Abstract
Background
In the present study, the prognostic impact of minimal residual disease during treatment on time to progression and overall survival was analyzed prospectively in patients with mantle cell lymphoma treated on the Cancer and Leukemia Group B 59909 clinical trial.
Design and Methods
Peripheral blood and bone marrow samples were collected during different phases of the Cancer and Leukemia Group B 59909 study for minimal residual disease analysis. Minimal residual disease status was determined by quantitative polymerase chain reaction of IgH and/or BCL-1/JH gene rearrangement. Correlation of minimal residual disease status with time to progression and overall survival was determined. In multivariable analysis, minimal residual disease, and other risk factors were correlated with time to progression.
Results
Thirty-nine patients had evaluable, sequential peripheral blood and bone marrow samples for minimal residual disease analysis. Using peripheral blood monitoring, 18 of 39 (46%) achieved molecular remission following induction therapy. The molecular remission rate increased from 46 to 74% after one course of intensification therapy. Twelve of 21 minimal residual disease positive patients (57%) progressed within three years of follow up compared to 4 of 18 (22%) molecular remission patients (P=0.049). Detection of minimal residual disease following induction therapy predicted disease progression with a hazard ratio of 3.7 (P=0.016). The 3-year probability of time to progression among those who were in molecular remission after induction chemotherapy was 82% compared to 48% in patients with detectable minimal residual disease. The prediction of time to progression by post-induction minimal residual disease was independent of other prognostic factors in multivariable analysis.
Conclusions
Detection of minimal residual disease following induction immunochemotherapy was an independent predictor of time to progression following immunochemotherapy and autologous stem cell transplantation for mantle cell lymphoma. The clinical trial was registered at ClinicalTrials.gov: NCT00020943.
Keywords: minimal residual disease, progression-free survival, MCL, induction, chemotherapy
Introduction
Mantle cell lymphoma (MCL) is an aggressive form of non-Hodgkin’s lymphoma (NHL) with a median overall survival (OS) of approximately 4.8 years following standard combination chemotherapy.1 Current treatment strategies include the addition of rituximab to different combination chemotherapy regimens including CHOP and Hyper-CVAD.2–3 Several phase II studies and one phase III study demonstrated that remission induction with combination chemotherapy followed by dose intensification with autologous stem cell transplantation (ASCT) prolongs progression-free survival (PFS) for patients with previously untreated MCL.4–6
The prognostic role of minimal residual disease (MRD) has been established in MCL.7 The introduction of rituximab to high-dose Ara-C led to successful in vivo purging of MCL cells from peripheral blood cells containing CD34 harvested from patients with MCL, resulting in a high molecular remission (MRD-) rate of over 90% and improved clinical outcome.8–9 Recently, MRD was evaluated retrospectively by quantitative real-time polymerase chain reaction (RQ-PCR) of clonal IgH rearrangements in 29 patients with MCL treated with high-dose radio-chemotherapy and ASCT. Molecular remission after ASCT strongly predicted for improved outcome.7 Andersen et al. showed that molecular relapse may occur many years after ASCT in MCL, and pre-emptive treatment using rituximab for MRD detected by RQ-PCR was able to re-induce molecular remission, and delay or prevent clinical relapse.10
CALGB 59909 was a recently published prospective trial that demonstrated the efficacy and tolerability of aggressive induction immunochemotherapy followed by ASCT for patients up to 70 years of age with previously untreated MCL.11 In the present study, the prognostic impact of MRD during treatment on time to progression (TTP) and overall survival (OS) was analyzed prospectively in patients with MCL treated on the CALGB 59909 clinical trial. Our study demonstrates for the first time that MRD status after induction immunochemotherapy predicted TTP independently of other previously defined prognostic factors.
Design and Methods
Patients’ characteristics and sample collection for minimal residual disease
Seventy-eight patients began treatment according to the CALGB 59909 clinical protocol.11 The study was approved by the Institutional Review Board of the participating institutions. All the participants signed an institutional review board-approved informed consent document. The study was conducted in accordance with the Declaration of Helsinki and was registered on clinicaltrials.gov (NCT00020943).
