Abstract
Background
Interactions with the microenvironment, such as bone marrow mesenchymal stromal cells and nurse-like cells, protect chronic lymphocytic leukemia cells from spontaneous and drug-induced apoptosis. This protection is partially mediated by the chemokine SDF-1α (CXCL12) and its receptor CXCR4 (CD184) present on the chronic lymphocytic leukemia cell surface.
Design and Methods
Here, we investigated the ability of AMD3100, a CXCR4 antagonist, to sensitize chronic lymphocytic leukemia cells to chemotherapy in a chronic lymphocytic leukemia/mesenchymal stromal cell based or nurse-like cell based microenvironment co-culture model.
Results
AMD3100 decreased CXCR4 expression signal (n=15, P=0.0078) and inhibited actin polymerization/migration in response to SDF-1α (n=8, P<0.01) and pseudoemperipolesis (n=10, P=0.0010), suggesting that AMD3100 interferes with chronic lymphocytic leukemia cell trafficking. AMD3100 did not have a direct effect on apoptosis when chronic lymphocytic leukemia cells were cultured alone (n=10, P=0.8812). However, when they were cultured with SDF-1α, mesenchymal stromal cells or nurse-like cells (protecting them from apoptosis, P<0.001), chronic lymphocytic leukemia cell pre-treatment with AMD3100 significantly inhibited these protective effects (n=8, P<0.01) and decreased the expression of the anti-apoptotic proteins MCL-1 and FLIP. Furthermore, combining AMD3100 with various drugs (fludarabine, cladribine, valproïc acid, bortezomib, flavopiridol, methylprednisolone) in our mesenchymal stromal cell co-culture model enhanced drug-induced apoptosis (n=8, P<0.05) indicating that AMD3100 could mobilize chronic lymphocytic leukemia cells away from their protective microenvironment, making them more accessible to conventional therapies.
Conclusions
Taken together, these data demonstrate that interfering with the SDF-1α/CXCR4 axis by using AMD3100 inhibited chronic lymphocytic leukemia cell trafficking and microenvironment-mediated protective effects. Combining AMD3100 with other drugs may, therefore, represent a promising therapeutic approach to kill chronic lymphocytic leukemia cells.
Keywords: AMD3100, chronic lymphocytic leukemia, microenvironment, mesenchymal stromal cells, nurse-like cells, apoptosis
Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in Western countries, is characterized by the accumulation of mature CD19, CD5, CD23 positive (CD19+CD5+CD23+) B cells which present a weak proliferation index compared with normal B cells1 and a defect in apoptosis.2 However, these cells rapidly undergo spontaneous apoptosis when they are cultured in vitro,3 suggesting that in vivo factors contribute to their prolonged survival, and reinforcing the importance of the microenvironment in this context.4–6 Indeed, microenvironments in the bone marrow, lymph nodes and other secondary lymphoid organs have been shown to inhibit spontaneous CLL cell apoptosis and enhance chemoresistance.5 In 1998, our group showed that bone marrow stromal cells could rescue CLL cells (but not normal B cells) from apoptosis,7 and in 2000, Burger et al. observed that nurse-like cells (NLC) derived from CD14+ cells of CLL patient blood could also protect CLL cells from apoptosis.8 These pro-survival effects are largely dependent on microenvironment/CLL cell contact but also on chemokines released in the milieu.
One such chemokine is stromal-derived factor-1α (SDF-1α, also known as chemokine (C-X-C motif) ligand 12 -CXCL12), which is produced by mesenchymal stromal cells (MSC)9 and NLC.8 This chemokine and its receptor (chemokine (C-X-C motif) receptor 4, CXCR4), which is present on the CLL cell surface, play a crucial role in CLL cell trafficking and survival. Furthermore, Burger et al. demonstrated that SDF-1α not only attracts CLL cells to the supportive microenvironment but also directly stimulates CLL cell survival.8 Several studies have shown that CLL cells can interact with their microenvironment through the CXCR4/SDF-1α axis.9–10 CLL cells express high levels of CXCR4 surface receptors compared to normal B cells, making them more sensitive to this signal.10 For these reasons, the CXCR4/SDF-1α axis has been considered as a potential target for new therapeutic strategies.11
AMD3100 (also known as Plerixafor or Mozobil®) is a bicyclam molecule and a specific antagonist to the CXCR4 receptor, preventing the binding of SDF-1α.12 AMD3100 was initially studied for its capacity to inhibit HIV virus entry12 and is currently used as a hematopoietic stem cell mobilization agent.13 In the present study, we hypothesized that AMD3100 could disrupt the MSC-based and NLC-based microenvironment/CLL cell crosstalk by interfering with the adhesion and homing of CLL cells via inhibition of the SDF-1α/CXCR4 axis. The aim of this study was to demonstrate that AMD3100 could increase CLL cell sensitivity to different currently used drugs (such as fludarabine, cladribine, etc.) or others (valproic acid, flavopiridol, etc.) under investigation in CLL treatment and could, therefore, be considered as a potential novel adjuvant therapy.
