Abstract
Background
Several studies of autologous stem cell transplantation in primary refractory myeloma have produced encouraging results. However, the outcome of primary refractory patients with stable disease has not been analyzed separately from the outcome of patients with progressive disease.
Design and Methods
In the Spanish Myeloma Group 2000 trial, 80 patients with primary refractory myeloma (49 with stable disease and 31 with progressive disease), i.e. who were refractory to initial chemotherapy, were scheduled for tandem transplants (double autologous transplant or a single autologous transplant followed by an allogeneic transplant). Patients with primary refractory disease included those who never achieved a minimal response (≥25% M-protein decrease) or better. Responses were assessed using the European Bone Marrow Transplant criteria.
Results
There were no significant differences in the rates of partial response or better between patients with stable or progressive disease. However, 38% of the patients with stable disease at the time of transplantation remained in a stable condition or achieved a minimal response after transplantation versus 7% in the group with progressive disease (P=0.0017) and the rate of early progression after transplantation was significantly higher among the group with progressive disease at the time of transplantation (22% versus 2%; P=0.0043). After a median follow-up of 6.6 years, the median survival after first transplant of the whole series was 2.3 years. Progression-free and overall survival from the first transplant were shorter in patients with progressive disease (0.6 versus 2.3 years, P=0.00004 and 1.1 versus 6 years, P=0.00002, respectively).
Conclusions
Our results show that patients with progressive refractory myeloma do not benefit from autologous transplantation, while patients with stable disease have an outcome comparable to those with chemosensitive disease. (ClinicalTrials.gov:NCT00560053)
Keywords: multiple myeloma, primary refractory disease, progressive disease, stable disease, outcome
Introduction
The outcome of patients with multiple myeloma treated with conventional therapy is unsatisfactory with a low proportion of such patients becoming long-term survivors. For this reason, high-dose therapy followed by autologous stem cell transplantation (SCT) has become the standard of care for up-front therapy in younger patients with multiple myeloma.1–4 In fact, it has been shown that the survival of patients younger than 60 years has improved in the more recent years and this has been attributed, at least in part, to the benefit of autologous SCT.5–7
Before the introduction of novel drugs, particularly thalidomide, bortezomib and lenalidomide, pre-transplant induction therapy consisted of regimens based on alkylating agents and/or dexamethasone. With these regimens, the pre-transplant complete response rate ranged from 5–10% and increased up to 35% after transplantation.1–2, 8–10 In fact, the benefit of autologous SCT in terms of survival was attributed to the higher frequency of complete response obtained after the intensive therapy.1,2 In addition, tandem autologous transplants have been shown to be of benefit for patients failing to achieve at least very good partial response with the first transplant.11–12
Whether autologous SCT is beneficial for the majority of patients or the overall benefit comes from certain subsets of patients is still an unresolved issue.4,13 In this regard, it has been claimed that patients with primary resistant disease are the most likely to benefit from early autologous SCT.14,15 However, there is a paucity of published evidence to support this claim.15–20 Furthermore, two different conditions should be distinguished among myeloma patients in whom primary therapy fails: (i) refractory myeloma, with progressive disease while on therapy and (ii) stable disease, in which the M-protein size does not change significantly and there is no clinical progression.21 These two different categories have not been analyzed separately in previous studies.
In the Spanish PETHEMA/GEM-2000 trial,22 patients were treated with alternating vincristine, carmustine (BCNU), melphalan, cyclophosphamide, prednisone (VBMCP) and vincristine, BCNU, adriamycin, dexamethasone (VBAD) chemotherapy or, in those with severe renal failure, with vincristine, adriamycin, dexamethasone (VAD), followed by high-dose therapy and autologous SCT.
Primary refractory patients were initially scheduled to received tandem transplants [double autologous SCT or autologous SCT followed by an allogeneic reduced-intensity conditioning (RIC) SCT]. The aim of our study was to investigate the efficacy, in terms of response rate and survival, of early autologous SCT in patients with truly primary refractory myeloma. The outcomes of patients with progressive disease were compared to those of patients with stable disease (also known as no change or non-responding, non-progressive disease).
