Abstract
Background
The levels and clinical relevance of Th17 cells and other interleukin-17-producing cells have not been analyzed in chronic lymphocytic leukemia. The objective of this study was to quantify blood and tissue levels of Th17 and other interleukin-17-producing cells in patients with this disease and correlate blood levels with clinical outcome.
Design and Methods
Intracellular interleukin-17A was assessed in blood and splenic mononuclear cells from patients with chronic lymphocytic leukemia and healthy subjects using flow cytometry. Interleukin-17A-producing cells were analyzed in formalin-fixed, paraffin-embedded spleen and lymph node sections using immunohistochemistry and immunofluorescence.
Results
The absolute numbers of Th17 cells in peripheral blood mononuclear cells and the percentages of Th17 cells in spleen cell suspensions were higher in patients with chronic lymphocytic leukemia than in healthy subjects; in six out of eight paired chronic lymphocytic leukemia blood and spleen sample comparisons, Th17 cells were enriched in spleen suspensions. Circulating Th17 levels correlated with better prognostic markers and longer overall survival of the patients. Two “non-Th17” interleukin-17-expressing cells were identified in chronic lymphocytic leukemia spleens: proliferating cells of the granulocytic lineage and mature mast cells. Granulocytes and mast cells in normal spleens did not express interleukin-17. Conversely, both chronic lymphocytic leukemia and healthy lymph nodes contained similar numbers of interleukin-17+ mast cells as well as Th17 cells.
Conclusions
Th17 cells are elevated in chronic lymphocytic leukemia patients with better prognostic markers and correlate with longer survival. Furthermore, non-Th17 interleukin-17A-expressing cells exist in chronic lymphocytic leukemia spleens as maturing granulocytes and mature mast cells, suggesting that the microenvironmental milieu in leukemic spleens promotes the recruitment and/or expansion of Th17 and other IL-17-expressing cells. The pathophysiology of Th17 and non-Th17-interleukin-producing cells in chronic lymphocytic leukemia and their distributions and roles in this disease merit further study.
Keywords: Th17, IL-17, T lymphocyte, microenvironment, myeloid differentiation, chronic lymphocytic leukemia
Introduction
Pro-survival signals from tissue microenvironments are required to maintain leukemic clones in patients with chronic lymphocytic leukemia (CLL). These signals involve intricate cross-talk between CLL cells and CD4+ T cells, monocyte-derived nurse-like cells, and mesenchymal stromal cells as well as various cytokines and chemokines,1 promoting resistance of CLL cells to chemotherapy and contributing to persistence of disease after therapy.
The role of immune dysregulation in CLL is not well defined. Effective anti-tumor immunity depends on CD4+ T cells that direct differentiation of other immune cells in response to tumor antigens. CD4+ T cells are divided into several major subsets: T helper type 1 (Th1), T helper type 2 (Th2), and T regulatory (Treg) cells. In addition, an interleukin (IL)-17-secreting CD4+ T-cell subset (Th17) exists;2–4 these cells play a crucial role in the development of inflammatory and autoimmune diseases.5
IL-17A is one of six members (A–F) of the IL-17 family.6 A corresponding IL 17 receptor family of five members (A–E) exists.6 IL-17 is produced by Th17 cells and other cells: CD8+ T cells, γδ T cells, invariant NKT cells, mast cells, and granulocytes.7 IL-17 has pleiotropic functions and multiple targets, mostly explored in mouse models and increasingly linked to human diseases.8–11 Generation of human Th17 cells from naïve precursors is co-promoted by IL-6 and IL-1β and expansion of human Th17 cells is maintained by IL-23;9 a role for transforming growth factor-β (TGF β) in human Th17-cell differentiation is controversial.12–15
IL-17 has pro- and anti-tumor actions.16,17 Functions of IL-17 relevant to cancer include angiogenesis,18 granulopoiesis,19 osteoclast induction,20 and induction of cytokines such as IL-6, TGF-β, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF), as well as matrix metalloproteinase and intercellular adhesion molecule-1 in a variety of cell types, including bone marrow stromal cells.21,22 Because of its multitude of actions, the Th17/IL-17 axis may affect development of solid tumors (ovarian, lung, and liver cancers)23–25 and hematologic cancers (myeloma, acute myeloid leukemia, and non-Hodgkin’s lymphoma).26–30 Having previously shown that various cytokines are elevated in the sera of CLL patients and that clusters of certain cytokines associate with distinct clinical courses,31 we explored the IL-17/Th17 axis in patients with CLL as IL-17 was in a cluster associated with better prognosis.
Design and Methods
Blood and lymphoid tissue samples
The Institutional Review Board of the North Shore-Long Island Jewish Health System approved these studies. After obtaining informed consent in accordance with the Declaration of Helsinki, frozen peripheral blood mononuclear cells (PBMC) were collected from 66 CLL patients identified according to the International Workshop on CLL diagnostic criteria,32 and 15 age-matched healthy volunteers. Of the 66 CLL patients, 44 eventually received some form of therapy and 19 died. Blood samples were collected prior to any treatment from 55 patients and after treatment from 11 patients. Archived, formalin-fixed, paraffin-embedded sections from spleens of CLL patients (n=6) and of normal subjects removed after trauma (n=4) were studied by immunohistochemistry and immunofluorescence. Paired spleen mononuclear cell suspensions from eight CLL and five healthy subjects were available for flow cytometry analysis. Formalin-fixed, paraffin-embedded sections from CLL (n=14) or non-CLL (n=4) lymph nodes were also studied. The clinical and laboratory characteristics of the patients from whom blood and spleen tissue samples were obtained are reported in Online Supplementary Tables S1 and S2, respectively.