Design and Methods
Patients, reagent and antibodies
This study was approved by the Bordet Institute Ethics Committee and was conducted using peripheral blood samples obtained with written informed consent from 20 CLL patients who presented with a typical CD19+CD5+CD23+ phenotype. Patients were either untreated or had received no treatment for at least six months before the study. A summary of patients’ characteristics is presented in the Online Supplementary Table S1. Cytoplasmic ZAP70 expression was determined by 3-color flow cytometry (CD3/ZAP70/CD19) and confirmed by quantitative real-time PCR.14 CD38 expression analysis, standard karyotype analysis and interphase FISH screening for the most common genetic aberrations and the IGHV gene mutation were performed as previously described.15 In the present patient population, the mean percentage of CD19+/CD5+ was 97.40±0.71% (range 91.25–99.97) and the normal B-cell (CD19+/CD5−) population was considered to be negligible. All reagents (source and excipient) and antibodies (epitope and reference) used in this work are detailed in the Online Supplementary Appendix.
Cell culture, AMD3100 treatment, MSC and NLC establishment, and phenotypic analysis
Mononuclear cells (MNC) were isolated from peripheral blood samples using density gradient centrifugation (Linfosep, Biomedics, Spain). The bone marrow stromal layer and NLC were prepared as previously described.8,16 AMD3100 was added to the MNC from CLL patient suspension (2×106 cells/mL) 30 min before each experiment. Details of the culture conditions and phenotypic analysis of the MSC and NLC are presented in the Online Supplementary Appendix. “Myeloid cell leukemia sequence 1” (MCL-1) and “CASP8 and FADD-like apoptosis regulator” (CFLAR or FLIP) protein staining was performed on CD19-labeled cells, using the Fix and Perm Permeabilization kit according to the manufacturer’s recommendations.
MSC layer establishment
Bone marrow samples were collected from healthy donors after obtaining written informed consent. MNC isolated from normal subjects were plated in Dulbeco’s Modified Eagle Medium – low glucose (DMEM-LG) (Lonza Europe) supplemented with 15% FCS (Sigma-Aldrich) to obtain a stromal layer composed of mesenchymal stromal cells (MSC), as previously described.16
NLC generation and co-culture
The NLC generation protocol was adapted from the protocol previously published by Burger et al.8 MNC of CLL patients were suspended in 500 μL of complete medium (as described above) to a final concentration of 2×107 cells/mL. After 14 days of culture, the non-adherent MNC from CLL patients were harvested by vigorously pipetting the content of the well and subsequently rinsing the plates with complete RPMI medium. Harvested cells were washed, and residual NLC were removed by a positive purification procedure using a CD14 magnetic bead system (MidiMACS, Miltenyi Biotec, Bergish Gladbash, Germany) according to the manufacturer’s instructions. Mean CD19 purity was thereafter over 98%. MNC from CLL patients were then suspended at a concentration of 2×106 cells/mL in complete medium and pre-treated or not with AMD3100 for 30 min; 200 μL of these cell suspensions (with or without AMD3100) were then cultured in a 24-well plate alone or with the previously generated NLC. Viability and apoptosis were evaluated after 48 h.
Viability and apoptosis assays
Cell viability was determined by 3,3′-dihexyloxacarbocyanine iodide (DiOC6)/propidium iodide (PI) staining after gating on lymphoid population based on forward and side scatter, as previously described.17 Apoptosis was measured with Annexin-V/7-Aminoactinomycin D (7-AAD) and PE-conjugated CD19 monoclonal (MoAb) antibodies, using the protocol described in the ApoTarget Kit (Biosource, Nivelles, Belgium).
Measure of CXCR4 expression on MNC from CLL patients
Untreated or AMD3100-treated MNC from CLL patients were incubated for 30 min with a fluorescein isothiocyanate (FITC)-conjugated anti-CD19 MoAb and phycoerythrin (PE)-conjugated anti-CXCR4 (epitope 12G5, BD Biosciences Pharmigen, San Diego, CA, USA). CXCR4 expression on the CD19+ population was then evaluated by a comparison with cells incubated with an isotype control antibody.
Actin polymerization assay
Actin polymerization was tested as previously described.18 Briefly, 1.25×106/mL were suspended in RPMI-1640 medium with 0.5% BSA at 37°C and incubated with 100 ng/mL SDF-1α between 0 and 240 sec. To determine the rate of actin polymerization in MNC from CLL patients after AMD3100 treatment (30 min at 5 μg/mL), MNC from CLL patients were relabeled with an allophycocyanin (APC)-conjugated anti-CD19 MoAb (Miltenyi Biotec). Pertussis toxin (200 ng/mL), which prevents G proteins from interacting with G protein-coupled receptors, was used as a control. At the indicated time points, 400 μL of the cell suspension was added to 100 μL of a solution containing 4×10−7 mol/L FITC-labeled phalloidin, 0.5 mg/mL 1-α-lysophosphatidylcholine (both from Sigma), and 18% formaldehyde in phosphate-buffered saline (PBS). The fixed cells were analyzed by flow cytometry, and all time points were plotted relative to the mean fluorescence of the sample before addition of the chemokine.
