Abstract
Tumor-necrosis factor alpha activity has been correlated to ineffective erythropoiesis in lower risk myelodysplastic syndromes. Infliximab (Remicade®) is an anti-tumor necrosis factor alpha chimeric antibody that is used in the treatment of patients with rheumatoid arthritis or Crohn’s disease. Forty-six patients with myelodysplastic syndromes and a relatively low risk of developing acute leukemia were included in a randomized phase II study assessing the therapeutic activity of two dosages of infliximab administration (3 mg/kg vs. 5 mg/kg). The primary end point was the response rate. Responses were observed in 3 of 22 patients (13.1%) randomized to the 3 mg/kg arm, versus 0 of 21 patients randomized in the 5 mg/kg arm. According to the statistical design of the current study, neither of the two infliximab dose schedules tested showed sufficient activity as a single agent in this cohort of unselected patients with early myelodysplastic syndrome.
Keywords: infliximab, tumor necrosis factor alpha, TNF-α, MDS, myelodysplastic syndrome, phase II, randomized
Introduction
Myelodysplastic syndromes (MDS) represent a collection of early hematopoietic progenitor cell clonal disorders characterized by ineffective hematopoiesis and low peripheral blood counts.1 Although lenalidomide, azacitidine and decitabine are all Food and Drug Administration (FDA)-approved agents to treat MDS in the US, allogeneic hematopoietic cell transplantation has remained the only potential cure for MDS patients.2
Increased apoptotic death of hematopoietic cells has been observed in early MDS, and has been associated with ineffective hematopoiesis.3–4 Tumor necrosis factor alpha (TNF-α) is a pro-inflammatory cytokine that inhibits normal hematopoiesis and induces programmed cell death of both normal total bone marrow cells and normal CD34+ cells.5 Several observations have suggested an important role of TNF-α in the pathophysiology of low-risk MDS.6–9
Infliximab (Remicade®) is a chimeric human / murine antibody combining the variable region of the murine monoclonal antibody A2 recognizing human TNF-α and human immunoglobulin G1 constant regions. Clinical phase III studies in rheumatoid arthritis and in Crohn’s disease have shown that infliximab binds to TNF-α, prevents TNF-α binding to both the p55 and p75 TNF receptors, and is effective in both disorders.10–11 Dosages applied in most trials were 3 mg/kg, 5mg/kg or 10 mg/kg, given intravenously at weeks 0, 2 and 6, and then every 4–8 weeks thereafter.10–11 Few data on the efficacy of infliximab in MDS patients have been reported so far. Stasi and Amadori observed sustained erythroid response in 2 of 2 patients with low-/intermediate-risk MDS, isolated anemia, and elevated circulating levels of TNF-α, after administration of infliximab given intravenously at the dose of 3 mg/kg.12 Raza et al. administered infliximab at the dose of 5 mg/kg (n=18) or 10 mg/kg (n=19) intravenously every four weeks for 4 cycles to patients with refractory anemia (RA)/refractory anemia with ringed sideroblasts (RARS) (n=28), or refractory anemia with excess blasts (RAEB, n=9).13 The drug was generally well tolerated, and 8 patients (including 3 patients given inflixamab at the dose of 5 mg/kg and 5 patients given infliximab at 10 mg/kg) achieved hematologic responses.
Here, we report the results of a randomized phase II study assessing therapeutic activity and adverse event profile of two dosages of infliximab administration (3 mg/kg vs. 5 mg/kg) in patients with myelodysplastic syndrome and a relatively low risk of developing acute leukemia.
Design and Methods
Study design
Study design is detailed in the Online Supplementary Appendix. Main eligibility criteria included MDS intermediate or good IPSS risk score14 and FAB type RA, RARS, or RAEB with 10% or under bone marrow blasts; no poor cytogenetic characteristics; 6 weeks or over prior to randomization without treatment for MDS other than supportive care only; WHO performance status 0–2; Hb <10 g/dL or RBC transfusion dependent and/or neutrophil count <1.5×109/L and/or platelet count <100×109/L or platelet transfusion dependent; no severe cardiac or pulmonary dysfunction; serum bilirubin and creatinine ≤1.5 × ULN; and absence of current or prior active or latent tuberculosis infection.15 Signed written informed consent was obtained according to ICH/GCP and national/local regulations. The study protocol was approved by the EORTC Protocol Review Committee and by the Ethical Committee of each participating center. Eligible patients were randomized 1:1 to receive infliximab 3 mg/kg (arm A) or infliximab 5 mg/kg (arm B). Infliximab was injected intravenously on days 1, 15 and then every four weeks until Month 6 (for a total of 8 infusions).