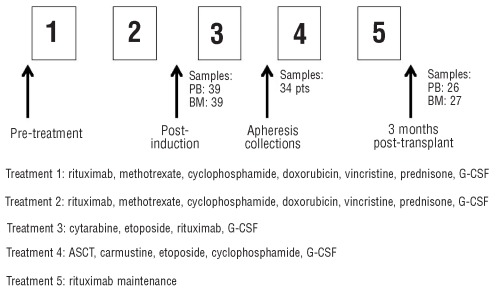
Briefly, patients received two cycles of induction chemotherapy using rituximab, high-dose methotrexate and augmented-dose CHOP chemotherapy, which was followed by high-dose consolidation and stem cell mobilization using high-dose cytarabine, etoposide, and rituximab administered as an in vivo purge. Patients subsequently underwent ASCT using a cyclophosphamide, BCNU, and etoposide (CBV) preparative regimen. Two weekly doses of maintenance rituximab were administered following recovery from ASCT (Figure 1). Sixty-seven patients (86%) received the protocol transplant.
Figure 1.
CALGB 59909 treatment schema and sample collection.
In 11 patients, no clone specific molecular marker was identified; therefore, 84% of the patients had successful identification of an allele specific primer-probe set for MRD detection. Seventeen patients had inadequate sample collection at sequential time points (n=15) or were not transplanted (n=2) on the protocol. Thus, a total of 39 patients had sequential samples and were evaluated for MRD during treatment on CALGB 59909.
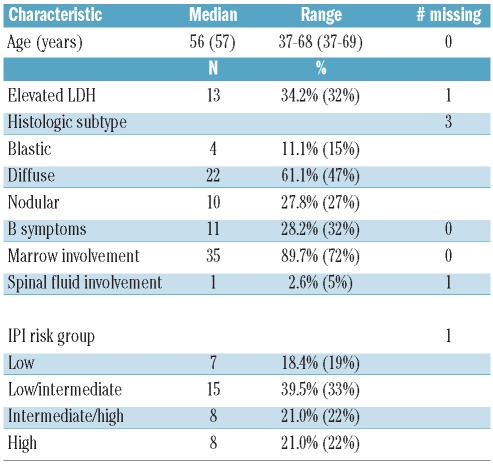
MRD status was determined by RQ-PCR using paired peripheral blood (PB) and bone marrow (BM) biopsy samples post induction treatment from the peripheral blood stem cell apheresis collection, and in PB and BM samples three months post ASCT (samples from PB or BM at each time point are listed in Figure 1). All the patients gave their signed informed consent for sample collection. The study entry characteristics for these patients are shown in Table 1. The characteristics of these 39 patients are highly comparable to the clinical characteristics from all the patients enrolled on CALGB 59909 (Table 1).
Table 1.
Baseline clinical characteristics (n=39). (The data in parenthesis are the clinical characteristics from all the patients in CALGB 59909).
Collection of samples for minimal residual disease evaluation
A 10 mL blood sample was collected for MRD studies in 2 EDTA tubes. Five mL of bone marrow aspirate was also collected in an EDTA tube at the time points described above. Samples were shipped by overnight mail to the CALGB leukemia/lymphoma tissue bank at the Ohio State University where they were cryopre-served using standard CALGB tissue bank procedures. Samples were later batched for shipment for MRD analysis in Dr. Stock’s laboratory which serves as an MRD reference laboratory for leukemia/lymphoma studies in the CALGB.
WBC isolation and DNA extraction
Ficoll-Paque Plus solution (Amersham Pharmacia Biotech, Uppsala, Sweden) was used for the isolation of mononuclear cells. Genomic DNA was extracted from purified cells according to standard procedures in the Puregene DNA Isolation kit (Gentra Systems, Minneapolis, MN, USA).