Migration in response to SDF-1α
These assays were performed using 5-μm diameter pore filters. To examine cell migration towards SDF-1α, 100 ng/mL of SDF-1α in RPMI was added to the lower chamber of the transmigration chamber (24-well, Corning Inc., New York, USA). Cells were pre-treated or not with AMD3100 (5 μg/mL) for 30 min. Untreated or AMD3100-treated MNC from CLL patients (5×105) were added to the upper chamber and incubated for 3 h under culture conditions. The cells in the lower chamber were then collected, labeled with an APC-conjugated CD19 MoAb (Miltenyi Biotec), and 100 μL of the cell suspension was counted with the MACSQUANT® flow cytometer, using the absolute volumetric cell counting function. The absolute number of CD19+ cells was then determined. Migration index was calculated as the number of cells transmigrating in the presence of the chemoattractant divided by the number of transmigrating cells in the absence of the chemoattractant.
In vitro migration of MNC from CLL patients into a stromal layer (pseudoemperipolesis)
We suspended 5×106 untreated or AMD3100-treated cells in 1 mL RPMI and these were then added to stromal layers that were established from normal subjects. After a 3-h incubation, non-adherent cells in suspension in the medium were removed. The stromal layer containing cells that had migrated was carefully washed twice with PBS in order to remove adherent cells. Transmigrated MNC from CLL patients were harvested using Tryple Select treatment (Gibco, Invitrogen, Merelbeke, Belgium), labeled with an APC-conjugated CD19 MoAb (Miltenyi Biotec) and 100 μL of the cell suspension was counted with the MAC-SQUANT® flow cytometer, using the absolute volumetric cell counting function. The absolute number of CD19+ cells was then determined.
CLL/MSC-based microenvironment co-culture model and drug treatment
After a pre-treatment with AMD3100 for 30 min, MNC from CLL patients (2×106/mL) were plated alone or with SDF-1α, MSC or NLC, and viability and apoptosis were evaluated after 48 h. To evaluate the potential of AMD3100 to sensitize MNC from CLL patients to drug-induced apoptosis and death, we performed the same experiment in a CLL/MSC co-culture model with or without various drugs at concentrations adapted from our previous study or the literature: fludarabine (3 μM),19 cladribine (0.5 μM),20 methylprednisolone (10 μM),21 valproic acid (1 mM),22 bortezomib (5 nM)22 and flavopiridol (50 nM).22 Viability and apoptosis were then evaluated after 48 h.
Statistical analysis
Wilcoxon’s signed ranks test was used to analyze the statistical significance of the experimental results. All tests were two-sided. P<0.05 was considered statistically significant, and all analyzes were performed with GraphPad Prism 5.0 software. For normalized data, the P values presented in this paper were obtained from primary data (before normalization) because P cannot be calculated for data with the same rank.
Results
AMD3100 interferes with 12G5 antibody for binding membrane CXCR4
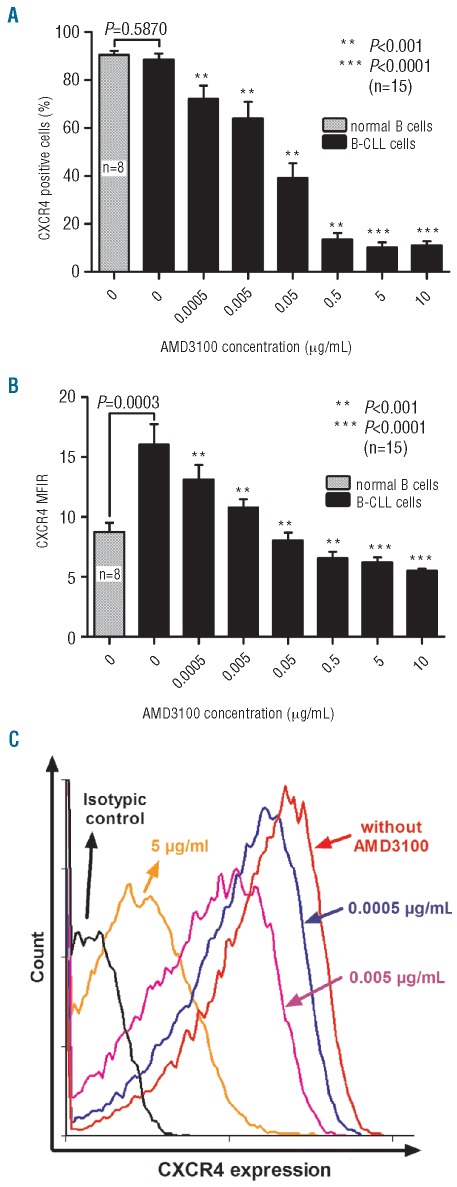
In order to interfere efficiently with the SDF-1α/CXCR4 axis in MNC from CLL patients, we first determined the optimal dose of AMD3100 to use in vitro. To monitor the binding of AMD3100 to CXCR4 on the MNC from CLL patients’ cell surface, cells were incubated with increasing concentrations of AMD3100 (0–10 μg/mL), and the CXCR4 expression signal was measured by flow cytometry. As the CXCR4 antibody used and AMD3100 bind to the same epitope (12G5), this competitive effect could be used to monitor AMD3100 binding. An AMD3100 concentration of 5 μg/mL in a suspension of 2×106 cells/mL decreased the percentage of CXCR4+ cells from 88.7±2.3% to 13.5±2.8% (n=15, P<0.0001) (Figure 1A), and the mean fluorescence intensity ratio (MFIR) representing CXCR4 signal over isotypic signal was also decreased from 16.0±1.7 to 6.2±0.4 (n=15, P<0.0001) (Figure 1B and C). We therefore decided to use a dose of 5 μg/mL AMD3100 for the subsequent experiments. We also confirmed that MNC from CLL patients have a higher MFIR than normal B cells (n=8, P=0.0003), but there was no significant difference in the percentage of CD19+/CXCR4+ cells (n=8, P=0.5870).
