Abstract
Background:
Exposure of animals to xenobiotics may or may not trigger adverse response at cellular levels. Aqueous extract of unripe Carica papaya is consumed by sickle cell patients as antisickling agent in Western Nigeria.
Aim:
This study was undertaken to investigate the effects of Carica papaya on certain organs in Wister albino rats exposed to aqueous extract of unripe Carica papaya.
Materials and Methods:
Different doses of aqueous extract of unripe Carica papaya were administered orally daily for 42 days to six groups of rats. At the end of exposure, the animals were sacrificed and tissue sections were prepared from livers, kidneys, hearts and small intestines using standard techniques.
Results:
Histopathological results showed that no pathological changes were observed in tissue sections of experimental animals when compared with tissue sections of the same organs in control animals.
Conclusion:
No pathological changes were elicited in the organs of rats exposed to aqueous extract of unripe Carica papaya.
Keywords: Carica papaya, histopathological changes, organs, exposure
Introduction
Carica papaya is regarded as a wholesome fruit, the daily requirements of some of the essential nutrients like proteins, minerals and vitamins can be met from this fruit.
The vitamin C content increases as the maturity progresses. Its carbohydrate content is mainly in invert sugar which is a form of predigested food[1]. Its main medicinal use is as a digestive agent; it is prescribed for people who have difficulty digesting protein and is used to break up blood clots after surgery, which is due to the presence of enzyme papain in the plant's latex. The latex from the trunk of the tree is also applied externally to speed the healing of wounds, ulcers, boils, and warts. The seed is used to expel worm and the flower may be taken in an infusion to induce menstruation[1,2]. It has been documented that black seeds of papaya are highly beneficial in the treatment of cirrhosis of the liver caused by alcoholism, malnutrition etc[1]. It has also been reported that annonaceous acetogenins derived from the extracts of the twigs of the pawpaw tree may be good chemotherapeutic agents for cancer as these compounds inhibit enzymes necessary for metabolic processes in tumor cells[3]. Ripe papaya has also been reported to be highly valuable in enlargement of the spleen[1].
Aqueous extract of unripe Carica papaya has been documented to possess antisickling properties. Oduola et al[5] confirmed this property and established the minimum concentration of the unripe Carica papaya that achieved maximum antisickling to be 1g/ml in physiological saline. Solvent partitioning revealed that the antisickling agent resides in the ethyl acetate fraction of the extract[5]. The results of the acute oral toxicity study in Wister albino rats showed the LD50 of the aqueous extract of the unripe Carica papaya to be 2520mg/kg[6]. Ingestion of aqueous extract of unripe Carica papaya has been reported to have no toxic effect on liver, kidney and bone marrow functions in Wister albino rats[6]. Nontoxic effect of ingestion of aqueous extract of unripe Carica papaya on liver functions in sickle cell patients of different age groups had also been documented[7]. The effect of ingestion of aqueous extract of unripe Carica papaya on kidney function in sickle cell patients was also reported to be normal[8]. Hematological parameter was also established to be normal in sickle patients who ingested unripe Carica papaya aqueous extract for 6 months[9].
The cell is the pivotal unit in the response to chemicals. It is the central unit of organization, which together with the extra cellular matrix, a modulator of many cellular functions, controls and maintains at a steady-state the internal environment of all functions/structures and the dispensing of energy in the immediate cell neighborhood and even at sites remotes to a given tissue[10]. Hence the present study is designed to assess histological changes in Wister albino rats exposed to oral administration of unripe Carica papaya aqueous extract.
Materials and Methods
Matured fresh unripe Carica papaya fruit was obtained in a local garden in Ile-Ife, and was authenticated at the herbarium of the Botany Department, Obafemi Awolowo University, Ile-Ife, the herbarium number is 14729. The fruit was cut into pieces; extracted with 5 liters of methanol at room temperature for 72 hours and concentrated to dryness in-vacuo on a rotary evaporator to obtain the crude methanolic extract.
Male and Female Wister albino rats weighing 195-225g (X 205.6g) obtained from the animal house of the faculty of pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria were used for the study. They were housed in plastic rat cages in groups of six rats per cage in a room with temperature of 25 ± 2°C, 12 hours natural light and 12hours darkness with free access to tap water and dry rat pellet (purchased at Ogo Oluwa Enterprises, Ile-Ife, Nigeria). They were allowed to acclimatize for three days prior to the experiment.