Polymerase chain reaction for identification of IgH or BCL-1/JH gene rearrangements
PCR was performed according to methods described in the Invivoscribe IGH Clonality Assay and BCL1/JH Translocation Assay kits (San Diego, CA 92121, USA). Following amplification, clonal PCR products were confirmed on 6% TBE gels and clonal fragments were excised from the 2% agarose gels following SYBR green staining, and purified according to Rapid Gel Extraction assay kit (Life Technologies, Rockville, MD, USA). Minimal residual disease monitoring in 11 patients was carried out using BCL-1/JH primer-probe sets and 28 patients were evaluated using IgH clonality.
Patient plasmid construction and DNA sequencing
Purified monoclonal fragments were cloned using the pGEM-T Vector Kit (Promega, Madison, WI, USA). In order to identify clones harboring recombinant plasmids, 6–10 colonies were selected and analyzed by PCR amplification. Recombinant plasmids were purified with the Concert Rapid Miniprep DNA Purification system (Life Technologies, Rockville, MD, USA) sequenced according to the ABI Rhodamine Terminator Cycle Sequencing procedure, and analyzed with an ABI 377 DNA Sequencer (PE Biosystems, Foster City, CA, USA). Primers complementary to the M13 region of the pGEM-T vector were used to determine sequence information. VH, DH, JH, and N-nucleotide insertions were identified by BLAST search analysis and antisense; CDRIII region, allele specific oligonucleotides were designed with annealing temperatures of approximately 60°C for quantitative PCR analysis. Patient specific primers were synthesized by Biosearch (Lewisville, TX, USA).
Real-time quantitative polymerase chain reaction
Quantitative PCR of IgH gene rearrangements or BCL-1/JH was performed using previously described methods12 which have been optimized for analysis using a LightCycler instrument (Roche Diagnostics, Indianapolis, IN, USA). Patients’ specimens, serial dilutions of standard templates and no template controls were amplified in triplicate.
Quantitation of IgH or BCL-1/JH copy number using real-time polymerase chain reaction
The patient recombinant IgH or BCL-1/JH plasmids, described above, were diluted 10-fold and were amplified simultaneously with patients’ specimens. For the standard curve, the copy number of plasmid was from ~10 to 106 which was further converted according to the ratio of the size of the PCR product and the whole genome size to give a copy number ranging from 3.1×108 to 3.1×1013. Following amplification, all patient and negative control CT (threshold cycle) values were averaged within each triplicate set of reactions. The absolute IgH or BCL-1/JH copy number was determined by comparing the average CT value of each patient sample to the respective standard curve. In order to compensate for differences in DNA quantities from sample to sample, the absolute IgH or BCL-1/JH copy number was normalized to the absolute copy number determined by amplification of the GAPDH gene. The GAPDH copy number was calculated for each patient sample in a method similar to the IgH or BCL-1/JH quantitation described above; however, a standard curve was generated using serial 5-fold (copy number ranging from 4.2×108 to 1.3×1012) dilutions of human placenta DNA. The normalized IgH or BCL-1/JH copy number was determined by dividing the absolute IgH or BCL-1/JH copy number by the GAPDH copy number. The sensitivity of detection of MRD with these methods ranges from 10−4–10−5, tested by analyzing 10-fold serial dilutions from the patient specific plasmid in genomic DNA derived from mononuclear cells of healthy donors. MRD negative was defined as the absence of a detectable signal following 40 amplification cycles under our real-time PCR settings with the sensitivity of detection of MRD from 10−4 to 10−5.
Immunohistochemistry staining of Ki-67, PIM1 and MIPI scoring system
The details of the immunohistochemistry staining of the Ki-67 and PIM1 have been described previously.13 MRD results were correlated with Ki-67, PIM1 and the MIPI (MCL international prognostic index) score.14
Statistical analysis
Cox’s regression model was used to determine associations between MRD and outcome.15 For a robust inference, Cox’s regression model uses MRD presence or absence (binary end point), instead of the raw value, as a covariate.16 Time to progression (TTP, time until progression, censoring deaths due to treatment or other causes) and overall survival were used as clinical end points. Kaplan-Meier and log rank testing were used for graphical representation of TTP and overall survival analysis.17 The statistical analysis was performed by CALGB statisticians.