Figure 1.
AMD3100 decreases CXCR4 expression signal. MNC isolated from CLL patients were incubated with AMD3100 for 30 min and then incubated with anti-CXCR4 antibody and analyzed by flow cytometry. Percentage of CXCR4+ cells (A) and mean fluorescence intensity ratio (MFIR representing the CXCR4 signal/isotypic signal) (B) for normal B cells (n=8) or CLL cells (n=15) treated with increasing concentrations of AMD3100. (C) Representative histogram showing the decrease in CXCR4 expression signal after AMD3100 treatment. Each bar represents the mean ± SEM of 8–15 experiments.
AMD3100 reduces actin polymerization and migration of MNC from CLL patients in response to SDF-1α and beneath a stromal layer
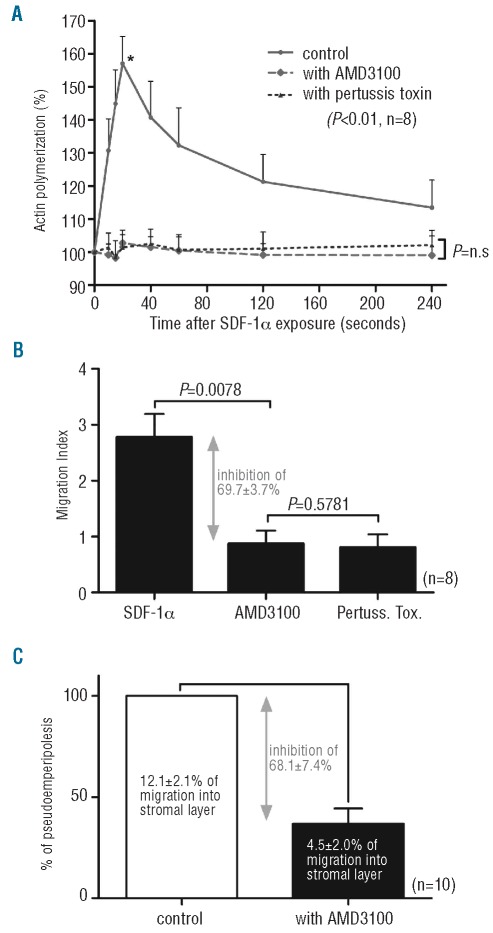
Knowing that SDF1-α/CXCR4 axis plays an important role in CLL cell trafficking, we first investigated its inhibition on cytoskeletal rearrangement and particularly on actin polymerization. In response to 100 ng/mL SDF-1α, MNC from CLL patients were cultured without any drug, with AMD3100 (30 min at 5 μg/mL) or with pertussis toxin (3 h at 200 ng/mL). We detected a transient increase in filamentous actin levels a few seconds after the addition of the chemokine and a maximal increase after 20 sec. This increase was completely abolished with AMD3100 exposure (n=8, P<0.01) as well as pertussis toxin treatment, which was used as a control (Figure 2A). We next evaluated the effect of AMD3100 on MNC from CLL patient chemotaxis in response to SDF-1α (n=8). As shown in Figure 2B, AMD3100 strongly decreased the cell migration index in response to SDF-1α: 2.8±0.4 without AMD3100 vs. 0.9±0.2 with AMD3100 (P=0.0078). These results correspond to an inhibition of 69.7±3.7% compared with the control cell migration. Moreover, we compared the migration of untreated or AMD3100-treated MNC from CLL patients (n=10) into a bone marrow stromal layer established from normal subjects; of the input MNC from CLL patients (5×106), an average of 12.1 ±2.1% migrated into the stromal layer. Compared with the untreated control samples, 30 min of 5 μg/mL AMD3100 treatment reduced the number of migrating cells to only 4.5±2.0%, corresponding to an inhibition of 68.18±7.4% (n =10, P=0.0020) compared with the control cell pseudoemperipolesis (Figure 2C).