Flow cytometric analysis of absolute numbers of Th17 cells in peripheral blood
PBMC from CLL patients and healthy subjects were isolated by density gradient centrifugation through Ficoll Paque (GE Healthcare, Piscataway, NJ, USA) and used thawed after having been frozen viable in fetal calf serum plus 10% dimethylsulfoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA). For the detection of IL-17A, peripheral blood or splenic mononuclear cell suspensions from CLL patients and healthy subjects were stimulated for 5 h with 10 mg/mL phorbol-12-myristate-13-acetate and 1.5 mg/mL ionomycin in the presence of monensin (BD Intracellular Staining Kit; BD Biosciences, San Jose, CA, USA). Stimulated cells were centrifuged at 1200g for 7 min at 10ºC, washed, and then surface stained by incubating cells (1–2×106/mL) for 30 min at room temperature in the dark with anti-human monoclonal antibodies: anti-CD3-APC-H7, -CD4-APC, -CD8-PerCP (all BD Biosciences), and -CD161-PerCPCy 5.5 (BD Biolegend, San Diego, CA, USA). Cells were subsequently fixed and stained intracellularly with anti-human IL-17A AlexaFluor 488 (eBioscience Inc., San Diego, CA, USA) or appropriate isotype-matched monoclonal antibodies using a Cell Fixation/Permeabilization Kit (BD Biosciences) according to the manufacturer’s instructions. Stained cells were quantified using LSR II (BD Biosciences), and data were analyzed with FlowJo software (version 8.8.6).
Immunohistochemistry and immunofluorescence analyses of lymphoid tissues
Serial sections of formalin-fixed, paraffin-embedded spleen and lymph node tissues were analyzed by immunohistochemistry for IL-17A-containing cells after deparaffinization, re-hydration, and antigen retrieval. Goat or rabbit anti-human IL-17A polyclonal antibodies were used to detect IL-17A+ cells; for double staining, a panel of antibodies reactive with CD3, CD4, CD20, CD21, CD23, CD31, pan-CD45, CD56, CD68, CD123, CD138, CD235a, PAX5, CD2AP, LMP-1, LANA-1, CD57, vimentin, S-100, Ki-67, CD38, and MPO were used (Online Supplementary Table S3). Isotype matched monoclonal antibodies were used as negative controls. Horseradish peroxidase activity was visualized using ImmPACT DAB and vector SG substrate kits (Vector Lab, Burlingame, CA, USA). Sections were counterstained with nuclear fast red (Vector Lab). After dehydration and mounting, stained samples were examined and images taken by light microscope (Zeiss Axiovert 200M, Axiovision 4 software).
Immunofluoresence studies were performed with similar methods using mouse anti-human CD3 and CD15, mouse or rabbit anti-human Ki-67, and rabbit anti-human CD13 (Online Supplementary Table S3). After overnight incubation, sections were washed with phosphate-buffered saline-Tween (PBS-T) and incubated in a humidified chamber for 2 h with a mixture of secondary antibodies: DyLight conjugated donkey anti-rabbit (488, 594, 649), donkey anti-goat (649 and 488), or donkey anti-mouse (594 and 649) (Jackson Research, West Grove, PA, USA). After washing with PBS-T and mounting with Prolong Gold Antifade Reagent (Invitrogen, Carlsbad, CA, USA), images were taken with a confocal laser scanning microscope (Olympus Fluoview 300; Olympus America Inc., NY, USA). In a subset analysis, immunofluorescence triple staining for CD20/Ki-67/IL-17A was performed, and proliferation centers identified as areas with high (> median) numbers of Ki-67+CD20+ cells (Ki-67High). Localization of IL-17A-containing cells was analyzed with respect to Ki-67High versus Ki-67Low fields.
Statistical analysis
Group comparisons were carried out using either the Mann-Whitney or Kruskal-Wallis test, as appropriate. Overall survival and time-to-first treatment were estimated using the Kaplan-Meier product-limit method, and compared using the log-rank test. Time-to-first treatment and survival were calculated from the date of first diagnosis to the date of first therapy. Pairwise comparisons were carried out using a Bonferroni-type adjustment (P<0.01). A Cox proportional hazard regression analysis with backward elimination was used to determine which variables were associated with overall survival. Statistical analyses were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA) and SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Chronic lymphocytic leukemia peripheral blood mononuclear cells contain increased absolute numbers of CD3+CD4+IL-17A+ (Th17) cells
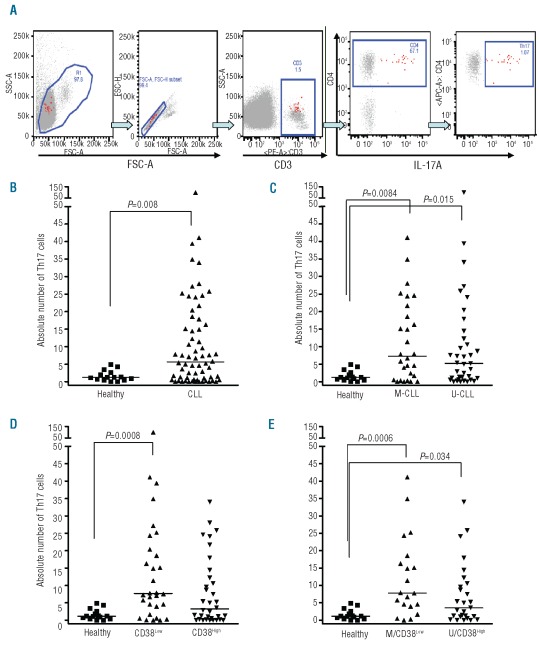
We quantified and compared Th17 cell frequencies among PBMC from CLL patients and healthy subjects by intracellular flow cytometry. After gating on CD3+ cells, CD3+CD4+ cells containing IL-17A were analyzed (Figure 1A) and the absolute number of Th17 cells per mm3 determined (Online Supplementary Table S1). The absolute numbers of Th17 cells were significantly higher in CLL patients (median=5.6; range, 0.0–99.9) than in healthy volunteers (median=1.2; range, 0.0–5.0; P=0.008; Figure 1B). PBMC were collected prior to any treatment from 55 patients and after treatment from 11 patients; those patients whose samples were collected after therapy were distributed equally between the Th17High (Th17≥median, 5.6) and Th17Low (<5.6) subsets. Furthermore, when the absolute numbers of Th17 cells were analyzed in samples drawn before or after therapy and compared independently to those from healthy subjects (n=15), the numbers of Th17 cells were significantly higher in patients with CLL (P<0.05 for both). The percentages of Th17 cells were also significantly different between CLL patients (median=0.64%; range, 0.0–3.6%) and healthy subjects (median=0.16%; range, 0.0–0.8%; P=0.010).
