/blood-2006-01-009613. [DOI] [PubMed] [Google Scholar]
- 35.Rufo PA, Jiang L, Moe SJ, Brugnara C, Alper SL, Lencer WI. The antifungal antibiotic, clotriamzole, inhibits Cl− secretion by polarized monolayers of human colonic epithelial cells. J. Clin. Invest. 1996;98:2066–2075. doi: 10.1172/JCI119012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl-secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am. J. Physiol. 1996;271(5 Pt 1):L775–784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- 37.Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzel RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelia cells. J. Gen. Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warth R, Hamm K, Bleich M, et al. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflugers Arch. 1999;438(4):437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- 39.Eichler I, Wibawa J, Grgic I, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br. J. Pharmacol. 2003;138(4):594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol. Sci. 2002;23(8):374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 41.Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses -- relevance to cardiovascular pathologies and drug discovery. Br. J. Pharmacol. 2009;157(4):509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brahler S, Kaistha A, Schmidt VJ, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119(17):2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. * Study showing that KCa2.3 and KCa3.1 play a critical role in mediating the EDHF response.
- 43.Si H, Heyken WT, Wolfle SE, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ. Res. 2006;99(5):537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 44.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ. Res. 2008;102(10):1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ledoux J, Taylor MS, Bonev AD, et al. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc. Natl. Acad. Sci. USA. 2008;105(28):9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grgic I, Kaistha BP, Paschen S, et al. Disruption of the Gardos channel (KCa3.1) in mice causes subtle erythrocyte macrocytosis and progressive splenomegaly. Pflugers Arch. 2009;458(2):291–302. doi: 10.1007/s00424-008-0619-x. [DOI] [PubMed] [Google Scholar]
- 47.Dufer M, Gier B, Wolpers D, Krippeit-Drews P, Ruth P, Drews G. Enhanced glucose tolerance by SK4 channel inhibition in pancreatic beta-cells. Diabetes. 2009;58(8):1835–1843. doi: 10.2337/db08-1324. ** Study suggesting that KCa3.1 might be a target for increasing insulin secretion.
- 48.Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr. Med. Chem. 2007;14(13):1437–1457. doi: 10.2174/092986707780831186. [DOI] [PubMed] [Google Scholar]
- 49.Kharrat R, Mabrouk K, Crest M, et al. Chemical synthesis and characterization of maurotoxin, a short scorpion toxin with four disulfide bridges that acts on K+ channels. Eur. J. Biochem. 1996;242(3):491–498. doi: 10.1111/j.1432-1033.1996.0491r.x. [DOI] [PubMed] [Google Scholar]
- 50.Castle NA, Lodon DO, Creech C, Fajloun Z, Stocker JW, Sabatier J-M. Maurotoxin - a potent inhibitor of the intermediate conductance Ca2+-activated potassium channel. Mol. Pharmacol. 2003;63:409–418. doi: 10.1124/mol.63.2.409. [DOI] [PubMed] [Google Scholar]
- 51.Rauer H, Lanigan MD, Pennington MW, et al. Structure-guided transformation of charybdotoxin yields an analog that selectively targets Ca(2+)-activated over voltage-gated K(+) channels. J. Biol. Chem. 2000;275:1201–1208. doi: 10.1074/jbc.275.2.1201. [DOI] [PubMed] [Google Scholar]
- 52.Alvarez J, Montero M, Garcia-Sancho J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J. Biol. Chem. 1992;267(17):11789–11793. [PubMed] [Google Scholar]
- 53.Brugnara C, Armsby CC, Sakamoto M, Rifai N, Alper SL, Platt O. Oral administration of clotrimazole and blockade of human erythrocyte Ca(++)-activated K+ channel: the imidazole ring is not required for inhibitory activity. J. Pharmacol. Exp. Ther. 1995;273:266–272. [PubMed] [Google Scholar]
- 54.De Franceschi L, Saadane N, Trudel M, Alper SL, Brugnara C, Beuzard Y. Treatment with oral clotrimazole blocks Ca2+-activated K+ transport and reverses erythrocyte dehydration in transgenic SAD mice. A model for therapy of sickle cell disease. J. Clin. Invest. 1994;93:1670–1676. doi: 10.1172/JCI117149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brugnara C, Gee B, Armsby CC, et al. Therapy with oral clotrimazole induces inhibition of the Gardos channel and reduction of erythrocyte dehydration in patients with sickle cell disease. J. Clin. Invest. 1996;97(5):1227–1234. doi: 10.1172/JCI118537. ** Study showing that KCa3.1 blockade with clotrimazole reduces erythrocyte dehydration in patients with sickle cell anemia.