Figure 2.
AMD3100 interferes with CLL cell trafficking. (A) Intracellular F-Actin was measured using FITC-labeled phalloidin in CD19-pre-labeled CLL cells after the addition of SDF-1α (100 ng/mL) at different time points without any drugs, in the presence of AMD3100 (5 μg/mL) or in the presence of pertussis toxin (200 ng/mL) as control. All time points are plotted relative to the mean fluorescence of the sample before addition of the chemokine. (B) CLL cells were pre-treated or not with AMD3100 for 30 min before being plated onto 5-μm Transwell microporous membranes for the migration assay. Results are expressed as the mean ± SEM migration index of 8 experiments. Migration index was calculated as the number of cells transmigrating in the presence of the chemoattractant divided by the number of transmigrating cells in the absence of the chemoattractant. (C) 5×106 untreated or AMD3100-treated cells were added to stromal layers, and after a 3-h incubation, cells that had migrated to the stromal layer were counted as described in the Online Supplementary Appendix.
Altogether, these results demonstrated that AMD3100, by interfering with SDF1-α, is a strong inhibitor of CLL cell cytoskeleton rearrangement and blocks the chemo-taxis and migration of the leukemia cells.
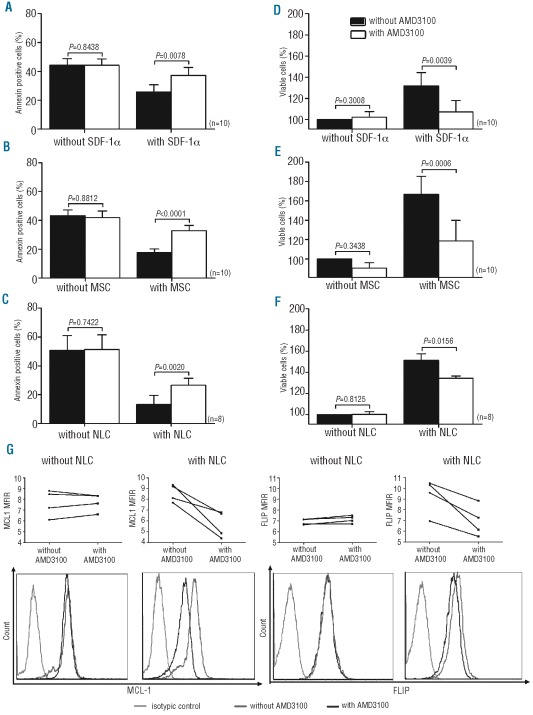
AMD3100 reduces viability and restores apoptosis in MNC from CLL patients cultured with SDF-1α, MSC or NLC
To better analyze the role of AMD3100 on MNC from CLL patients in various cellular microenvironments, we decided to co-culture them with the MSC and NLC, which were previously characterized (see Online Supplementary Appendix for results). MNC from CLL patients were pre-treated or not with AMD3100 and cultured in the presence or absence of SDF-1α (Figure 3A and D), an MSC layer (Figure 3B and E) or NLC (Figure 3C and F). After a 48-h incubation, apoptosis (% annexin positive cells including early and late apoptosis) and cell viability (% viable cells) were evaluated. Our results showed that spontaneous apoptosis was significantly reduced, and cell viability was improved with SDF-1α or in the presence of both cellular microenvironments (n=8, P<0.0001). Moreover, when MNC from CLL patients were cultured alone, AMD3100 did not have a significant effect on apoptosis or viability (n=8–10, P>0.7422). In contrast, when MNC from CLL patients were cultured with SDF-1α or an MSC-based or NLC-based microenvironment, apoptosis was significantly increased and viability decreased after AMD3100 treatment (n=8–10, P<0.01) (Figure 3). A representative annexin V/PI staining image is presented in the Online Supplementary Appendix. Interestingly, AMD3100 did not have an effect on the expression of two anti-apoptotic proteins (MCL-1 and FLIP) when MNC from CLL patients were cultured alone but clearly decreased the expression of these two proteins in a CLL/NLC co-culture (n=4). A representative case is shown in Figure 3G.
Figure 3.
AMD3100 restores apoptosis and decreases the viability of CLL cells in the presence of an MSC-based or NLC-based microenvironment or SDF-1α. MNC isolated from CLL patients were treated or not with AMD3100 and then cultured alone or with SDF-1α (A, D), MSC (B, E) or NLC (C, F), and apoptosis and cell viability were measured after 48 h. The rates in the figure include early and late apoptosis. Viability rates were obtained after normalizing the results to those for untreated cells. Each bar represents the mean ± SEM of 8–10 experiments. (G) MCL1 and FLIP expression were evaluated by flow cytometry in 4 patients in different conditions (with/without AMD3100; with/without NLC) and MFIR representing MCL1 or FLIP expression over isotypic signal were plotted for each case. A representative case of intra-cellular staining for MCL-1 and FLIP protein on CD19+ cells is provided for the different situations.