Graded doses of the extract were administered orally to six groups of rats on daily basis for 42days. Rats in group 1 served as control and received normal saline (0.85% NaCl). The animals in groups II, III, IV, V and VI received 50mg, 100mg, 150mg, 200mg and 250mg/kg body weight respectively, (these doses were based on previous report[6]). At the end of 42-day extract exposure, the animals were sacrificed by cervical dislocation under ether anesthesia. The livers, kidneys, hearts and small intestines of the rats were harvested and fixed in 10% formol saline for 48hours and processed for paraffin wax embedding with an automatic tissue processor by dehydrating through 70%, 90%, 95% and two changes of absolute ethanol for 90minutes each. Clearing was achieved through two changes of xylene for 2hours each; and infiltrating with two changes of paraffin wax for 2hours. Sections were cut at 5μm with a rotary microtome. The sections were stained by haematoxylin and eosin (H&E) method[11], examined and photographed using a light microscope.
Results
The photomicrographs of liver, kidney, heart and small intestine tissue sections from control and experimental rats stained with haematoxylin and eosin are shown below. The tissue sections of the experimental animals were essentially normal when compared with the control sections (Fig. 1).
Fig. 1.
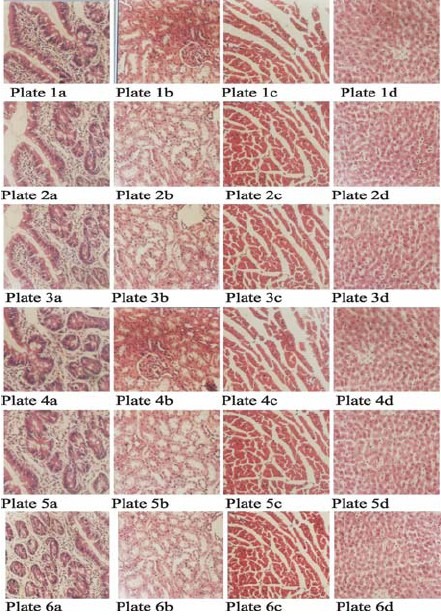
The histopathological examination of all the tissue sections of the control and experimental animals were essentially normal as no pathological lesions were observed.
Plates 1a, 1b, 1c and 1d (×400) were sections of intestine, kidney, heart and liver respectively of control animals treated with normal saline and stained with H&E. Plates 2a, 2b, 2c and 2d (×400) were sections of intestine, kidney, heart and liver respectively of group II animals that received 50mg/kg of the extract. Plates 3a, 3b, 3c and 3d (×400) were sections of intestine, kidney, heart and liver respectively of group III animals that received 100mg/kg of the extract. Plates 4a, 4b, 4c and 4d (×400) were sections of intestine, kidney, heart and liver respectively of group IV animals that received 150mg/kg of the extract. Plates 5a, 5b, 5c and 5d (×400) were sections of intestine, kidney, heart and liver respectively of group V animals that received 200mg/kg of the extract. Plates 6a, 6b, 6c and 6d (×400) were sections of intestine, kidney, heart and liver respectively of group VI animals that received 250mg/kg of the extract.
Discussion
It has been reported that the safety assessment in experimental animals of both medicinal and non-medicinal biologically active chemicals has been very successful in predicting toxicity in humans[4]. It has also been documented that the major advantages of preclinical safety assessment studies are the known responses of experimental species, the controlled conditions under which they can be maintained and the establishment of appropriate metrics, such as tissue volume rates, which can be applied to extrapolation of findings in laboratory animals to assessment of possible human effects. In general, the response(s) of humans is similar to that of experimental animals, with notable exceptions, such as peroxisome proliferators[13] and α2u globulin nephropathy inducers[14] that do not elicit in humans the same effects as in rodents. Liver and kidney are important organs of metabolism, detoxification, storage and excretion of xenobiotics and their metabolites and especially vulnerable to damage[15]. Small intestine and heart just like any other organ can also be affected by toxic effect of chemicals.
From the present study, it was shown that the tissue section of liver, kidney, heart and small intestine of control animals were essentially normal. There were also no lesions (pathological changes) in the tissues of the animals that received various doses of the extract. The present finding is in agreement with the previous reports on biochemical and hematological response in Wister albino rats exposed to aqueous extract of unripe Carica papaya[6]. In conclusion, ingestion of aqueous extract of unripe Carica papaya has no adverse effect on the tissues of the organs studied in the rats. However, more work is suggested at the sub cellular levels and characterization of the active substance in the aqueous extract of unripe Carica papaya.
Acknowledgments
The authors are grateful to Mr. Solomon and Mr. Owolabi, of Animal House, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, for rendering technical assistance and Professor V.O Anosa, Faculty of Veterinary Medicine, University of Ibadan, Ibadan for reporting the slides.
All authors made substantial contributions right from inception to the end of the study. There is no conflict of interest in whatever form.