Design and Methods
Study design
Patients diagnosed with symptomatic multiple myeloma between October 1, 1999 and December 31, 2004, who were younger than 70 years were included in the PETHEMA/GEM-2000 trial. Induction therapy consisted of six cycles of alternating VBMCP/VBAD chemotherapy or VAD for patients with severe renal failure (serum creatinine ≥4 mg/dL), followed by high-dose therapy intensification with busulfan 12 mg/kg plus melphalan-140 or with melphalan-200. The diagnosis of multiple myeloma was made according to the criteria of the Chronic Leukemia Myeloma Task Force.23 Patients with asymptomatic multiple myeloma were not included.
All patients who were primary refractory to the induction therapy were also scheduled to receive a second autologous SCT or an allogeneic RIC SCT, independently of the response status after the first autologous transplant. The treatment assignment to a second autologous transplant or to an allogeneic one with RIC was based on the availability or not of an HLA-identical sibling donor.
This study was approved by the Ethics Committee of the Hospital Clínic and informed consent was obtained from patients in accordance with the Declaration of Helsinki. The study was registered at ClinicalTrials.gov with number NCT00560053.
High-dose therapy
Peripheral blood stem cells were collected after cycle 4 using granulocyte colony-stimulating factor (16–24 μg/kg daily) for 5 days. The target number of CD34+ cells was at least 4×106/kg in order to have the possibility to perform two autologous transplants.
High-dose therapy consisted of BUMEL: oral busulfan 1 mg/kg every 8 h on days -6 to -3 (total dose 12 mg/kg) plus melphalan 140 mg/m2 in a single dose on day -2 or melphalan-200 (melphalan 200 mg/m2 in a single dose on day -2 or in two divided doses on days -3 and -2).
The high-dose therapy of the second autologous transplant consisted of CVB [cyclophosphamide 1.8 g/m2 on days -6 to -3, etoposide (VP-16) 200 mg/m2 on days -6 to -4 and BCNU 300 mg/m2 on day -6] or melphalan-200 as previously described. Patients receiving an allogeneic RIC transplant were conditioned with fludarabine 25 mg/m2 on days -7 to -3 and melphalan 70 mg/m2 on days -3 to -2. Graft-versus-host-disease prophylaxis consisted of cyclosporine A and methotrexate.22
Response criteria
Response, relapse and progression were assessed according to European Bone Marrow Transplant (EBMT) criteria.24 Complete response was defined as negative immunofixation in serum and urine, <5% plasma cells in a bone marrow aspirate as well as disappearance of soft tissue plasmacytomas and no increase in lytic bone lesions. Partial response was defined as a decrease in serum M-protein of ≥50% and 24-hour urinary light-chain excretion by ≥90% or to <200 mg and a reduction in extramedullary plasmacytomas of ≥50%. Minimal response required a 25–49% reduction in the level of serum M-protein and a 50–89% reduction in 24-hour urinary light-chain protein excretion. Responses had to be maintained for a minimum of 6 weeks. Relapse from complete response was defined as reappearance of serum or urinary para-protein on immunofixation, development of new extramedullary plasmacytomas, an increase in size or development of new lytic lesions or hypercalcemia. Progressive disease was defined by a greater than 25% increase in serum M-protein with an absolute increase of at least 5 g/L or a greater than 25% increase in urine M-protein and also an absolute increase ≥200 mg/24 h, and/or the appearance of soft-tissue plasmacytomas, new lytic bone lesions or hypercalcemia.