Taken together, these results suggest that interfering with the SDF1α/CXCX4 axis using AMD3100 antagonizes the protective effect of MSC-based or NLC-based microenvironment on MNC from CLL patients that become more sensitive to apoptosis.
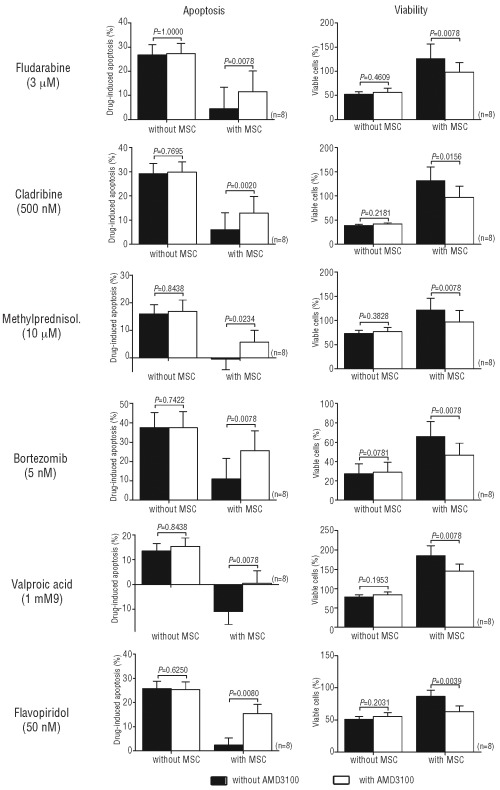
AMD3100 sensitizes MNC from CLL patients to drug-induced apoptosis in a CLL/MSC co-culture model
As suggested previously by Kurtova et al., a co-culture model with a protective microenvironment seems to be very important for studying drug-induced apoptosis of CLL cells.17 Therefore, we measured the viability and apoptosis of MNC from CLL patients cultured with or without an MSC layer without any drug, with or without AMD3100 (5 μg/mL), and with fludarabine (3 μM), cladribine (0.5 μM), methylprednisolone (10 μM), valproic acid (1 mM), bortezomib (5 nM) and flavopiridol (50 nM). After subtracting the spontaneous apoptosis, we observed that only in the presence of an MSC microenvironment, AMD3100 increased the degree of apoptosis induced by each drug (n=10, P<0.05), while viability (after normalization to cells plated alone without AMD3100 or any drugs) was statistically decreased (n=10, P<0.05) (Figure 4). A representative annexin V/PI staining image is presented in the Online Supplementary Appendix. These results demonstrated that antagonizing the SDF-1α/CXCR4 axis leads to higher drug-induced apoptosis in MNC from CLL patients when they are in a bone marrow stromal environment.
Figure 4.
AMD3100 sensitizes CLL cells to drug-induced apoptosis in a CLL/MSC co-culture model. MNC isolated from CLL patients were treated or not with AMD3100 and then cultured with or without various drugs. Apoptosis and cell viability were measured after 48 h, as described in the Design and Methods section. The rates in the figure include early and late apoptosis. The drug-induced apoptosis rates were obtained after subtracting the spontaneous apoptosis, while the viability rates were obtained after normalization to untreated cells. Each bar represents the mean ± SEM of 8 experiments.
Discussion
This last decade, increasing attention has been focused on the tumoral microenvironment and its contribution to the survival of malignant cells.23 Our group was among the first to observe the survival advantage conferred by an in vitro MSC microenvironment to CLL cells.4,7 In addition, Burger et al. showed that NLC and SDF-1α played a crucial role in the migration, homing and survival of CLL cells, and that this survival protection was mediated through the SDF-1α/CXCR4 axis.8 In the present work, we interfered with the SDF-1α/CXCR4 axis using AMD3100, a well-known and tolerated drug. AMD3100 has been used in humans for more than ten years24 and clinical phases have shown that AMD3100 has modest and tolerable side effects.25–26 Here, we observed that AMD3100 could interfere with CLL cell trafficking by inhibiting actin polymerization and cell migration in response to SDF-1α and pseudoemperipolesis. Results are in line with those observed by Burger et al.27
When CLL cells are cultured in vitro, they rapidly undergo spontaneous apoptosis; but this can be prevented by culturing them with SDF-1α, MSC or NLC.7–8 Here, we show that AMD3100 treatment leads to a higher rate of spontaneous apoptosis and decreased viability in each of these 3 situations. However, AMD3100 could only partially restore apoptosis levels compared with the single culture system, probably because of other unknown mechanisms, such as cytokines and other interactions likely involved in the survival of CLL cells. This phenomenon was previously observed by Burger et al. who did not detect a significant degree of apoptosis inhibition with another CXCR4 antagonist (T140 analog).8 In our study, we also found some cases in which cell apoptosis was only increased by about 5–10% after AMD3100 treatment. However, in each experiment, we show a partial restoration of apoptosis, making our data significant. These data were strengthened by the reduced expression of important anti-apoptotic proteins, such as MCL-1 or FLIP, when we cultured MNC from CLL patients with NLC, indicating again that AMD3100 was able to partially restore apoptosis.