Figure 1.
Increased absolute numbers of Th17 cells in CLL and CLL subgroups compared to normal PBMC. (A) Strategy to measure Th17 cells. After gating on CD3+ cells, CD3+CD4+ cells containing IL-17A were analyzed by flow cytometry. These plots illustrate gating of the terminally selected Th17 (CD3+ CD4+ IL-17+) population (rightmost plot, red highlighted cells) and subsequent back-gating, and show that the Th17 population (red highlighted cells in all plots) selected for analysis comprises singlet lymphocytes (two left plots – FSA-C versus SSC-A and FSC-A versus FSC-H plots) that are CD3+ (middle plot) and CD4+ (2nd plot from the right). (B) Absolute numbers of Th17 cells (CD3+CD4+IL 17A+ cells) among PBMC from CLL patients. PBMC from 66 patients and 15 healthy subjects were stimulated with PMA and ionomycin for 5 h in the presence of monensin and then incubated with fluo-rochrome-labeled antibodies as described in the Design and Methods section. Marked cells were measured by flow cytometry. Absolute numbers (per mm3) of Th17 cells are higher in CLL patients (P=0.008). The absolute number of Th17 cells in blood (per mm3) for each patient was determined according to the formula: [ALC (per mm3)] × [% CD3 positive MNC] × [% CD4 positive CD3 cells] × [%IL-17 positive CD4 cells]. ALC means absolute lymphocyte count and MNC means mononuclear cells. (C) Absolute numbers of circulating Th17 cells in M-CLL and U-CLL patients compared to healthy individuals. Absolute numbers of Th17 cells were higher in the blood of M-CLL (n=28) and U-CLL (n=37) patients than in healthy subjects (n=15) (P=0.008 and P=0.015, respectively). (D) Absolute numbers of circulating Th17 in CLL patients stratified by CD38 levels compared to healthy subjects. Absolute numbers of Th17 cells were significantly higher in CD38Low patients (n=30) than in healthy controls (n=15) (P=0.0008). (E) Absolute numbers of circulating Th17 cells in M-CLL/CD38Low and U-CLL/CD38High patients were higher than in healthy individuals. M-CLL/CD38Low CLL patients (n=20) and U-CLL/CD38High (n=27) patients had higher absolute numbers of Th17 cells as compared to controls (n=15) (P=0.0006) and (P=0.034). See Online Supplementary Table S1 for the patients’ features. All values determined by the Mann-Whitney test.
Significantly higher numbers of Th17 cells were found in both IGHV-mutated (M-CLL) and IGHV-unmutated (U-CLL) cases than in healthy subjects (Figure 1C; P=0.008 and 0.015, respectively). The numbers of Th17 cells did not differ significantly between M-CLL and U-CLL cases (P=0.512). Comparing CLL patients grouped according to CD38 expression, Th17 levels in patients with less than 30% of the clone expressing CD38+ cells (CD38Low) were significantly higher than those in healthy individuals (Figure 1D; P=0.0008). The CD38Low subgroup tended to have higher numbers of Th17 cells than did the CD38High subgroup (P=0.060).
When patients were divided based on combinations of better (M-CLL/CD38Low) or worse (U-CLL/CD38High) prognostic markers, both the M-CLL/CD38Low and U-CLL/CD38High subgroups of patients had significantly higher numbers of Th17 cells compared with healthy individuals (P=0.0006 and P=0.034, respectively; Figure 1E). Furthermore, the M-CLL/CD38Low subgroup tended to have higher Th17 cell numbers than did the U-CLL/CD38High subgroup (P=0.070). Mean fluorescent intensities of Th17 cells in CLL patients and healthy controls were similar (data not shown).