Criteria for primary refractory disease
The patients with primary refractory disease consisted of patients with stable disease and those with progressive disease, i.e. patients who never achieved a minimal response or better in whom there was no significant change in M protein and no evidence of clinical progression (no change or non-responsive, non-progressive, stable disease) and patients with primary refractory progressive disease who met the criteria for true progressive disease (serum and/or urine M-protein increase ≥25% and/or increase or development of skeletal involvement, anemia, plasmacytomas or hypercalcemia while on the initial chemotherapy).21
Statistical Methods
The χ2 test or Fisher’s exact test was used when required to assess the statistical significance of multiple comparisons. The Kaplan-Meier method was used to plot the survival curves, which were compared by a log-rank test.25
Results
Eighty patients with primary refractory myeloma (48 males, 32 females, median age 56 years) were included in this study. Induction chemotherapy consisted of VBMCP/VBAD in 78 patients and VAD in two. Sixty-eight patients underwent autologous SCT immediately after initial chemotherapy had failed, while 12 patients received a second-line treatment before transplantation: thalidomide (3 patients), bortezomib (1 patient), intermediate doses of melphalan (1 patient), VAD (4 patients), TaCyDex (thalidomide, cyclophosphamide, dexamethasone) (1 patient), DCEP (dexamethasone, cyclophosphamide, etoposide, cisplatin) (1 patient) and hyperCVAD (cyclophosphamide, doxorubicin, vincristine, dexamethasone) (1 patient). After salvage therapy, five patients achieved a partial response, two patients had a minimal response, three patients remained with stable disease and two patients showed progressive disease.
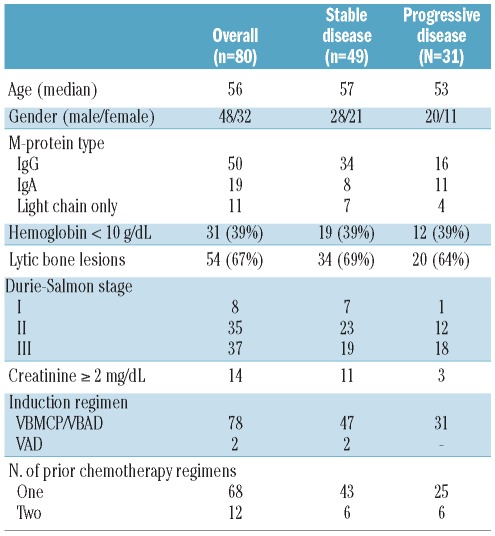
Among the 80 patients, 49 were classified as having stable disease and 27 had progressive disease while on treatment. Four additional patients who achieved a very transient partial response while receiving initial chemotherapy but who showed rapid progression before transplantation were included in the subgroup of patients with progressive disease. The prognostic features were similar in both subgroups of refractory patients (stable disease versus progressive disease). The initial characteristics of the patients are shown in Table 1.
Table 1.
Initial characteristics of the patients.
Response to first autologous transplant
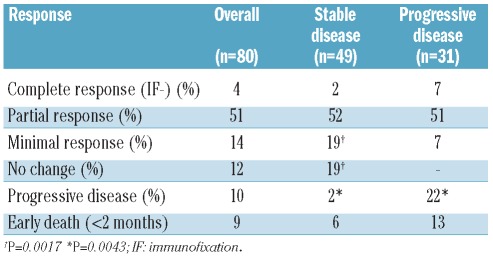
Twenty-seven patients received BUMEL as the conditioning regimen and 53 received MEL-200. Overall, 69% of the patients responded to the first autologous SCT (complete response: 4%, partial response: 51%, minimal response: 14%). Nine (12%) patients remained with stable disease after the procedure and eight (10%) had progressive disease after high-dose therapy. The transplant-related mortality rate was 9% with no significant differences between patients with stable disease and those with progressive disease (6% versus 13%). There were no significant differences in response rate (complete + partial responses) between the two subgroups of refractory patients (54% versus 58% in patients with stable disease and progressive disease, respectively). However, 38% of patients with stable disease at transplantation remained stable or achieved a minimal response after transplantation versus only 7% in the patients with progressive disease (P=0.0017). Moreover, the rate of early progression within the first 3 months after high-dose therapy was significantly higher among the patients initially with progressive disease than in those initially with stable disease (22.5% versus 2%, P=0.0043). The response rates after the first autologous SCT are summarized in Table 2.
Table 2.
Response rate after the first autologous transplant.
Flow-chart of patients undergoing a second transplant
Although refractory patients were scheduled to receive a tandem transplant (double autologous or autologous followed by allogeneic RIC SCT), 40 did not undergo the second high-dose procedure. The reasons for not proceeding with the second transplant were: death (n=8), poor performance status (n=8), progressive disease (n=7), physicians’ decision (n=8, four of these patients were in complete remission after the first transplant), patients’ refusal (n=3), insufficient CD34+ cells (n=2) and unknown (n=4).