Results
Kinetics of minimal residual disease on CALGB 59909
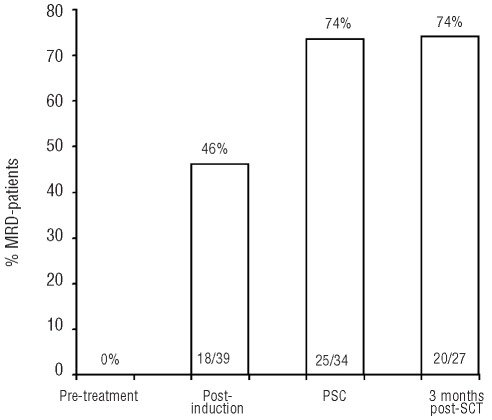
The kinetics of MRD during treatment were evaluated and are summarized in Figure 2. Using peripheral blood monitoring, 18 of 39 patients (46%) became MRD negative following induction immunochemotherapy, while 21 (54%) remained MRD positive. Twenty-five of 34 patients (74%) had MRD-negative leukapheresis products following stem cell mobilization with high-dose cytarabine, etoposide and rituximab. There was no further improvement in molecular CR rate three months after ASCT. Twenty of 27 patients (74%) were MRD negative following ASCT.
Figure 2.
Kinetics of MRD during treatment. The bar graphs demonstrate the percentage of patients who had an MRD-negative (y axis) sample at specified treatment time points (x axis).
Post-induction peripheral blood minimal residual disease status predicts TTP
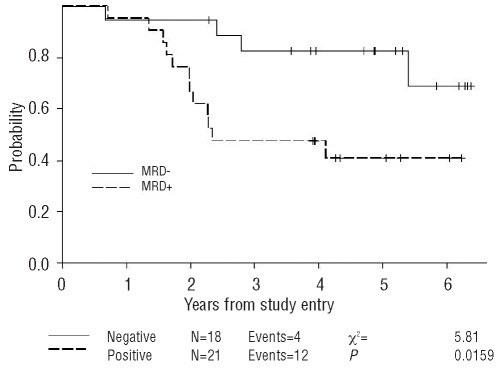
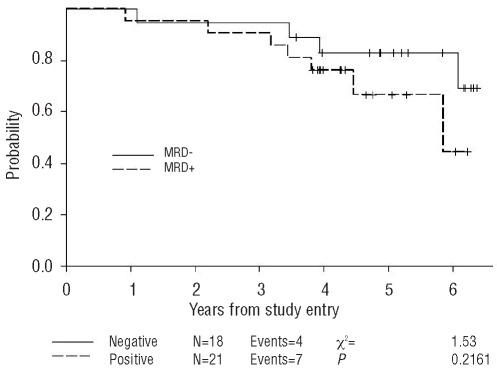
The prognostic significance of MRD detection at each of these treatment time points was evaluated. Notably, MRD status following 2 cycles of induction chemotherapy was predictive of TTP. Twelve of 21 patients who were MRD positive following induction therapy (57%) progressed within three years of follow up compared to only 4 of 18 (22%) who had become MRD negative (P=0.049). Detection of MRD in PB following induction therapy predicted disease progression with a hazard ratio of 3.7 (P=0.016, 95% CI 1.2, 11.8) (Figure 3) but not overall survival (Figure 4). The 3-year probability of remaining progression-free among those who were MRD negative after induction chemotherapy was 82% (95% CI 55%–94%), compared to 48% (95% CI 26%–67%) in patients who were MRD positive.
Figure 3.
Progression-free survival according to the MRD status in PB after induction immunochemotherapy. MRD was assessed in PB after the induction immunochemotherapy in MCL patients on the CALGB 59909 clinical trial.