References
- 1.Papaya Natural Benefits and Curative properties. (Accessed March 15, 2010, at http://www.best-home-remeies.com/herbalmedicine/fruits/papaya.htm. )
- 2.Ogoda, Onah J, Akubue Pl, Okide GB. The kinetics of reversal of presickled erythrocytes by the aqueous extracts of Cajanus cajan seeds. Phytother Res. 2002;16(8):748–750. doi: 10.1002/ptr.1026. [DOI] [PubMed] [Google Scholar]
- 3.Zhao GX, Hui YH, Rupprecht JK, Mclaughlin JL, wood KV. “Additional bioactive compounds and triobacin, a novel highly cytotoxic acetogenin, from the bark of Asimina trioba.”. J Nat Prod. 1992;52:347–356. doi: 10.1021/np50081a011. [DOI] [PubMed] [Google Scholar]
- 4.Zhao GX, Gu Zm, Zeng L, Chao JF, Wood KV, Kozoloski JF, Mclaughlin JL. The absolute configuration of trilobaxin and trilobin, a novel highly potent acetogenin from the stem bark of Asmina Trioba (Annonacae) Tetrahedron. 1995;51:7149–7160. [Google Scholar]
- 5.Reiser MJ, Hui YH, Rupprecht JK, Kozolowski JF, Wood KV, McLaughlin JL, Hoye TR, Hanson PR, Zhuang ZP. Determination of absolute configuration of stereogenic carbinol centres in annonaceous aceto-enis by IH- and F-NMR analysis of Mosher ester derivatives. J Am Chem Soc. 1992;114:10203–10213. [Google Scholar]
- 6.Thomas KD, Ajani B. Antisickling agent in an extract of unripe pawpaw fruit (Carica papaya) Trans R Soc Trop Med Hyg. 1987;81:510–511. doi: 10.1016/0035-9203(87)90180-5. [DOI] [PubMed] [Google Scholar]
- 7.Oduola T, Adeniyi FAA, Ogunyemi EO, Bello IS, Idowu TO. Antisickling agent in an extract of unripe pawpaw (Carica papaya): Is it real? African J Biotechnol. 2006;5:1947–1949. [Google Scholar]
- 8.Oduola T, Adeniyi FAA, Ogunyemi EO, Bello IS, Idowu TO, Subair HG. Toxicity studies on an aqueous extract of unripe Carica papaya: Biochemical and haematological effect in Wistar albino rats. J Med Plant Res. 2007;1(1):1–4. [Google Scholar]
- 9.Oduola T, Adeniyi FAA, Ogunyemi EO, Idowu TO, Bello IS. Evaluation of the effects of intake of extract of unripe pawpaw (Carica papaya) on liver function in sickle cell patients. World J Med Sci. 2007;2(1):28–32. [Google Scholar]
- 10.Oduola T, Adeniyi FAA, Ogunyemi EO, Bello IS, Idowu TO. Ingestion of aqueous extract of unripe Carica papaya has no adverse effect on kidney function. World J Med Sci. 2008;3(2):89–92. [Google Scholar]
- 11.Oduola T, Adeniyi FAA, Adenaike FA, Ogunyemi EO, Bello IS, Idowu TO. Haematological response to intake of unripe Carica papaya aqueous extract. IJMR. 2009;(1):20–25. [Google Scholar]
- 12.Iatropoulos MJ. Endocrine considerations in toxicologic pathology. Exp Toxicol Pathol. 1994;45:391–410. doi: 10.1016/S0940-2993(11)80365-9. [DOI] [PubMed] [Google Scholar]
- 13.Avwioro OG. Histochemistry and Tissue Pathology: Principles and techniques. 1st ed. Claverianum Centre; 2002. [Google Scholar]
- 14.Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van Denn K, Smith P, Berger B, Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regal Toxicol pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 15.Views and expert opinions of an IARC working group. Lyon, France: IARC. IARC Technical Report No 24; 1995. International Agency for Research on cancer. Peroxisome proliferation and its role in carcinogenesis. [Google Scholar]
- 16.Rice JM, Baan RA, Blettner M, Genevois – Charneau C, Grosse Y, McGregor DB, Partensky C, Wilbourn JD. Rodent tumours of urinary bladder, renal cortex, and thyroid gland in IARC monographs evaluations of carcinogenic risk to humans. Toxicol Sci. 1999;49:166–171. doi: 10.1093/toxsci/49.2.166. [DOI] [PubMed] [Google Scholar]
- 17.Brzoska MM, Moniuszko-Jakonium J, Pilat-Marcinkiewicz B, Sawcki B. Liver and kidney function and histology in rats exposed to cadmium and ethanol. Alcohol Alcoholism. 2003;38:2–10. doi: 10.1093/alcalc/agg006. [DOI] [PubMed] [Google Scholar]