Primary end point was the response rate (complete response, partial response and hematologic improvement) as defined by Cheson et al.16 Secondary end points included the definition of toxicity profile of infliximab in MDS patients and the duration of response. Overall and progression-free survivals, although not specifically indicated as secondary end points in the protocol, were also evaluated prospectively.
Statistical analysis
The statistical design of the study is detailed in the Online Supplementary Appendix. Briefly, the amended protocol was based on a Simon 2-stage design. P0 was 15%; P1 was 35%; beta was 0.05; and alpha was 0.15. This implies entering and evaluating the first 18 patients for each infliximab arm. If 2 responses or less were observed (2 of 18=11.1%), the trial would conclude that infliximab (at the given dose level, according to randomization) should not be further investigated in this patient population, while if 3 responses or more were observed, one should continue the accrual for 18 additional patients, in the arm(s) which passed the 1st step. Since 36 (2 × 18) patients with a follow up longer than six months were already entered at the time of the amendment, an interim analysis was performed on complete data for all these patients. The study remained closed after the interim analysis given the demonstrated lack of sufficient efficacy of the two schedules of infliximab evaluated (see below).
Statistical analyses
Survival duration was calculated from the date of randomization until death irrespective of the cause. Progression-free survival was calculated from the date of randomization until the date of first progression or until death. The Kaplan-Meier method was used to estimate survival-type distributions.17 The 95% confidence interval of the median was obtained using the reflected method.18 Cox’s Proportional Hazard Model has been used to determine the prognostic importance of IPSS risk group (3-ordered categorical variable) and to obtain an estimate of the treatment hazard ratio (HR) adjusted by IPSS risk group along with its corresponding 95% confidence interval (CI).17 Analyses were performed according to the intent-to-treat principle. The cut-off date was June 2010. SAS 9.2 statistical software (SAS Institute®, Cary, North Carolina, USA) was used for analysis.
Results and Discussion
Patients’ characteristics
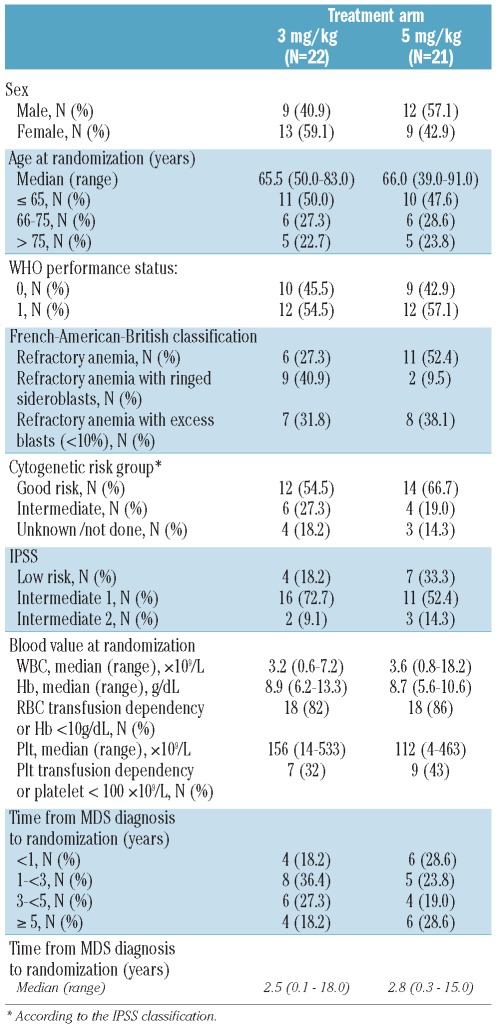
Between February 2004 and March 2006, a total of 46 patients were randomized by 17 centers. The study coordinator considered 3 patients to be ineligible (2 patients because of poor risk cytogenetics and the remaining patient for bilirubin level > 1.5 × ULN). These patients were excluded from all analyses. Patients’ characteristics were well balanced in the two treatment arms with the exception of a slight imbalance regarding IPSS score with more good risk patients being randomized in the 5 mg/kg arm (7 of 21) than in the 3 mg/kg arm (4 of 22) (Table 1).
Table 1.
Patients’ characteristics.
Adherence to protocol treatment
Treatment administration was generally performed according to protocol guidelines (Online Supplementary Appendix).