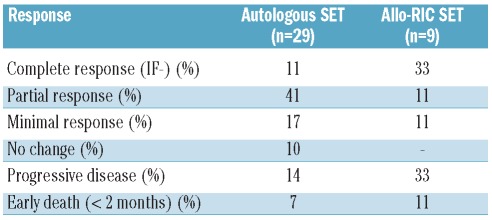
Overall 40 (50%) patients underwent the second high-dose procedure: 29 had a second autologous SCT and 11 an allogeneic procedure. Conditioning for the second autologous transplant consisted of MEL-200 (5 patients), BUMEL (1 patient) and CBV (23 patients). In the allogeneic group, one patient received a conventional allogeneic transplant, one patient was transplanted after non-myeloablative conditioning (micro-allogeneic RIC with fludarabine and total body irradiation 200 cGy) and nine patients received RIC. In order to report on a homogeneously treated series of patients, only the nine patients who underwent allogeneic RIC SCT were analyzed for response rate.
Response to second transplant
Response rates after the second transplant are detailed in Table 3. Only 11% and 33% of patients achieved complete response after tandem autologous SCT and autologous + allogeneic RIC SCT, respectively. The transplant-related mortality rate with the two strategies was 7% and 11%, respectively.
Table 3.
Response rate after the second transplant.
Survival
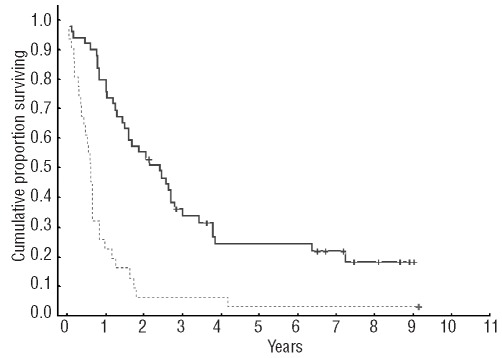
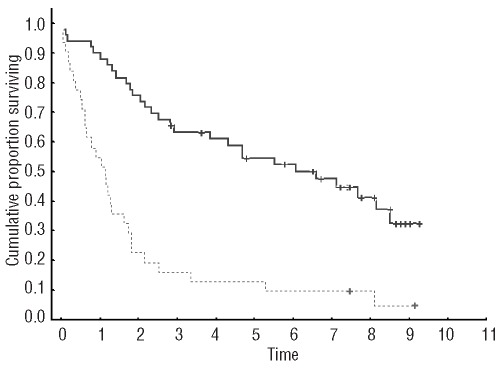
After a median follow-up for alive patients of 6.6 years, the median survival of the whole series after the first transplant was 2.3 years. Progression-free survival from the first autologous SCT was significantly shorter in patients initially with progressive disease than in those initially with stable disease (0.6 versus 2.3 years, P<0.00004) (Figure 1). Patients progressing under therapy (progressive disease group) had a significantly shorter overall survival from the first transplant than that of the stable disease group (median 1.1 versus 6 years, P=0.00002) (Figure 2).
Figure 1.
Progression-free survival from the first autologous SCT in patients with progressive disease as compared to those with stable disease (median 0.6 versus 2.3 years, P=0.00004)
Figure 2.