Th17 cells in spleens from patients with chronic lymphocytic leukemia
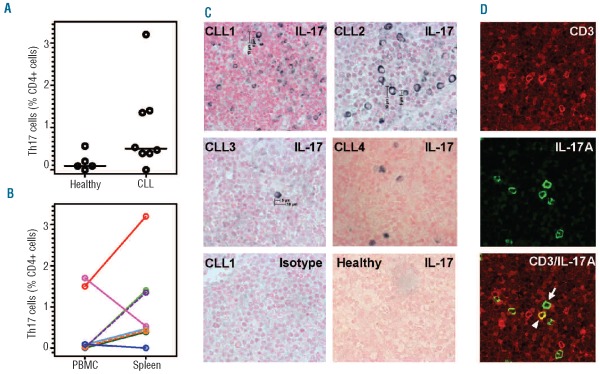
Dissociated CLL splenic mononuclear cells contained a mean frequency of 1.0% (range, 0–3.2%) Th17 cells, compared to 0.2% (range, 0–0.5 %) in spleens from healthy subjects (Figure 2A) as determined by intracellular flow cytometry. Furthermore, in six out of eight paired splenic mononuclear cell and PBMC samples, Th17 cells were enriched in the spleen (Figure 2B). Online Supplementary Figure S1 shows a representative plot of total IL-17A-producing cells in spleen suspensions.
Figure 2.
Th17 cells (CD3+CD4+IL-17A+ cells) in CLL splenic mononuclear cell (MNC) suspensions and in fixed tissues. (A) Th17 cell percentages in splenic MNC suspensions. There is a trend towards more Th17 cells in CLL spleen cell suspensions (n=8) than in healthy splenic cell suspensions (n=5) (P=0.09). (B) Comparison of Th17 cell percentages in paired PBMC and splenic MNC suspensions. Paired PBMC and splenic MNC suspensions from CLL patients (n=8) were analyzed using flow cytometry. (C) Immunohistochemistry (IHC) analysis of IL-17A expression in CLL spleen sections. IL-17A-expressing cells were found in all CLL spleens by IHC. Images from four of six CLL spleens stained for IL-17A are shown (upper 4 panels). Similar analyses of healthy spleens did not reveal any IL-17A-expressing cells (lower right panel shows a representative image). Lower left panel shows background (isotype control) staining. High magnification 60× images were taken using 60× oil immersion objective lens. (D) Identification of CD3+IL-17A+ cells in CLL spleen (Th17 cells). The upper panel shows CD3+ cells (red) and the middle panel shows IL-17A+ cells (green). The lower panel (merged image) shows several CD3/IL-17A coexpressing cells (yellow). The white arrowhead in the lower panel identifies a Th17 cell (yellow) and the thin while arrow identifies a non-Th17, IL 17A+ cell (green). Images were obtained at magnification 600× using 60× oil immersion objective lens and a confocal laser scanning biological microscope at room temperature.
Immunohistochemistry analysis of formalin-fixed, paraffin-embedded spleen sections for IL-17A-expressing cells showed IL-17A+ cells in all six CLL spleen sections studied, albeit to variable extents (Figure 2C, upper panels). No IL-17A-containing cells were found in the four healthy spleens studied (Figure 2C, lower right panel and data not shown).
To determine whether IL-17A+ cells were Th17 cells, double immunofluorescence staining of spleen sections with monoclonal antibodies specific for CD3 and IL-17A was performed. CD3+IL-17A+ (Th17) cells were observed in five of six CLL spleens (0.1, 0.2, 0.4, 0.7, and 2.3%; Figure 2D shows a representative field); we assume these are comparable to the CD3+CD4+ Th17 cells detected in blood (Figure 1) and dissociated spleen cells (Figure 2B). We noted, however, the presence of IL-17A+ cells negative for CD3 (identified by thin white arrow), suggesting additional “non-Th17” IL-17A-expressing cells in spleens of CLL patients.
Presence of non-Th17 interleukin-17A+ cells in chronic lymphocytic leukemia spleens
The non-Th17 IL-17A-expressing cells in CLL spleens did not display CD68, CD33, CD56, CD20, Pax5, and CD138 (Online Supplementary Figure S2) or CD21, CD23, CD14, CD11b, CD123, CD2AP, CD34, CD38, CD31, BDCA2, and pan-CD45 (data not shown), indicating that they were not macrophages, B cells, NK cells, dendritic cells, or plasma cells.
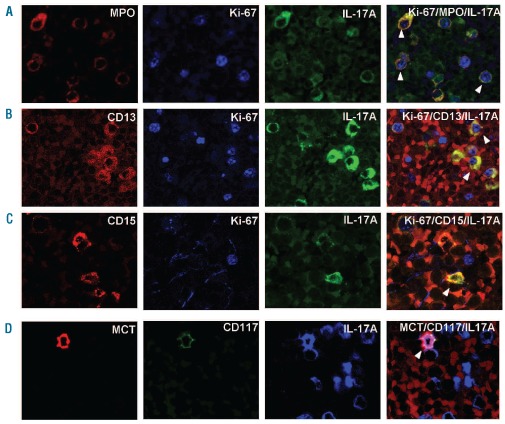
However, immunohistochemistry and immunofluorescence staining revealed that the majority of the non-Th17 IL-17A+ cells in each CLL spleen co-expressed myeloper-oxidase (MPO) (mean percentage of MPO+: 73.8%; range, 52.6–89.5%). Complementary immunofluorescence analyses showed that a portion of the IL-17A+MPO+ cells contained nuclear Ki-67 (Figure 3A; mean: 61.3%; range, 46.2–77.8%), indicating ongoing/recent proliferation. These large brightly stained, IL-17A+MPO+ cells were found in clusters, often near sinusoids and occasionally in and around vessels (Online Supplementary Figure S3).
Figure 3.