Reasons for discontinuing the treatment
Median time to off-protocol treatment was 0.5 years in both arms, while 14 of 22 (64%) patients in the 3 mg/kg group versus 11 of 21 (52%) patients in the 5 mg/kg group completed 8 courses of infliximab. In the 3 mg/kg group, 8 patients went prematurely off-protocol due to either disease progression (n=3), adverse event/toxicity (n=3) or patient refusal (n=2). In the 5 mg/kg group, reasons for going off-protocol before the normal completion of the study included disease progression (n=7), death not due to malignant disease (n=1), patient refusal (n=1), and other (n=1).
Adverse events
The incidence of grade 3 or over adverse events reported during the treatment period was relatively low, apart for grade 3–5 infections that occurred in 9 of 22 (41%) patients in the 3 mg/kg arm versus in 4 of 21 (19%) patients in the 5 mg/kg arm. Two of these infections, both on 3 mg/kg, were lethal, and have been considered as likely to be related to protocol treatment. The maximum grade 3 or over adverse events by treatment arm are shown in the Online Supplementary Table S1. The fact that infliximab was relatively well tolerated in both the 3 and 5 mg/kg arms apart for grade 3–5 infections might open a window for further studies combining TNF-α blockade with other MDS active agents. Indeed, encouraging results have been observed by Scott et al. by combining TNF-α blockade with azacitidine19 or anti-thymocyte globulin.20
Activity and outcome
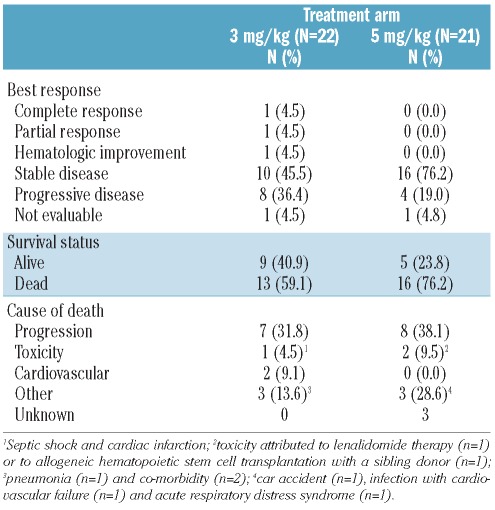
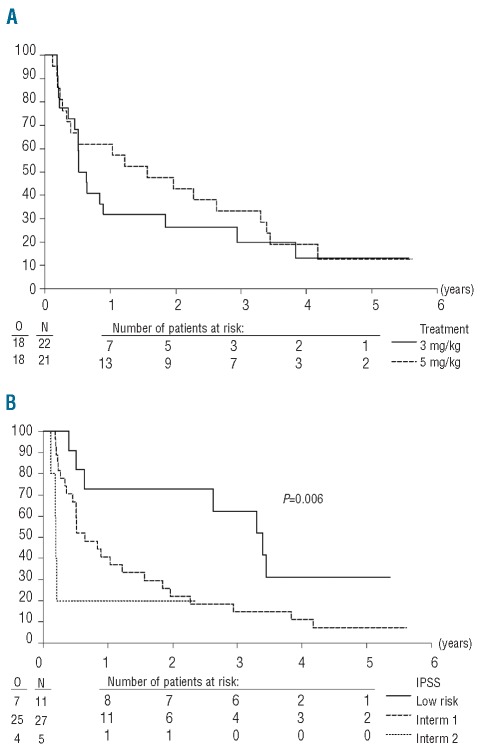
The response rate (complete remission, partial remission or hematologic improvement) was low in both arms: 3 of 22 (13.6%) patients in the 3 mg/kg arm versus 0 of 21 patients in the 5 mg/kg arm (Table 2). For both arms, the 95% confidence interval of the response rate was 2.9–34.9% and 0–16.1% for the 3 and 5 mg/kg arms, respectively. This response rate did not cover the targeted rate of 35%. The 3 responders had either IPSS intermediate-1 (n=2) or intermediate-2 (n=1) and were randomized 0.4, 3.2 and 3.4 years after MDS diagnosis. As shown in Figure 1, the outcomes of the 5 patients with intermediate-2 IPSS patients were poor, with 4 of them progressing on treatment 45–76 days after randomization. If we exclude these 5 patients, the response rate was 2 of 20 patients (10%; 95% CI, 1.2%–31.7%) in the 3 mg/kg arm, versus 0 of 18 patients (0%; 95% CI, 0–18.5%) in the 5 mg/kg arm. Risk of progression or death on treatment was associated with a higher IPSS score (Figure 1) but not with a longer time from diagnosis to randomization (Online Supplementary Figure S1). The response rates observed in the current study were lower than response levels observed in that carried out by Raza et al. in which 8 of 37 patients (22%) achieved a hematologic response.13 In agreement with our data, Deeg et al. observed a low frequency of response when etanercept (a soluble TNF-α receptor fusion protein; Enbrel®) was administered alone in patients with MDS.21 The relatively long duration of MDS (2.7 years) might be an explanation for the low response rates in our study, since apoptosis plays a more important role in early MDS than in more advanced disease. Furthermore, lower response rate in patients with longer duration of MDS has also been observed after treatment with lenalidomide or immunosuppressive therapy.22–23 Taken together, these observations support the hypothesis that TNF-α blockade alone has insufficient activity in patients with MDS.