In 2009, Kurtova et al. proposed a co-culture model based on diverse bone marrow stromal cell types to study in vitro the effect of drugs in a more reliable and reproducible manner.17 Therefore, we investigated the effect of a combination of different drugs that are currently being investigated or used for hematologic malignancies, with or without AMD3100, and in the presence/absence of an MSC stromal layer, mimicking CLL cell/MSC interactions that occur in the bone marrow. For all drugs tested, we demonstrate that AMD3100 increased the degree of drug-induced apoptosis and decreased cell viability. As for our previous experiments, the restoration of apoptosis was not complete due to the reasons discussed above, but the effect was clearly significant. Burger et al. obtained similar results with fludarabine and another CXCR4 antagonist (T140 analog).27 In the present study, we show that the adjuvant effect of a CXCR4 antagonist such as AMD3100 could be observed not only with fludarabine but also with other drugs (cladribine, methylprednisolone, valproic acid, bortezomib and flavopiridol) currently used or under investigation for CLL treatment. This is also particularly important in the context of lymph node (LN) niches;23 indeed, the LN microenvironment (including NLC) may provide a safe haven from cytotoxic anticancer drugs thus serving as a tumor reservoir from which relapse occurs, and where CLL cells could proliferate and accumulate genetic mutations that favor disease progression. Since AMD3100 disrupts the cross-talk between MNC of CLL patients and NLC, we can expect that it will also mobilize CLL cells from protective LN niches.
As mentioned previously, different CXCR4 antagonists are available.11 Several interesting studies have been per-formed with the T140 analogs, small peptide inhibitors of CXCR4 receptors.27–28 However, T140 analogs have not yet been approved by the Food and Drug Administration (FDA). In contrast, AMD3100 has been FDA-approved since 2009; it has been investigated in clinical trials and displays moderate and tolerable side effects.25–26 For all these reasons, we decided to investigate AMD3100 instead of other CXCR4 antagonists such as T140 analogs. In addition, a currently ongoing clinical phase I/II trial combining AMD3100 with rituximab in CLL patients mirrors our choice of CXCR4 inhibitor. Indeed, the preliminary results of this study presented at the American Society of Hematology 2010 meeting indicate that this combination is safe, well tolerated and could potentially provide interesting results.
In conclusion, we suggest the potential benefit of CXCR4 antagonist therapies using AMD3100 in CLL. AMD3100 inhibits CLL cell trafficking, mobilizes CLL cells from tissues, thereby disrupting the cell/MSC-based or NLC-based microenvironment interactions and partially blocking survival stimuli, and also interferes with the survival signal provided by SDF-1α. Through these mechanisms, AMD3100 enhanced the sensitivity of CLL cells to all tested drugs. Taken together, these data demonstrate that using AMD3100 to interfere with the SDF-1α/CXCR4 axis could represent a new therapeutic modality, and that combining AMD3100 with other conventional agents may thus be a promising therapeutic approach for killing CLL cells.
Supplementary Material
Footnotes
Funding: this research was supported by the “Fonds Germaine Eisendrath-Dubois”, the “Wallonie-Bruxelles International”, the “Fonds National de la Recherche Scientifique – FNRS” (F.R.S-F.N.R.S.), the French Ministry of Foreign and European affairs, and the Ministry of Higher Education and Research in the framework of a Hubert Curien partnership. B.S. (Postdoctoral Researcher), C.dB. (Scientific Research Worker), and L.L. (Senior Research Associate) are members of the F.N.R.S.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Defoiche J, Debacq C, Asquith B, Zhang Y, Burny A, Bron D, et al. Reduction of B cell turnover in chronic lymphocytic leukaemia. Br J Haematol. 2008;143(2):240–7. doi: 10.1111/j.1365-2141.2008.07348.x. [DOI] [PubMed] [Google Scholar]
- 2.Dighiero G, Travade P, Chevret S, Fenaux P, Chastang C, Binet JL. B-cell chronic lymphocytic leukemia: present status and future directions. French Cooperative Group on CLL. Blood. 1991;78(8):1901–14. [PubMed] [Google Scholar]
- 3.Collins RJ, Verschuer LA, Harmon BV, Prentice RL, Pope JH, Kerr JF. Spontaneous programmed death (apoptosis) of B-chronic lymphocytic leukaemia cells following their culture in vitro. Br J Haematol. 1989;71(3):343–50. doi: 10.1111/j.1365-2141.1989.tb04290.x. [DOI] [PubMed] [Google Scholar]
- 4.Lagneaux L, Delforge A, De Bruyn C, Bernier M, Bron D. Adhesion to bone marrow stroma inhibits apoptosis of chronic lymphocytic leukemia cells. Leuk Lymphoma. 1999;35(5–6):445–53. doi: 10.1080/10428199909169609. [DOI] [PubMed] [Google Scholar]