Immunofluorescence analyses of IL-17A+ myeloid cells in CLL spleen. (A) Some IL-17A+ cells (green) in CLL spleens coexpress MPO (red) and Ki-67 (blue) indicating that these cells are proliferating myeloid cells. (B) IL-17A+Ki-67+ cells in CLL spleen can coex-press CD13. (C) IL-17A+Ki-67+ cells in CLL spleen can also coexpress CD15. These immunofluorescence data (B and C) suggest that a subset of IL-17A+ cells have followed a granulocytic differentiation pathway. (D) A small number of IL-17A+ cells (blue) in CLL spleens coexpress CD117 and MCT, suggesting that a minority of the non-Th17, IL-17A-expressing cells in CLL spleens are mast cells. White arrowheads indicate triple positive cells in respective panels. All images were obtained at magnification 600× using a 60× oil immersion objective lens and a confocal laser scanning biological microscope (Olympus Fluoview 300, Fluoview software) at room temperature.
Using a triple staining immunofluorescence approach, we demonstrated that the IL-17A+MPO+ cells present in CLL spleens expressed CD13, CD15, or both CD13 and CD15 to variable extents (Figures 3B and 3C). The mean percentages (6 spleens) of CD13+, CD15+, and CD13+CD15+ cells expressing IL-17A were 49.4%, 84.5%, and 54.1%, respectively.
The frequencies of CD13+ and CD15+ myeloid cells, irrespective of IL-17A expression, were compared in CLL (n=6) and healthy (n=4) spleens by double immunofluorescence staining (1000 cells counted per myeloid marker). The mean percentages of CD13+, CD13+CD15+, and CD15+ cells were 4.7% (range, 0.7–9.2%), 1.5% (range, 0–3.9%), and 3.3% (range, 1.3–5.5%), respectively, in CLL spleens and 0.9% (range, 0.3–2%), 0%, and 8.7% (range, 4.9–15.7%), respectively, in healthy spleens. Of note, the majority of these myeloid cells in CLL spleens expressed IL-17A, while no cells expressing IL-17A were detected in healthy spleens. Thus, most of the proliferating, IL-17A+MPO+ cells in CLL spleens were of the granulocyte lineage.
While IL-17A+MPO+ cells comprised the major portion of the non-Th17 IL-17A+ cells in CLL spleens, a minor population of these cells were MPO-negative, suggesting the presence of an additional IL-17A-expressing cell type. Because mast cells can also produce IL-17A,33 we determined the percentages of mast cells in the same CLL and healthy spleens and screened these cells for IL-17A expression. The mean percentages of mast cells did not differ between normal and CLL spleens (CLL: CD117+ - 0.2%, range: 0.2–0.3 % and MCT+ - 0.3%, range 0.2–0.3 %; normal: CD117+ 0.4%, range: 0.3–0.5 %, and MCT+ - 0.5%, range 0.4–0.6 %). However, in CLL spleens 94.2% of the CD117+ cells co-expressed IL-17A (range, 76.9–100%) and 91.7% of the MCT+ cells co-expressed IL-17A (range, 66.7–100%; Figure 3D), while none of the mast cells in healthy spleens expressed IL-17A.
We explored whether spleen Th17 cells were present at higher frequency in proliferation centers, defined experimentally by the presence of high numbers of Ki-67-expressing CD20+ cells (CLL B cells). Using triple (CD3/IL-17A/Ki-67) immunofluorescence staining, we observed that Th17 and non-Th17 cells in CLL spleen sections tended to accumulate within proliferation centers (Ki-67High versus Ki-67Low fields), although this did not reach statistical significance (not shown).
Presence of Th17 and non-Th17 interleukin-17A-containing cells in leukemic and non-leukemic lymph nodes
We next analyzed lymph nodes for the presence of Th17 cells by immunofluorescence. Among 14 CLL and 4 non-CLL reactive lymph nodes, we found that Th17 cell percentages varied but were similar in CLL (mean - 0.39%; range, 0.0–1.4 %,) and non-CLL (mean - 0.18%; range, 0.1–0.3 %) lymph nodes (P=0.9). Both CLL and non-CLL lymph nodes exhibited large, brightly staining CD3-IL-17A+ cells. Immunohistochemistry and immunofluorescence analyses indicated that the majority of these cells in CLL lymph nodes co-expressed CD117/IL-17A (mean: 82.7%; range, 43.5–100%) and MCT/IL-17A (mean: 98.4 %; range, 93.8–100%; Online Supplementary Figure S4, panels A and B), consistent with mast cells as a major source of the IL-17A in CLL lymph nodes. CD117+ mast cells were also present in non-CLL lymph nodes, at mean percentages somewhat lower than in the CLL nodes (CD117+IL-17A+ cells - 53.7%, range, 5.9–90%, and MCT+IL-17A+ cells - 76%, range, 50–100%). Few, if any, of the IL 17A+ cells in lymph nodes expressed CD13, CD15, or MPO; furthermore, these cells did not express Ki-67. Therefore, the large, brightly stained IL-17A-expressing cells in CLL and normal lymph nodes are predominantly terminally differentiated, non-cycling mast cells. In CLL lymph nodes, non-Th17 IL-17A+ cells were more plentiful in Ki-67High areas, but Th17 cells were found at equal frequencies within and outside the Ki-67High areas (proliferation centers).