Table 2.
Outcome by treatment arm.
Figure 1.
Progression-free survival from randomization according to infliximab dosage arm (A) or IPSS score (B). P value given by the Wald test (Cox’s model).
The median progression-free survival from randomization was 0.6 (95% CI, 0.5–3.0) years in the 3 mg/kg arm versus 1.6 (0.4–3.4) years in the 5 mg/kg arm (Figure 1A), while the median survival was 2.6 (1.9-not reached) years versus 3.4 (2.2–5.3) years in the 3 mg/kg and 5 mg/kg arms, respectively (Online Supplementary Figure 2A). As expected, IPSS score was of prognostic importance for both progression-free (P=0.006; Figure 1B) and overall (P=0.04; Online Supplementary Figure 2B) survival. The longer median progression-free and overall survival in the 5 mg/kg arm versus the 3 mg/kg arm could be explained by the imbalance in favor of the 5 mg/kg regarding the IPSS risk group. Indeed, the estimated hazard ratio (5 mg/kg vs. 3 mg/kg) adjusted by the IPSS risk group was 1.05 (95% CI, 0.52–2.11) years for progression-free survival and 1.25 (95% CI, 0.60–2.60) years for overall survival, respectively.
In summary, our results demonstrated that neither of the two infliximab dose schedules tested in the current study showed sufficient activity in unselected patients with MDS and a relatively low risk of developing acute leukemia to warrant further investigation as a single agent in phase III studies.
Supplementary Material
Appendix
The following investigators/centers participated in this EORTC trial 06023: Drs. Bron and Feremans - H. Univ. Bordet-Erasme, Brussels (B); Dr. Selleslag - A.Z. St Jan, Brugge (BE); Dr. Berneman - U.Z. Antwerpen (BE); Dr. Vermeulen - C.H. La Tourelle, Verviers (B); Drs. Verhoef and Delforge - U.Z. Gasthuisberg, Leuven (B); Drs. Thyss - Centre Lacassagne, Nice (F); Drs. Marie and Vekhoff - Hotel-Dieu, Paris (F); Drs. De Witte and Muus - Radboud UMC, Nijmegen (NL); Dr. Ossenkoppele - Vrije Universiteit MC, Amsterdam (NL); Dr. Willemze - Univ Med Ctr Leiden; Dr. Wijermans - Haga Zkh Leyenburg (NL); Dr. Dengler - Univ.Klin.Heidelberg (DE); Dr. Driessen - Eberhard Karls University, Tuebingen (DE); Dr. Cermak - Institute Hematology, Prague (CZ); Dr. Demuynck -H. Hartziekenhuis, Roeselaere (BE); Dr. Indrak - University Hospital, Olomouc (CZ); Drs. Amadori and Buccisano - H. Tor Vergata, Roma (IT). We thank the EORTC Headquarters’ members who took care of this study: data managers (Mr Filip Beeldens, Mrs Aurore Theys, Mrs Liv Meert), clinical research physicians (Drs. Liliana Baila, Jocelyne Flament and Matthias Karrasch) and the project manager (Mrs Hilde Breyssens).