- 5.Caligaris-Cappio F. Role of the microenvironment in chronic lymphocytic leukaemia. Br J Haematol. 2003;123(3):380–8. doi: 10.1046/j.1365-2141.2003.04679.x. [DOI] [PubMed] [Google Scholar]
- 6.Stamatopoulos B, Haibe-Kains B, Equeter C, Meuleman N, Soree A, De Bruyn C, et al. Gene expression profiling reveals differences in microenvironment interaction between patients with chronic lymphocytic leukemia expressing high versus low ZAP70 mRNA. Haematologica. 2009;94(6):790–9. doi: 10.3324/haematol.2008.002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91(7):2387–96. [PubMed] [Google Scholar]
- 8.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–63. [PubMed] [Google Scholar]
- 9.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94(11):3658–67. [PubMed] [Google Scholar]
- 10.Mohle R, Failenschmid C, Bautz F, Kanz L. Overexpression of the chemokine receptor CXCR4 in B cell chronic lymphocytic leukemia is associated with increased functional response to stromal cell-derived factor-1 (SDF-1) Leukemia. 1999;13(12):1954–9. doi: 10.1038/sj.leu.2401602. [DOI] [PubMed] [Google Scholar]
- 11.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23(1):43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 12.Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4(1):72–7. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 13.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728–30. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 14.Stamatopoulos B, Meuleman N, Haibe-Kains B, Duvillier H, Massy M, Martiat P, et al. Quantification of ZAP70 mRNA in B cells by real-time PCR is a powerful prognostic factor in chronic lymphocytic leukemia. Clin Chem. 2007;53(10):1757–66. doi: 10.1373/clinchem.2007.089326. [DOI] [PubMed] [Google Scholar]
- 15.Stamatopoulos B, Meuleman N, Haibe-Kains B, Saussoy P, Van Den Neste E, Michaux L, et al. microRNA-29c and micro-RNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification. Blood. 2009;113(21):5237–45. doi: 10.1182/blood-2008-11-189407. [DOI] [PubMed] [Google Scholar]
- 16.Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23(8):1105–12. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 17.Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114(20):4441–50. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamatopoulos B, Meuleman N, De Bruyn C, Delforge A, Bron D, Lagneaux L. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces apoptosis, down-regulates the CXCR4 chemokine receptor and impairs migration of chronic lymphocytic leukemia cells. Haematologica. 2010;95(7):1136–43. doi: 10.3324/haematol.2009.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina-Arcas M, Bellosillo B, Casado FJ, Montserrat E, Gil J, Colomer D, et al. Fludarabine uptake mechanisms in B-cell chronic lymphocytic leukemia. Blood. 2003;101(6):2328–34. doi: 10.1182/blood-2002-07-2236. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Galan P, Marzo I, Giraldo P, Rubio-Felix D, Lasierra P, Larrad L, et al. Role of caspases and apoptosis-inducing factor (AIF) in cladribine-induced apoptosis of B cell chronic lymphocytic leukemia. Leukemia. 2002;16(10):2106–14. doi: 10.1038/sj.leu.2402650. [DOI] [PubMed] [Google Scholar]
- 21.Boelens J, Lust S, Van Bockstaele F, van Gele M, Janssens A, Derycke L, et al. Steroid effects on ZAP-70 and SYK in relation to apoptosis in poor prognosis chronic lymphocytic leukemia. Leuk Res. 2009;33(10):1335–43. doi: 10.1016/j.leukres.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Stamatopoulos B, Meuleman N, De Bruyn C, Mineur P, Martiat P, Bron D, et al. Antileukemic activity of valproic acid in chronic lymphocytic leukemia B cells defined by microarray analysis. Leukemia. 2009;23(12):2281–9. doi: 10.1038/leu.2009.176. [DOI] [PubMed] [Google Scholar]
- 23.Munk Pedersen I, Reed J. Micro-environmental interactions and survival of CLL B-cells. Leuk Lymphoma. 2004;45(12):2365–72. doi: 10.1080/10428190412331272703. [DOI] [PubMed] [Google Scholar]
- 24.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44(6):1667–73. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendrix CW, Collier AC, Lederman MM, Schols D, Pollard RB, Brown S, et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37(2):1253–62. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 26.Cashen A, Lopez S, Gao F, Calandra G, MacFarland R, Badel K, et al. A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(11):1253–61. doi: 10.1016/j.bbmt.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106(5):1824–30. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 28.Buchner M, Brantner P, Stickel N, Prinz G, Burger M, Bar C, et al. The microenvironment differentially impairs passive and active immunotherapy in chronic lymphocytic leukaemia - CXCR4 antagonists as potential adjuvants for monoclonal antibodies. Br J Haematol. 2010;151(2):167–78. doi: 10.1111/j.1365-2141.2010.08316.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.