The absolute numbers of circulating Th17 cells in chronic lymphocytic leukemia patients correlates with overall survival
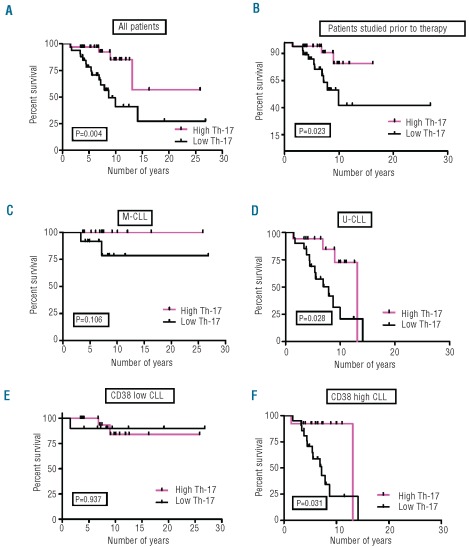
Finally, we divided patients into two groups based on the median number of blood Th17 cells (median=5.6): group 1 with less than 5.6 Th17 cells (Th17Low; n=33) and group 2 with ≥5.6 Th17 cells (Th17High; n=33). Notably, patients in group 2 had a significantly longer survival than those in group 1 (P=0.004; Figure 4A); specifically, the median overall survival of the Th17High CLL patients (group 2) was not reached, whereas it was 8.7 years for the Th17Low patients (group 1). Since certain treatments can alter Th17 cell numbers,34,35 we compared overall survival in patients whose samples were taken prior to any therapy (n=55) (Figure 4B). Overall, patients whose PBMC were collected before therapy and who fell into the Th17High category (n=28) had a significantly longer median overall survival compared to that of Th17Low (n=27) CLL patients (not reached versus 9.96 years; P=0.023).
Figure 4.
Relationship between absolute numbers of circulating Th17 cells and survival of CLL patients. (A) Correlation of absolute numbers of Th17 cells with survival in all patients. Patients were divided into two groups based on the median value of absolute numbers of Th17 cells: Th17Low (<5.6, Group 1; n=33) and Th17High (≥5.6, Group 2; n=33). Th17High patients had a longer median overall survival (OS) (P=0.004) compared to Th17Low patients. Median survival for Th17High patients was not reached, while it was 8.7 years for Th17Low patients. (B) Correlation of absolute numbers of Th17 cells with survival in those patients who were untreated at the time of blood collection. Patients whose blood was collected prior to any treatment were divided into two groups based on the median value of absolute number of Th17 cells as above: Th17Low (<5.8; n=27) and Th17High (≥5.8; n=28). Th17High patients had a significantly longer median OS (P=0.023) compared to Th17Low patients. Median survival for Th17High patients was not reached, but was 9.96 years for Th17Low untreated patients. (C) Correlation of absolute numbers of Th17 cells with survival in all M-CLL patients. Median survival for both M-CLL/Th17Low (n=28) and M-CLL/Th17High (n=12) subgroups was not reached. (D) Correlation of absolute numbers of Th17 cells with survival in all U-CLL patients. U-CLL patients with higher levels of Th17 cells (n=17; median OS 13.1 years) had a significantly longer median OS compared to Th17Low U-CLL patients (n=20; median OS 7.87 years; P=0.028). (E) Correlation of absolute numbers of Th17 cells with survival in all CD38Low CLL patients. Median survival for both CD38Low Th17Low (n=10) and CD38Low Th17High (n=20) subgroups was not reached. (F) Correlation of absolute numbers of Th17 cells with survival in all CD38High CLL patients. CD38High CLL patients with higher levels of Th17 cells (n=13; median OS 13.1 years) had a significantly longer median OS compared to Th17Low CD38High patients (n=21; median OS 7.18 years; P=0.031).
When U-CLL and M-CLL patients were considered separately and each group was subdivided based on Th17 cell numbers and then analyzed for survival, higher Th17 levels were associated with better survival in both groups (Figure 4C and 4D). When CD38High and CD38Low patients were similarly analyzed, higher Th17 numbers were associated with better survival in the CD38High group (Figure 4E and 4F). It is noteworthy that U-CLL/Th17High patients and CD38High/ Th17High patients had a significantly better outcome compared to U-CLL/Th17Low and CD38High/Th17Low patients (P=0.028; Figure 4D and P=0.031; Figure 4F). These correlations lost significance when patients whose samples were acquired after treatment were removed (Online Supplementary Figure S5B and S5D), possibly due to decreased numbers of patients in the analysis. For M-CLL and CD38Low patients, time-to-first treatment was not significantly different between Th17High and Th17Low patients and the median overall survival was not reached in either subgroup of patients (Figure 4C and 4E).
Furthermore, Th17High patients (group 2) were more likely to express better prognostic markers (mutated IGHV and low percentages of CD38+ CLL cells; Online Supplementary Table S1). Conversely, consistent with their shorter survival (Figure 4A), Th17Low patients (group 1) tended to have more advanced Rai stages and less favorable karyotypes (del17p, del11q; Online Supplementary Table S1). The absolute number of Th17 cells was a significant prognostic marker in univariate, but not multivariate, analysis (data not shown).
Discussion
The biological and clinical relevance of microenviron-mental networks is under intense investigation in CLL. In this study, we demonstrated that the absolute numbers of blood Th17 cells are significantly higher in CLL patients than in healthy subjects. We also found that the levels of Th17 cells among dissociated spleen cells were often higher than those in accompanying peripheral blood samples. Furthermore, we identified non-Th17 cells expressing IL-17A in CLL spleens; these IL-17A+ non-Th17 cells were of myeloid origin, representing both immature granulocytes and mature mast cells. When these three types of IL-17A+ cells were examined in CLL and non-CLL lymph nodes, no significant differences were found. Finally, we demonstrated that higher absolute numbers of circulating Th17 cells correlated with more favorable prognostic markers and longer median overall survival in CLL.