Footnotes
Funding: this publication was supported by grants ns. 5U10 CA11488-32 through 2U10 CA011488-41 from the National Cancer Institute (Bethesda, Maryland, USA) and by Fonds Cancer (FOCA) from Belgium. Its content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Cancer Institute. Centocor R&D, Inc., Great Valley Parkway, Malvern, PA, US, provided substantial financial support and Remicade® free of charge.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Scott BL, Deeg HJ. Myelodysplastic syndromes. Annu Rev Med. 2010;61:345–58. doi: 10.1146/annurev.med.051308.132852. [DOI] [PubMed] [Google Scholar]
- 2.de Witte T, Hermans J, Vossen J, Bacigalupo A, Meloni G, Jacobsen N, et al. Haematopoietic stem cell transplantation for patients with myelo-dysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2000;110(3):620–30. doi: 10.1046/j.1365-2141.2000.02200.x. [DOI] [PubMed] [Google Scholar]
- 3.Raza A, Gezer S, Mundle S, Gao XZ, Alvi S, Borok R, et al. Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndromes. Blood. 1995;86(1):268–76. [PubMed] [Google Scholar]
- 4.Kerbauy DMB, Lesnikov V, Abbasi N, Seal S, Scott B, Deeg HJ. NF-kB and FLIP in arsenic trioxide (ATO)-induced apoptosis in myelodysplastic syndromes (MDSs) Blood. 2005;106(12):3917–25. doi: 10.1182/blood-2005-04-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selleri C, Sato T, Anderson S, Young NS, Maciejewski JP. Interferon-gamma and tumor necrosis factor-alpha suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol. 1995;165(3):538–46. doi: 10.1002/jcp.1041650312. [DOI] [PubMed] [Google Scholar]
- 6.Musto P, Matera R, Minervini MM, Checchia-de Ambrosio C, Bodenizza C, Falcone A, et al. Low serum levels of tumor necrosis factor and interleukin-1 beta in myelodysplastic syndromes responsive to recombinant erythropoietin. Haematologica. 1994;79(3):265–8. [PubMed] [Google Scholar]
- 7.Verhoef GE, De Schouwer P, Ceuppens JL, van Damme J, Goossens W, Boogaerts MA. Measurement of serum cytokine levels in patients with myelodysplastic syndromes. Leukemia. 1992;6(12):1268–72. [PubMed] [Google Scholar]
- 8.Stasi R, Brunetti M, Bussa S, Conforti M, Martin LS, La Presa M, et al. Serum levels of tumour necrosis factor-alpha predict response to recombinant human erythropoietin in patients with myelodysplastic syndrome. Clin Lab Haematol. 1997;19(3):197–201. [PubMed] [Google Scholar]
- 9.Gersuk GM, Beckham C, Loken MR, Kiener P, Anderson JE, Farrand A, et al. A role for tumor necrosis factor-a, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol. 1998;103(1):176–88. doi: 10.1046/j.1365-2141.1998.00933.x. [DOI] [PubMed] [Google Scholar]
- 10.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354(9194):1932–9. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 11.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 12.Stasi R, Amadori S. Infliximab chimaeric anti-tumour necrosis factor alpha mono-clonal antibody treatment for patients with myelodysplastic syndromes. Br J Haematol. 2002;116(2):334–7. [PubMed] [Google Scholar]
- 13.Raza A, Candoni A, Khan U, Lisak L, Tahir S, Silvestri F, et al. Remicade as TNF suppressor in patients with myelodysplastic syndromes. Leuk Lymphoma. 2004;45(10):2099–104. doi: 10.1080/10428190410001723322. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes (erratum appears in Blood. 1998;91(3):1100) Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 15.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–4. [PubMed] [Google Scholar]
- 17.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. John Wiley; 2002. [Google Scholar]
- 18.Slud EV, Byar DP, Green SB. A comparison of reflected versus test-based confidence intervals for the median survival time, based on censored data. Biometrics. 1984;40(3):587–600. [PubMed] [Google Scholar]
- 19.Scott BL, Ramakrishnan A, Storer B, Becker PS, Petersdorf S, Estey EH, et al. Prolonged responses in patients with MDS and CMML treated with azacitidine and etanercept. Br J Haematol. 2010;148(6):944–7. doi: 10.1111/j.1365-2141.2009.08061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott BL, Ramakrishnan A, Fosdal M, Storer B, Becker P, Petersdorf S, et al. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br J Haematol. 2010;149(5):706–10. doi: 10.1111/j.1365-2141.2010.08145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeg HJ, Gotlib J, Beckham C, Dugan K, Holmberg L, Schubert M, et al. Soluble TNF receptor fusion protein (etanercept) for the treatment of myelodysplastic syndrome: A pilot study. Leukemia. 2002;16(2):162–4. doi: 10.1038/sj.leu.2402356. [DOI] [PubMed] [Google Scholar]
- 22.Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 23.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26(15):2505–11. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.