Overall survival from the first autologous SCT in patients with progressive disease as compared to those with stable disease (median 1.1 versus 6 years, P=0.00002)
Discussion
Autologous SCT is considered the standard of care in younger patients with newly diagnosed symptomatic multiple myeloma.1–4 The beneficial effect of autologous SCT likely results from the greater tumor reduction attained as compared to that achieved with conventional chemotherapy (i.e. complete response 40% versus <10%).20,26,27 The identification of factors that can predict achievement of a complete response is important in order to limit autologous SCT to those patients most likely to benefit from the procedure. In our experience28 and in that of Alexanian et al.,26 the tumor burden, determined by the serum and urine M-protein size at the time of transplantation, is the most important predictor of complete response post-transplant.26,28
Patients with primary resistant disease have a poor prognosis with standard chemotherapy, with a median overall survival between 1.5 and 3 years.15,29 It has been suggested that these patients are the most likely to benefit from ASCT provided that the rescue transplant is performed early in the course of the disease before the emergence of resistant clones. In six published series, the reported complete response rate was between 8–40% and the overall survival ranged from 4 to 6 years.15–19 Thus, in a series from the M. D. Anderson,15 the median event-free survival and overall survival of 27 patients with primary resistant disease who received a transplant during the first year after initiation of therapy were 3.5 and 6 years, respectively. In addition, in one study from the University of Arkansas,16 the median event-free survival and overall survival in 72 patients with primary unresponsive disease were 21 and 47 months, respectively. In a study at the Royal Marsden Hospital, the complete response rate after transplantation was 40% in a series of 43 patients with primary refractory disease: in this series, the complete response was only assessed by immunoelectrophoresis. In a series of patients studied by Kumar et al.,19 the post-transplant response rate was 92%, including 20% complete responses defined either by immunoelectrophoresis or immunofixation, with no significant differences in event-free and overall survival rates at 1 year post-transplant between chemosensitive patients and patients with primary refractory disease. In the largest series of 89 patients, published by Alexanian et al.,20 the complete response rate post-transplant was 16% and the overall survival longer than 7 years for those patients who achieved a complete response. These “good” results are in contrast with those reported by Rajkumar et al.17 for a small series of 12 patients with primary refractory disease who had a post-transplant complete response rate of 17% and median overall and progression-free survivals of only 30 and 26 months post-transplant, respectively.
In our study, patients with primary refractory disease had a poor prognosis with a median progression-free survival of 1.2 years and an overall survival of 2.3 years. This could be explained by the low complete response rate achieved after transplantation (5% after the first autologous SCT, 11% after double autologous transplants and 33% after tandem autologous/allogeneic RIC transplants). There are several possible explanations for the differences between our results and those reported for the other series. First, the criteria used to define “primary refractory disease” were different. In our study, only patients with stable disease or progressive disease as defined by the EBMT24 criteria were included. In contrast, in the previous series patients failing to achieve a minimal response15,20,26 or even a partial response18,19 according to the EBMT criteria were considered to have primary refractory disease. Second, in all the other series the primary therapy was mainly steroid-based while our patients had received combination chemotherapy mainly based on alkylating agents, steroids and doxorubicin. Finally, the two categories of patients considered as having primary refractory disease (i.e., primary unresponsive with progressive disease versus no change without clinical progression) should be analyzed separately.21 Thus, in our series, refractory patients with initially progressive disease had an extremely poor prognosis, with median progression-free survival and overall survival of only 7 and 13 months post-transplant, respectively. In contrast, refractory patients with initially stable disease had a progression-free survival of 27 months and a median overall survival of 73 months after a median follow-up of 6.6 years, which is similar to that reported for chemosensitive patients.30 It is of note that although the achievement of at least a partial response after the first autologous SCT was similar in the two subgroups of patients (54% versus 58%) the duration of response was very limited in the group with progressive disease. In addition, 38% of the group of patients with non-responding/non-progressive disease achieved a minimal response or remained with stable disease while almost one-fourth of patients with unresponsive progressive disease while on initial chemotherapy developed progressive disease after the first transplant. In contrast, Singhal et al.18 found no significant differences between 13 patients who developed progressive disease during C-VAMP chemotherapy compared to the 30 who had stable disease or a suboptimal response. Of note, the transplant-related mortality of almost 9% with the first transplant is higher than that reported in chemosensitive patients; this likely resulted from an increased risk in the refractory population.
In the era of new drugs, high overall and complete response rates can be achieved pre-transplantation.31–33 However, the issue of primary refractory disease is not yet resolved and thus our analysis is relevant at present. In fact, in the current Spanish trial comparing four cycles of VBMCP/VBAD plus two cycles of bortezomib versus six cycles of thalidomide/dexamethasone versus six cycles of bortezomib, thalidomide and dexamethasone, the rates of progressive disease are as high as 12%, 23% and 7%, respectively.34 Although it is not yet known whether patients refractory to novel agents will benefit from autologous SCT, it would be reasonable to offer these patients salvage regimens or experimental therapies to substantially decrease the tumor burden before transplantation, which is the crucial factor associated with complete response after transplantation which, in turn, is the main surrogate for prolonged survival.