We not only documented, for the first time, a positive correlation between circulating Th17 cell numbers and survival in CLL (Figure 4A and 4B), we also observed this for U-CLL and CD38High patients (Figure 4D and 4F), suggesting that Th17 cells exert direct or indirect anti-tumor actions in CLL, even in patients with more aggressive disease. Of note, elevated levels of Th17 cells have been found in patients with a favorable response to therapy in breast and prostate cancer and melanoma,36 and acute myeloid leukemia.28 Eleven of our 66 patients analyzed had received treatment (Online Supplementary Table S1), raising the possibility that therapy could influence these numbers, and indeed certain treatments, such as lenalidomide, can lead to higher Th17 levels,34 whereas others, such as rituximab, can lower Th17 cell numbers.35 However, because treated patients were equally distributed between the Th17High and Th17Low subsets (Online Supplementary Table S1) and because absolute numbers of Th17 cells were significantly higher for patients whose samples were taken before receiving any form of therapy when compared to healthy subjects (P<0.05), treatment is unlikely to be a major cause of the high Th17 findings in our study. Furthermore, because Th17 cells secrete a variety of cytokines (e.g., IL-17A, IL-17F, CCL20, IL-21, and IL-22), the beneficial effects in CLL could be due to the actions of any one or a combination of these. Previous reports indicated that three patients with CLL who responded to lenalidomide had higher levels of Th17 cells and lower levels of Treg34 and that IL-21, which can be secreted by Th17 cells, promotes apoptosis of CLL cells.37 Our preliminary analysis (not shown) on IL-17 receptor family members (RA, RB, RC and RD) suggest that IL-17 receptors are expressed at varying levels on CLL and normal B cells, T cells, and monocytes, in increasing order. Thus, Th17 cells might affect CLL cells directly or indirectly via T cells and monocytes, inhibiting leukemic cell proliferation, Treg formation or action, and angiogenesis or by promoting apoptosis of CLL cells.
It is intriguing that among the Th17Low subgroup there were eight patients with del17p and/or del11q (Online Supplementary Table S1), six of whom died, whereas of the six patients with the same genotypes (determined by fluorescent in situ hybridization) in the Th17High group, only two died. Thus, Th17 cells may favorably modify the clinical course of patients with CLL, regardless of prognostic or genetic subgroup; this is consistent with our demonstration that IL-17 as well as IL-6 and IL-1β, which promote human Th17 cell differentiation, are members of clusters of cytokines that correlate with better prognosis in CLL.31
Because secondary lymphoid tissues play essential roles in CLL cell activation and proliferation,38 we determined the frequency of Th17 cells in spleens and lymph nodes from CLL patients and healthy subjects. Th17 cells were identified among dissociated splenic mononuclear cells and in situ in fixed tissues. When we analyzed paired samples of CLL spleen mononuclear cells and PBMC, we found more Th17 cells in the spleen mononuclear cells in six out of eight instances (Figure 2B), suggesting that the cytokine milieu in CLL spleens promotes Th17 cell recruitment and/or expansion. Th17 cells were also identified in formalin-fixed, paraffin-embedded spleen tissues (Figure 2D), often localizing within proliferation centers, indicating that Th17 cells are present at sites within CLL spleens where they might directly or indirectly influence CLL cell proliferation.
In addition to Th17 cells, we identified non-Th17 IL-17A+ cells of the myeloid lineage in CLL spleens (Figure 2-D). The majority of these were maturing granulocytes, many of which were proliferating (Figure 3A); a minority were mature, resting mast cells. Our analysis revealed that non-Th17 IL-17A+ cells were present at higher frequency within proliferation centers in CLL spleens. Furthermore, these IL-17A-containing cells were frequently located as aggregates near and adjacent to vessels (Online Supplementary Figure S3), perhaps because IL-17 can promote angiogenesis.18
Although present in lower numbers than the granulocytic IL-17A+ cells, most mast cells in CLL spleens contained IL-17A (Figure 3D). Expression of IL-17A by spleen mast cells was not found in normal individuals, suggesting that the splenic microenvironment in CLL supports IL-17A production by these myeloid lineage cells. Although mast cells in synovial tissues of patients with rheumatoid arthritis can produce IL-17,33 published reports on IL-17A+ mast cells in human tumor microenvironments are lacking.
Expression of IL-17A in cells of the granulopoietic pathway has not been reported in human cancers, although such cells were found in human atheromatous plaques.7 It has been reported that myeloid-derived suppressor cells can express IL-17 and promote tumor development in a murine hepatocarcinoma model.39 Because all CLL spleens had higher numbers of immature myeloid cells than spleens from healthy individuals, a previously unrecognized splenic granulopoiesis may occur in CLL. Since we did not detect erythroid precursors in CLL spleens (not shown), we do not believe that this granulopoiesis was a manifestation of extramedullary trilineage hematopoiesis. It is not known whether myeloid cells developing in bone marrow during normal granulopoiesis produce IL-17A or whether this only occurs in disease, with or without granulocyte colony-stimulating factor (G-CSF). Of note, we did not detect IL-17A+ cells in spleens from patients with documented extramedullary hematopoiesis secondary to non-neoplastic diseases (not shown). Although the role of myeloid-derived IL-17A+ cells in CLL spleens is unclear, these cells may mediate a feed-forward process, because IL-17 can promote G-CSF production resulting in granulopoiesis19 and the spleens we found to have higher percentages of IL-17A+ cells were in two patients who had received G-CSF. Although elevated levels of IL-17A are associated with autoimmune diseases,5 only one of the CLL patients whose spleens we studied exhibited autoimmune phenomena.