- 56.Suzuki S, Kurata N, Nishimura Y, Yasuhara H, Satoh T. Effects of imidazole antimycotics on the liver microsomal cytochrome P450 isoforms in rats: comparison of in vitro and ex vivo studies. Eur. J. Drug Metab. Pharmacokinet. 2000;25(2):121–126. doi: 10.1007/BF03190078. [DOI] [PubMed] [Google Scholar]
- 57.Luo G, Cunningham M, Kim S, et al. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab. Dispos. 2002;30(7):795–804. doi: 10.1124/dmd.30.7.795. [DOI] [PubMed] [Google Scholar]
- 58.Wulff H, Miller MJ, Haensel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proc. Natl. Acad. Sci USA. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wulff H, Gutman GA, Cahalan MD, Chandy KG. Delination of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel IKCa1. J. Biol. Chem. 2001;276:32040–32045. doi: 10.1074/jbc.M105231200. [DOI] [PubMed] [Google Scholar]
- 60.McNaughton-Smith GA, Burns JF, Stocker JW, et al. Novel inhibitors of the Gardos channel for the treatment of sickle cell disease. J. Med. Chem. 2008;51(4):976–982. doi: 10.1021/jm070663s. [DOI] [PubMed] [Google Scholar]
- 61.Ataga KI, Orringer EP, Styles L, et al. Dose-escalation study of ICA-17043 in patients with sickle cell disease. Pharmacotherapy. 2006;26(11):1557–1564. doi: 10.1592/phco.26.11.1557. [DOI] [PubMed] [Google Scholar]
- 62.Abbassi M, Shresta S, Raman G, et al. Formulation-based approach to support early drug discovery and development efforts: a case study for entereric microencapsulation of a novel immunosuppressant TRAM-34. J. Microencapsulation. 2009 doi: 10.3109/03639040903329554. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urbahns K, Horvath E, Stasch JP, Mauler F. 4-Phenyl-4H-pyrans as IK(Ca) channel blockers. Bioorg. & Med. Chem. Lett. 2003;13(16):2637–2639. doi: 10.1016/s0960-894x(03)00560-2. [DOI] [PubMed] [Google Scholar]
- 64.Urbahns K, Goldmann S, Kruger J, et al. IKCa-channel blockers. Part 2: discovery of cyclohexadienes. Bioorg. & Med. Chem. Lett. 2005;15(2):401–404. doi: 10.1016/j.bmcl.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 65.Mauler F, Hinz V, Horvath E, et al. Selective intermediate-/small-conductance calcium-activated potassium channel (KCNN4) blockers are potent and effective therapeutics in experimental brain oedema and traumatic brain injury caused by acute subdural haematoma. Eur. J. Neurosci. 2004;20(7):1761–1768. doi: 10.1111/j.1460-9568.2004.03615.x. * Study showing that a cyclohexadiene-type KCa3.1 blocker has beneficial effects in traumatic brain injury.