In summary, our results show that patients with unresponsive progressive disease do not benefit from autologous SCT. In consequence, novel treatment approaches, including experimental drugs, should be offered to these patients. In contrast, patients with non-responding, non-progressive disease have a good outcome with an overall survival comparable to that of patients with chemosensitive disease. However, whether this is due to the benefit of high-dose therapy or to the natural history of a more indolent disease is uncertain.
Supplementary Material
Footnotes
Funding: this work was supported in part by Spanish grants from Instituto Carlos III RD06/0020/0005 and 0006, 0031,0101,1056 and grant 08/0147.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Attal M, Haroussau JL, Stopa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. Autologous bone marrow transplantation versus conventional chemotherapy in multiple myeloma. N Engl J Med. 1996;335(3):91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Phil D, et al. Medical Research Council Adult Leukaemia Working Party. High-dose chemotherapy stem-cell rescue for multiple myeloma. N Engl Med. 2003;348(19):1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Pineda-Roman M, Barlogie B, Anaissie E, Zangari M, Bolejack V, Van Rhee F, et al. High-dose melphalan-based autotransplants for multiple myeloma: the Arkansas experience since 1989 in 3077 patients. Cancer. 2008;112(8):1754–64. doi: 10.1002/cncr.23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bladé J, Rosiñol L, Cibeira MT, Rovira M, Carreras E. Hematopietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 2010;115(18):3655–63. doi: 10.1182/blood-2009-08-238196. [DOI] [PubMed] [Google Scholar]
- 5.Kristinsson SY, Landgren O, Dickman PW, Derolf AR, Bjorkholm M. Patterns of survival in multiple myeloma: a population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25(15):1993–9. doi: 10.1200/JCO.2006.09.0100. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111(5):2521–6. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 7.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy M, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and impact of novel therapies. Blood. 2008;111(5):2516–20. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bladé J, Rosiñol L, Sureda A, Ribera JM, Díaz-Mediavilla J, García-Laraña J, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the inicial chemotherapy: long-term results from a prospective randomized trial from the Spanish Cooperative Group PETHEMA. Blood. 2005;106(12):3755–9. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 9.Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, et al. High-dose therapy and autologous blood stem cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23(36):9227–33. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 10.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemotherapy for multiple myeloma: final results of phase III US Intergroup trial S9321. J Clin Oncol. 2006;24(6):929–36. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 11.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349(26):2495–502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 12.Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, et al. Prospective, randomized study of single compared with double autologous transplatation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25(17):2434–41. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 13.Bladé J, Vesole DH, Gertz M. Transplantation for multiple myeloma: who, when, how often? Blood. 2003;102(10):3469–77. doi: 10.1182/blood-2003-01-0073. [DOI] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Hester J, Huh Y, Champlin R, Alexanian R. Intensive chemotherapy with blood progenitor transplantation for primary resistant multiple myeloma. Br J Haematol. 1994;87(4):730–4. doi: 10.1111/j.1365-2141.1994.tb06731.x. [DOI] [PubMed] [Google Scholar]
- 15.Alexanian R, Dimopoulos MA, Hester J, Delasalle K, Champlin R. Early myeloablative therapy for multiple myeloma. Blood. 1994;84(12):4278–82. [PubMed] [Google Scholar]
- 16.Vesole DH, Barlogie B, Jagannath S, Cheson B, Tricot G, Alexanian R, et al. High-dose therapy for refractory multiple myeloma: improved prognosis with better supportive care and double transplants. Blood. 1994;84(3):950–6. [PubMed] [Google Scholar]
- 17.Rajkumar SV, Fonseca R, Lacy MQ, Witzig TE, Lust JA, Greipp PR, et al. Autologous stem cell transplantation for relapsed and primary refractory myeloma. Bone Marrow Transplant. 1999;23(12):1267–72. doi: 10.1038/sj.bmt.1701805. [DOI] [PubMed] [Google Scholar]