Conversely, within CLL and non-CLL lymph nodes, almost all non-Th17 IL-17A+ cells were mast cells (Online Supplementary Figure S4), with only a few cells of the granulocytic lineage. In general, these IL-17A+ mast cells localized to subcapsular regions of lymph nodes and did not differ in frequency. Unlike normal spleens in which mast cells did not produce IL-17A, those in normal lymph nodes did, again suggesting a unique pro-IL-17 microenvironment in CLL spleens. The literature regarding IL-17A+ mast cells in tumor microenvironments is sparse, and there are no reports of IL-17+ mast cells in CLL. Th17 cell numbers in CLL and normal lymph nodes were similar.
In conclusion, this study explored Th17 cell numbers in CLL, documented their association with a more favorable clinical course, and provided evidence for phenotypic heterogeneity (Th17 and non-Th17 myeloid cells) of IL-17A+ cells in CLL microenvironments (peripheral blood, spleen, and lymph node). Mechanisms by wich Th17 cells and IL-17A (and possibly other isoforms) influence CLL cells, locally and/or systemically, and whether these mechanisms mediate direct or indirect effects need to be determined. The roles of non-Th17 IL-17A-producing myeloid cells and mast cells expressing IL-17A, and the splenic granulopoiesis that appears to occur in certain CLL spleens, also need to be pursued.
Supplementary Material
Acknowledgments and funding
Wtudies were supported by the Karches Foundation, the Prince Family Foundation, the Marks Foundation, the Jerome Levy Foundation, the Leon Levy Foundation, the Andrew and Mona Albert Fund, Inc., and the Joseph Eletto Leukemia Research Fund. The authors extend special thanks to Sophia Yancopoulos for critical reading of the manuscript, Stella Stefanova for helping with flow cytometry, and Dr. Tarush Kothari for providing valuable tissue samples.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114(16):3367–75. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2(12):933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 3.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y-H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miossec P. Diseases that may benefit from manipulating the Th17 pathway. Eur J Immunol. 2009;39(3):667–9. doi: 10.1002/eji.200839088. [DOI] [PubMed] [Google Scholar]
- 6.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, van Maldegem F, et al. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol. 2010;220(4):499–508. doi: 10.1002/path.2667. [DOI] [PubMed] [Google Scholar]
- 8.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182(9):5296–305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien RL, Roark CL, Born WK. IL-17-producing gammadelta T cells. Eur J Immunol. 2009;39(3):662–6. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, et al. Cutting edge: mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184(7):3336–40. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 12.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 13.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 14.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 15.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5(6):325–31. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- 16.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183(7):4169–75. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 17.Maniati E, Soper R, Hagemann T. Up for Mischief? IL-17/Th17 in the tumour microenvironment. Oncogene. 2010;29(42):5653–62. doi: 10.1038/onc.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, et al. Tumor-infiltrating IL-17-producing gamma delta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40(7):1927–37. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 19.Jovcic G, Bugarski D, Krstic A, Vlaski M, Petakov M, Mojsilovic S, et al. The effect of interleukin-17 on hematopoietic cells and cytokine release in mouse spleen. Physiol Res. 2007;56(3):331–9. doi: 10.33549/physiolres.930944. [DOI] [PubMed] [Google Scholar]
- 20.Noonan K, Marchionni L, Anderson J, Pardoll D, Roodman GD, Borrello I. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116(18):3554–63. doi: 10.1182/blood-2010-05-283895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175(10):6676–85. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 23.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69(3):348–54. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50(5):980–9. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Dhodapkar KM, Barbuto S, Matthews P, Kukreja A, Mazumder A, Vesole D, et al. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17–1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112(7):2878–85. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115(26):5385–92. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Wang S, Wang F, Chen Q, Peng S, Zhang Y, et al. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clin Exp Immunol. 2009;158(2):199–204. doi: 10.1111/j.1365-2249.2009.04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2009;69(13):5522–30. doi: 10.1158/0008-5472.CAN-09-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripodo C, Gri G, Piccaluga PP, Frossi B, Guarnotta C, Piconese S, et al. Mast cells and Th17 cells contribute to the lymphoma-associated pro-inflammatory microenvironment of angioimmunoblastic T-cell lymphoma. Am J Pathol. 2010;177(2):792–802. doi: 10.2353/ajpath.2010.091286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan XJ, Li W, Yancopoulos S, Dozmorov I, Centola M, Allen SL, et al. Identification of distinct cytokine and chemokine clusters that correlate with outcome in B-cell chronic lymphocytic leukemia: implications for disease pathogenesis. Blood. 2010;116(21):1368. [Google Scholar]
- 32.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184(7):3336–40. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 34.Idler I, Giannopoulos K, Zenz T, Bhattacharya N, Nothing M, Dohner H, et al. Lenalidomide treatment of chronic lymphocytic leukaemia patients reduces regulatory T cells and induces Th17 T helper cells. Br J Haematol. 2010;148(6):948–50. doi: 10.1111/j.1365-2141.2009.08014.x. [DOI] [PubMed] [Google Scholar]
- 35.van de Veerdonk FL, Lauwerys B, Marijnissen RJ, Timmermans K, Di Padova F, Koenders MI, et al. The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum. 2011;63(6):1507–16. doi: 10.1002/art.30314. [DOI] [PubMed] [Google Scholar]
- 36.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10(4):248–56. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gowda A, Roda J, Hussain SR, Ramanunni A, Joshi T, Schmidt S, et al. IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody dependent cellular cytotoxicity in primary chronic lymphocytic cells. Blood. 2008;111(9):4723–30. doi: 10.1182/blood-2007-07-099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-{kappa}B activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z, Zhang B, Li D, Lv M, Huang C, Shen GX, et al. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS One. 2010;5(1):e8922. doi: 10.1371/journal.pone.0008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.