- 66.Pedersen KA, Schroder RL, Skaaning-Jensen B, Strobaek D, Olesen SP, Christophersen P. Activation of the human intermediate-conductance Ca2+-activated K+ channel by 1-ethyl-2-benzimidazolinone is strongly Ca2+-dependent. Biochim. Biophys. Acta. 1999;1420(1-2):231–240. doi: 10.1016/s0005-2736(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 67.Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K+ channels. Am. J. Physiol. Cell. Physiol. 2000;278(3):C570–581. doi: 10.1152/ajpcell.2000.278.3.C570. [DOI] [PubMed] [Google Scholar]
- 68.Pedarzani P, Mosbacher J, Rivard A, et al. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J. Biol. Chem. 2001;276(13):9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- 69.Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J. Pharmacol. Exp. Ther. 2001;296(2):600–611. [PubMed] [Google Scholar]
- 70.Strobaek D, Teuber L, Jorgensen TD, et al. Activation of human IK and SK Ca2+-activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim. Biophys. Acta. 2004;1665(1-2):1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 71.Sankaranarayanan A, Raman G, Busch C, et al. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol. Pharmacol. 2009;75(2):281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morimura K, Yamamura H, Ohya S, Imaizumi Y. Voltage-dependent Ca2+-channel block by openers of intermediate and small conductance Ca2+-activated K+ channels in urinary bladder smooth muscle cells. J. Pharmacol. Sci. 2006;100(3):237–241. doi: 10.1254/jphs.sc0060011. [DOI] [PubMed] [Google Scholar]
- 73.Chou CC, Lunn CA, Murgolo NJ. KCa3.1: target and marker for cancer, autoimmune disorder and vascular inflammation? Expert Rev. Mol. Diagn. 2008;8(2):179–187. doi: 10.1586/14737159.8.2.179. [DOI] [PubMed] [Google Scholar]
- 74.Brugnara C, de Franceschi L, Alper SL. Inhibition of Ca2+-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J. Clin. Invest. 1993;92:520–526. doi: 10.1172/JCI116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stocker JW, De Franceschi L, McNaughton-Smith GA, Corrocher R, Beuzard Y, Brugnara C. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101(6):2412–2418. doi: 10.1182/blood-2002-05-1433. * First study demonstrating that Senicapoc® is effective in an animal model of sickle cell disease.
- 76.Wulff H, Calabresi PA, Allie R, et al. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J. Clin. Invest. 2003;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. Potassium channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reich EP, Cui L, Yang L, et al. Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur. J. Immunol. 2005;35(4):1027–1036. doi: 10.1002/eji.200425954. [DOI] [PubMed] [Google Scholar]
- 79.Chou CC. IBC Assays and Cellular Targets. San Diego: 2005. Blocking ion channel KCNN4 alleviates inflammation symptoms by arresting the synthesis of key pro-inflammatory factors. [Google Scholar]
- 80.Wojtulewski JA, Gow PJ, Walter J, et al. Clotrimazole in rheumatoid arthritis. Ann. Rheum. Dis. 1980;39:469–472. doi: 10.1136/ard.39.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grgic I, Wulff H, Eichler I, Flothmann C, Kohler R, Hoyer J. Blockade of T-lymphocyte KCa3.1 and Kv1.3 channels as novel immunosuppression strategy to prevent kidney allograft rejection. Transplant. Proc. 2009;41(6):2601–2606. doi: 10.1016/j.transproceed.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di L, Srivastava S, Zhdanova O, et al. Inhibition of the K+ channel KCa3.1 ameliorates T cell mediated colitis. Proc. Natl. Acad. Sci. USA. 2010;107(4):1541–1546. doi: 10.1073/pnas.0910133107. ** Study describing the effect of KCa3.1 knock-out on T cell subset function and demonstration that KCa3.1 might constitute a therapeutic target for inflammatory bowl disease.
- 83.Duffy SM, Cruse G, Lawley WJ, Bradding P. Beta2-adrenoceptor regulation of the K+ channel iKCa1 in human mast cells. FASEB J. 2005;19(8):1006–1008. doi: 10.1096/fj.04-3439fje. [DOI] [PubMed] [Google Scholar]
- 84.Bradding P, Wulff H. The K+ channels K(Ca)3.1 and K(v)1.3 as novel targets for asthma therapy. Br. J. Pharmacol. 2009;157(8):1330–1339. doi: 10.1111/j.1476-5381.2009.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinette L, Abraham WM, Bradding P, et al. Senicapoc® (ICA-17043), a potent and selective KCa3.1 K+ channel blocker, attenuates allergen-induced asthma in sheep. 15th International Conference of the Inflammation Research Association; Chantilly, VA. 2008. * Conference report on the effect of Senicapoc® in a sheep model of asthma.