- 18.Singhal S, Powles R, Sirohi B, Treleaven J, Kulkarni S, Metha J. Response to induction chemotherapy is not essential to obtain survival benefit from high-dose melphalan and autotransplantation in myeloma. Bone Marrow Transplant. 2002;30(10):673–9. doi: 10.1038/sj.bmt.1703717. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Lacy MQ, Dispenzieri A, Rajkumar SV, Fonseca R, Geyer S, et al. High-dose therapy and autologous stem cell transplantation for multiple myeloma poorly responsive to initial therapy. Bone Marrow Transplant. 2004;34(2):161–7. doi: 10.1038/sj.bmt.1704545. [DOI] [PubMed] [Google Scholar]
- 20.Alexanian R, Weber D, Delasalle K, Handy B, Champlin R, Giralt S. Clinical outcomes with intensive therapy for patients with primary resistant multiple myeloma. Bone Marrow Transplant. 2004;34(3):229–34. doi: 10.1038/sj.bmt.1704562. [DOI] [PubMed] [Google Scholar]
- 21.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos D, Kyle RA, et al. Consensus recommendations for the uniform reporting of clinical trials. Report of the International Myeloma Workshop Consensus Panel I. Blood. 2011;117(18):4691–5. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosiñol L, Pérez-Simón JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112(9):3591–3. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 23.Chronic Leukemia-Myeloma Task Force. National Cancer Institute. Porposed guidelines for protocol studies. II. Plasma cell myeloma. Cancer Chemother Rep. 1973;4:145–58. [Google Scholar]
- 24.Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol. 1998;102(5):1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 25.Peto R, Pike MC. Conservation of the approximation sigma (O-E)2-E in the log rank test for survival data or tumor incidence data. Biometrics. 1973;29(3):579–84. [PubMed] [Google Scholar]
- 26.Alexanian R, Weber D, Giralt S, Dimopoulos M, Delasalle K, Smith T, et al. Impact of complete remission with intensive therapy in patients with responsive multiple myeloma. Bone Marrow Transplant. 2001;27(10):1037–43. doi: 10.1038/sj.bmt.1703035. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Delasalle K, Feng L, Thomas S, Giralt S, Qazilbash M, et al. CR represents an early index of potential long survival in multiple myeloma. Bone Marrow Transplant. 2010;45(3):498–504. doi: 10.1038/bmt.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadal E, Giné E, Bladé J, Esteve J, Rosiñol L, Fernández-Avilés, et al. High-dose therapy/autologus stem cell transplantation in patients with chemosensitive multiple myeloma: predictors of complete remission. Bone Marrow Transplant. 2004;33(1):61–4. doi: 10.1038/sj.bmt.1704313. [DOI] [PubMed] [Google Scholar]
- 29.Bladé J, San Miguel JF, Fontanillas M, Esteve J, Maldonado J, Alcalá J, et al. Increased conventional chemotherapy does not improve survival in multiple myeloma: long-term results of two PETHEMA trials including 914 patients. Hematol J. 2001;2(4):272–8. doi: 10.1038/sj.thj.6200115. [DOI] [PubMed] [Google Scholar]
- 30.Lahuerta JJ, Mateos MV, Martínez-López J, Rosiñol L, Sureda A, de la Rubia J, et al. Influence of pre- and postransplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26(35):5775–82. doi: 10.1200/JCO.2008.17.9721. [DOI] [PubMed] [Google Scholar]
- 31.Barlogie B, Anaissie E, van Rhee F, Haessler J, Hollnig K, Pineda-Román M, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Hematol. 2007;138(2):176–85. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 32.Cavo M, Tachetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before and consolidation therapy after double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomized phase 3 study. Lancet. 2010;376(9758):2075–85. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 33.Popat R, Oakervee HE, Hallan S, Curry N, Odeh L, Foot N, et al. Bortezomib, doxorubicin and dexamethasone (PAD) front-line treatment of multiple myeloma updated results after long-term follow-up. Br J Haematol. 2008;141(4):512–6. doi: 10.1111/j.1365-2141.2008.06997.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosinol L, Cibeira MT, Mateos MV, Martínez J, Oriol A, Teruel A, et al. A phase III PETHEMA/GEM study of induction therapy prior autologous stem cell transplantation (ASCT) in multiple myeloma: Superiority of VTD (bortezomib/thalidomide/dexamethasone) over TD and VBMCP/VBAD plus Bortezomib. Blood. 2010;116(21) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.