Figure 4.
Overall survival according to the MRD status in PB after induction immunochemotherapy. MRD was assessed in PB after the induction immunochemotherapy in MCL patients on the CALGB 59909 clinical trial.
Post-induction MRD detection of BM samples did not predict TTP. Similarly, MRD evaluation at later treatment time points was not significantly associated with either TTP or survival. This included evaluation of MRD in the apheresis specimens. Nine of the 34 (26%) patients had MRD positive apheresis specimens. Four (44%) of these patients eventually progressed compared to 11 of the 25 (44%) with MRD-negative specimens (P=1.00)
Post-induction peripheral blood minimal residual disease status predicts TTP independently of other prognostic factors
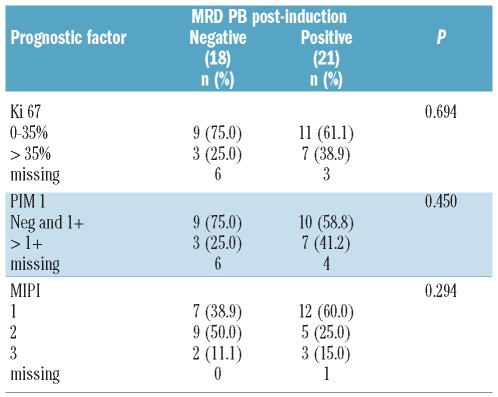
A recently published correlative study demonstrated that expression of Ki-67 and PIM1 were important prognostic markers in patients treated on CALGB 59909.13 Thus, the correlation of the post-induction MRD status with other prognostic factors was determined. Of the 39 patients with post-induction peripheral blood measurements, 30 had Ki-67 measurements, 29 had PIM1 measurements, and 38 had data to compute the MIPI. Table 2 shows the distribution of these prognostic factors by post-induction peripheral blood MRD. P values were computed using Fisher’s exact test. As seen in Table 2, there was no statistically significant correlation of MRD post-induction with the other 3 prognostic factors. Multivariable analyses showed the MRD post-induction fit with the other three prognostic factors. Post-induction PB MRD status predicted time to progression independently of the other prognostic factors.
Table 2.
Cross-tabulations of MRD in PB post-induction by prognostic factors.
Comparability of PB and BM for MRD detection
Paired PB and BM samples were collected for comparison of MRD level in PB and BM. Prior to the treatment, all the samples from PB and BM were MRD positive. After induction chemotherapy, of the 39 pairs of samples, 19 were positive for both and 12 were negative for both. Six patients were MRD negative in PB, but positive in BM, while 2 patients were MRD positive in PB and negative in BM which could be due to focal presentation of lymphoma in the bone marrow, or poor quality of bone marrow aspiration, or no bone marrow MCL involvement. There was 21% (8 of 39) discordance between PB and BM, and PB analysis underestimated MRD in approximately 15% (6 of 39) of patients compared to BM MRD. Among 23 sample pairs at three months post ASCT, the discordance rate was 26% (6 of 23), and PB analysis underestimated MRD in approximately 17% (4 of 23) of patients compared to BM MRD.
Discussion
We and others have demonstrated that monitoring MRD using sensitive RQ-PCR techniques to identify the malignant clone is an important tool for assessment of treatment response and outcome in MCL.7,9,18–19 Andersen et al.18 demonstrated that PFS following ASCT correlated with the ability to purge MRD from the harvested marrow using antibody “cocktails”. Pott et al. 7 demonstrated that the achievement of a molecular remission after high-dose chemotherapy with ASCT was associated with a median PFS of 92 months compared with only 21 months in the MRD positive group. Recently published MRD results from the European MCL intergroup study20 demonstrated that achievement of MRD negativity after induction chemotherapy correlated with significant improvement in response duration compared to patients with detectable MRD.