- 86.Tharp DL, Wamhoff BR, Wulff H, Raman G, Cheong A, Bowles DK. Local delivery of the KCa3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic podulation and limits stenosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:1084–1089. doi: 10.1161/ATVBAHA.107.155796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toyama K, Wulff H, Chandy KG, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J. Clin. Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. ** Study suggesting that KCa3.1 might constitute a target for atherosclerosis.
- 88.Grgic I, Kiss E, Kaistha BP, et al. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc. Natl. Acad. Sci. USA. 2009;106(34):14518–14523. doi: 10.1073/pnas.0903458106. * Study showing that genetic deletion and pharmacological blockade of KCa3.1 prevent kidney fibrosis.
- 89.Benzaquen LR, Brugnara C, Byers HR, Gattoni-Celli S, Halperin JA. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat. Med. 1995;1:534–540. doi: 10.1038/nm0695-534. [DOI] [PubMed] [Google Scholar]
- 90.Wang ZH, Shen B, Yao HL, et al. Blockage of intermediate-conductance-Ca2+-activated K+ channels inhibits progression of human endometrial cancer. Oncogene. 2007 doi: 10.1038/sj.onc.1210308. [DOI] [PubMed] [Google Scholar]
- 91.Albaqumi M, Srivastava S, Li Z, et al. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;74(6):740–749. doi: 10.1038/ki.2008.246. [DOI] [PubMed] [Google Scholar]
- 92.Flores CA, Melvin JE, Figueroa CD, Sepulveda FV. Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+ channel KCNN4. J. Physiol. (Lond.) 2007;583(Pt 2):705–717. doi: 10.1113/jphysiol.2007.134387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rufo PA, Merlin D, Riegler M, et al. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. Demonstration of efficacy in intact rabbit colon and in an in vivo mouse model of cholera. J. Clin. Invest. 1997;100(12):3111–3120. doi: 10.1172/JCI119866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ataga KI, Stocker J. Senicapoc® (ICA-17043): a potential therapy for the prevention and treatment of hemolysis-associated complications in sickle cell anemia. Expert Opin. Invest. Drugs. 2009;18(2):231–239. doi: 10.1517/13543780802708011. ** Review summarizing the safety, pharmacokinetics and phase-2 results for Senicapoc®.
- 95.Ataga KI, Smith WR, De Castro LM, et al. Efficacy and safety of the Gardos channel blocker, Senicapoc® (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111(8):3991–3997. doi: 10.1182/blood-2007-08-110098. [DOI] [PubMed] [Google Scholar]
- 96.Swerdlow P, Ataga KI, Smith W, Saunthararajah Y, Stocker JW. A 48-week open-label study of Sincapoc (ICA-17042), a Gardos channel blocker, in patients with sickle cell disease. Blood. 2006;108 Abstract 685. [Google Scholar]
- 97.Gribkoff VK, Starrett JE, Dworetzky SI, et al. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nature Med. 2001;7:471–477. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]
- 98.Jensen BS. BMS-204352: a potassium channel opener developed for the treatment of stroke. CNS drug reviews. 2002;8(4):353–360. doi: 10.1111/j.1527-3458.2002.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beschorner R, Schluesener HJ, Gozalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J. Neuroimmunol. 2002;126(1-2):107–115. doi: 10.1016/s0165-5728(02)00046-2. [DOI] [PubMed] [Google Scholar]
Websites
- 101.Company press release dated October 26. 2009 available at www.icagen.com.
Patents
- 201.Children Medical Center Corporation; Ion Pharmaceuticals. Inc.; WO9734589 President and Fellows of Harvard College. 1997
- 202.Children Medical Center Corporation; NuChem Pharmaceuticals Inc.; US6992079 President and Fellows of Harvard College. 2006
- 203.Children Medical Center Corporation; NuChem Pharmaceuticals Inc.; US7342038 President and Fellows of Harvard College. 2008
- 204.Icagen Inc.; US7119112 2006
- 205.Bayer A-G. Germany: DE-9619612645 1997