Our data confirm the findings from the European MCL intergroup study and our multivariable analysis further demonstrated that MRD status in blood samples following induction therapy is the most important predictor of TTP. This relationship was shown to be independent of other risk factors in multivariate modeling, including expression of Ki-67, PIM1 and the clinical factors incorporated into the MIPI score. The strong predictive value of MRD in PB following induction will facilitate identification of those patients who are particularly sensitive to immunochemotherapy, and could be used as a means of stratifying post-remission therapy. The efficacy of blood MRD detection other than bone marrow facilitates MRD monitoring.
In contrast to the predictive value of MRD in BM samples from the European MCL intergroup, the MRD status in BM samples after induction chemotherapy in our study failed to predict TTP. Interestingly, 6 of the 18 patients with MRD negative PB following induction were still MRD positive in BM, but at very low MRD levels. Only one of these patients progressed after completion of all treatment. One potential explanation for this was that patients on the CALGB 59909 received intensification chemotherapy (treatment 3) prior to apheresis collection and ASCT. Among these 6 patients, 5 had evaluable apheresis samples, and 4 of these patients had MRD negative apheresis collections. This suggests that intensification with high-dose cytarabine, etoposide and rituximab facilitated the mobilization of MRD negative CD34+ enriched progenitor cells and further reduced MRD to undetectable levels in the bone marrow.
Other studies have demonstrated that the presence of MRD in apheresis specimens was predictive of relapse of MCL after high-dose chemotherapy with ASCT.9 Similarly, MRD negative bone marrow harvest prior to ASCT for follicular lymphoma demonstrated that MRD negative bone marrow was associated with a markedly reduced relapse rate.21 The European MCL intergroup study did not monitor MRD in the apheresis product, but monitored MRD after ASCT in the younger MCL patient, or after maintenance therapy with either rituximab or interferon in older adults with MCL. They demonstrated that sustained molecular remission during the post-induction period was predictive for outcome in MCL after ASCT in younger patients (response duration at two years 100% vs. 65%; P=0.0007) and during maintenance in MCL in older patients (response duration at two years 76% vs. 36%; P=0.016).20 Our data indicated in vivo purging with etoposide, cytarabine and rituximab was very effective and significantly increased the percentage of MRD negative samples from 46% (18 of 39) after induction immunochemotherapy to 74% (25 of 34) in apheresis products measured for MRD. However, there was no correlation with TTP and MRD status of apheresis products or with MRD status three months after ASCT. The disparity between our results and those of the European MCL study group could be explained by the relatively small number of patients with MRD positive apheresis samples. Only 9 patients had MRD positive apheresis products, and 10 were found to be MRD positive three months post ASCT. It is also possible that the additional doses of rituximab that were given three months post rituximab on CALGB 59909 favorably impacted MRD status. Interestingly, pre-emptive therapy with rituximab at the time of molecular relapse following ASCT has been shown by others to induce molecular remissions that may have prevented overt clinical relapse.10
Many studies have demonstrated superior outcomes in MCL patients with continuous MRD negative PB or BM samples after ASCT.7,20,22 Since we did not have MRD data on the 6 patients with MRD positive apheresis product after conditioning chemotherapy but prior to stem cell infusion, it is impossible to determine if the persistent MRD positivity after ASCT was due to infusion of contaminated apheresis product or persistent residual disease after conditioning chemotherapy. Further study is also needed to determine the clinical importance of the addition of post-transplant immunotherapy on MRD modulation in this setting.
Kinetics of MRD in this intensive study showed a progressive decline in MRD detection with post-remission therapy. The 46% MRD negative rate after induction immunochemotherapy in our study is comparable with the 48% MRD negative rate after induction immunochemotherapy in a European MCL study group in younger patients.20 The MRD negative rate increased to 74% after in vivo purging during mobilization in our study. There was no further increase of MRD negative rate after high-dose chemotherapy and ASCT in our study. These data demonstrate that in vivo purging with cytarabine, etoposide, and rituximab is very effective in achieving a high molecular CR rate. Interestingly, there was no additional benefit of ASCT in further reducing the MRD detection rate. Recent studies from the MDACC demonstrated excellent time to failure and overall survival using intensive immunochemotherapy without transplant.23
Therefore, the results of our study raise some questions regarding the treatment of MCL. First, do patients who become MRD negative after intensive induction immunochemotherapy really require ASCT, or will they have prolonged CR with rituximab-based consolidation chemotherapy alone without ASCT? A recently published study demonstrated that intensive immunochemotherapy without stem cell transplantation was effective for untreated aggressive MCL, and the median overall survival (OS) for all patients had not been reached at ten years follow up.23 Second, will MRD positive patients after intensive induction immunochemotherapy benefit from allogeneic stem cell transplant (Allo-SCT) instead of ASCT at first remission? A recent retrospective study clearly demonstrated that age and the effects of prior therapies predict OS and NRM (non-relapse mortality) after RIC (reduced-intensity conditioning) Allo-SCT in patients with relapsed or refractory MCL,24 raising the potential value of considering Allo-SCT for the early phase of disease management. Third, could MRD status after intensive induction immunochemotherapy be used to stratify patients for maintenance therapy with either rituximab or another targeted agent, and if sequential monitoring of MRD status could be used to determine the duration of maintenance therapy? Only well designed prospective studies will answer these questions and integration of MRD measurements in future clinical trials in MCL should result in a refinement of treatment strategies to achieve better outcomes. With this in mind, the CALGB has recently completed a trial testing the addition of bortezomib as maintenance therapy in patients receiving immunochemotherapy followed by ASCT for front-line therapy of MCL (CALGB 50403). MRD monitoring is being carried out in a prospective fashion to evaluate the efficacy of bortezomib in eradication of MRD.
In summary, our study demonstrated for the first time that MRD status following induction immunochemotherapy predicted TTP on CALGB 59909 independently of other prognostic factors, including MIPI, Ki-67 and PIM1. MRD measurements after induction immunochemotherapy may be used for a risk-adapted treatment approach. The ultimate goal of treatment for MCL may be to enter molecular remission early during the treatment of MCL, perhaps to avoid emergence of a relatively chemo-resistant clone that could give rise to disease relapse. Thus, a potential strategy for future trials in MCL could be to incorporate novel agents25–26 during induction or early post-remission chemotherapy. The CALGB 59909 treatment approach is an ideal platform to evaluate the addition of novel agents to eradicate MRD with the goal of further improving survival in MCL.
Supplementary Material
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27(4):511–8. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 2.Schulz H, Bohlius JF, Trelle S, Skoetz N, Reiser M, Kober T, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99(9):706–14. doi: 10.1093/jnci/djk152. [DOI] [PubMed] [Google Scholar]
- 3.Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013–23. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 4.Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677–84. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 5.Khouri IF, Romaguera J, Kantarjian H, Palmer JL, Pugh WC, Korbling M, et al. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: an active regimen for aggressive mantle-cell lymphoma. J Clin Oncol. 1998;16(12):3803–9. doi: 10.1200/JCO.1998.16.12.3803. [DOI] [PubMed] [Google Scholar]
- 6.Vose JM, Bierman PJ, Weisenburger DD, Lynch JC, Bociek Y, Chan WC, et al. Autologous hematopoietic stem cell transplantation for mantle cell lymphoma. Biol Blood Marrow Transplant. 2000;6(6):640–5. doi: 10.1016/s1083-8791(00)70030-9. [DOI] [PubMed] [Google Scholar]
- 7.Pott C, Schrader C, Gesk S, Harder L, Tiemann M, Raff T, et al. Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood. 2006;107(6):2271–8. doi: 10.1182/blood-2005-07-2845. [DOI] [PubMed] [Google Scholar]
- 8.Magni M, Di Nicola M, Devizzi L, Matteucci P, Lombardi F, Gandola L, et al. Successful in vivo purging of CD34-containing peripheral blood harvests in mantle cell and indolent lymphoma: evidence for a role of both chemotherapy and rituximab infusion. Blood. 2000;96(3):864–9. [PubMed] [Google Scholar]
- 9.Gianni AM, Magni M, Martelli M, Di Nicola M, Carlo-Stella C, Pilotti S, et al. Long-term remission in mantle cell lymphoma following high-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen) Blood. 2003;102(2):749–55. doi: 10.1182/blood-2002-08-2476. [DOI] [PubMed] [Google Scholar]
- 10.Andersen NS, Pedersen LB, Laurell A, Elonen E, Kolstad A, Boesen AM, et al. Pre-emptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol. 2009;27(26):4365–70. doi: 10.1200/JCO.2008.21.3116. [DOI] [PubMed] [Google Scholar]
- 11.Damon LE, Johnson JL, Niedzwiecki D, Cheson BD, Hurd DD, Bartlett NL, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27(36):6101–8. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan JW, Ladetto M, Zou G, Neuberg D, Poor C, Bowers D, et al. Immunoglobulin heavy-chain consensus probes for real-time PCR quantification of residual disease in acute lymphoblastic leukemia. Blood. 2000;95(8):2651–8. [PubMed] [Google Scholar]
- 13.Hsi ED, Jung SH, Lai R, Johnson JL, Cook JR, Jones D, et al. Ki67 and PIM1 expression predict outcome in mantle cell lymphoma treated with high dose therapy, stem cell trans-plantation and rituximab: a Cancer and Leukemia Group B 59909 correlative science study. Leuk Lymphoma. 2008;49(11):2081–90. doi: 10.1080/10428190802419640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–65. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life tables (with discussion) J R Statist Soc B. 1972;34:187–220. [Google Scholar]
- 16.Jung SH, Owzar K, George SL. A multiple testing procedure to associate gene expression levels with survival. Stat Med. 2005;24(20):3077–88. doi: 10.1002/sim.2179. [DOI] [PubMed] [Google Scholar]
- 17.Peto R, Peto J. Asymptotically efficient rank invariant test procedures (with discussion) J R Statist Soc A. 1972;135:185–206. [Google Scholar]
- 18.Andersen NS, Donovan JW, Borus JS, Poor CM, Neuberg D, Aster JC, et al. Failure of immunologic purging in mantle cell lymphoma assessed by polymerase chain reaction detection of minimal residual disease. Blood. 1997;90(10):4212–21. [PubMed] [Google Scholar]
- 19.Corradini P, Ladetto M, Zallio F, Astolfi M, Rizzo E, Sametti S, et al. Long-term follow-up of indolent lymphoma patients treated with high-dose sequential chemotherapy and autografting: evidence that durable molecular and clinical remission frequently can be attained only in follicular subtypes. J Clin Oncol. 2004;22(8):1460–8. doi: 10.1200/JCO.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Pott C, Hoster E, Delfau-Larue MH, Beldjord K, Bottcher S, Asnafi V, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood. 2010;115(16):3215–23. doi: 10.1182/blood-2009-06-230250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman AS, Neuberg D, Mauch P, Soiffer RJ, Anderson KC, Fisher DC, et al. Long-term follow-up of autologous bone marrow trans-plantation in patients with relapsed follicular lymphoma. Blood. 1999;94(10):3325–33. [PubMed] [Google Scholar]
- 22.Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687–93. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romaguera JE, Fayad LE, Feng L, Hartig K, Weaver P, Rodriguez MA, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150(2):200–8. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 24.Cook G, Smith GM, Kirkland K, Lee J, Pearce R, Thomson K, et al. Outcome following Reduced-Intensity Allogeneic Stem Cell Transplantation (RIC AlloSCT) for relapsed and refractory mantle cell lymphoma (MCL): a study of the British society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2010;16(10):1419–27. doi: 10.1016/j.bbmt.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Goy A, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20(3):520–5. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867–74. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.