Abstract
Background
Phosphatidylserine exposure by red blood cells is acknowledged as a signal that initiates phagocytic removal of the cells from the circulation. Several disorders and conditions are known to induce phosphatidylserine exposure. Removal of phosphatidylserine-exposing red blood cells generally occurs by macrophages in the spleen and liver. Previously, however, we have shown that endothelial cells are also capable of erythrophagocytosis. Key players in the erythrophagocytosis by endothelial cells appeared to be lactadherin and αv-integrin. Phagocytosis via the phosphatidylserine-lactadherin-αv-integrin pathway is the acknowledged route for removal of apoptotic innate cells by phagocytes.
Design and Methods
Endothelial cell phagocytosis of red blood cells was further explored using a more (patho)physiological approach. Red blood cells were exposed to oxidative stress, induced by tert-butyl hydroperoxide. After opsonization with lactadherin, red blood cells were incubated with endothelial cells to study erythrophagocytosis and examine cytotoxicity.
Results
Red blood cells exposed to oxidative stress show alterations such as phosphatidylserine exposure and loss of deformability. When incubated with endothelial cells, marked erythrophagocytosis occurred in the presence of lactadherin under both static and flow conditions. As a consequence, intracellular organization was disturbed and endothelial cells were seen to change shape (‘rounding up’). Increased expression of apoptotic markers indicated that marked erythrophagocytosis has cytotoxic effects.
Conclusions
Activated endothelial cells show significant phagocytosis of phosphatidylserine-exposing and rigid red blood cells under both static and flow conditions. This results in a certain degree of cytotoxicity. We postulate that activated endothelial cells play a role in red blood cell clearance in vivo. Significant erythrophagocytosis can induce endothelial cell loss, which may contribute to vasopathological effects as seen, for instance, in sickle cell disease.
Keywords: phosphatidylserine, red blood cells, activated endothelial cells, HUVEC, lactadherin erythrophagocytosis
Introduction
A variety of hereditary and acquired conditions may shorten the survival of red blood cells (RBC). Premature clearance is often initiated by events causing alteration of RBC membrane integrity. When the induced membrane changes are severe, they result in abrupt intravascular lysis. In case of less acute and milder membrane alterations, RBC undergo a series of events leading to recognition, ingestion and, ultimately, destruction by phagocytes of the reticulo-endothelial system. Several mechanisms have been described by which RBC are removed from the circulation. These include oxidation of band 3, leading to naturally occurring antibody-mediated opsonization followed by Fc-receptor mediated phagocytosis, and programmed or suicidal cell death.1–3 Because RBC lack a nucleus and mitochondria, they do not undergo ‘classic’ apoptosis. However, features of programmed cell death have also been proposed for RBC (‘eryptosis’). These features include cell shrinkage, decreased cell deformability, vesiculation and membrane phospholipid scrambling by translocation of phosphatidylserine (PS) to the outer leaflet.1 The pathophysiological conditions initiating extravascular clearance of RBC are not yet fully elucidated. Loss of membrane phospholipid asymmetry has been recognized as a key trigger that leads to recognition and extravascular removal of senescent, disordered RBC, and transfused RBC that have been stored for a long time.4–6 This is in agreement with the clearance mechanism for apoptotic nucleated cells.7,8 PS exposure, as a result of membrane scrambling, can be induced on RBC in multiple ways such as oxidative damage, (inherited) intracorpuscular defects, infection, intravascular drugs or toxic compounds, and mechanical stress.4,9–11 Ultimately, this initiates recognition and subsequent removal, via phagocytosis, by phagocytes in the spleen and liver.
Upon exposure of PS by apoptotic nucleated cells or suicidal RBC, opsonization by lactadherin [also called milk fat globule epidermal growth factor 8 (MFG-E8) or SED1] is essential for recognition by macrophages and phagocytosis.6,12 Lactadherin is a protein that contains two distinct functional domains: a C-terminal domain that binds to anionic phospholipids such as PS, and an N-terminal domain with two epidermal growth factor (EGF)-repeats.13,14 Lactadherin is expressed by subsets of macrophages,15 and in and around blood vessels.16,17 The second EGF-repeat of lactadherin contains an Arg-Gly-Asp (RGD) motif that binds to αvβ3 and αvβ5 integrin expressed on macrophages, epithelial cells, and vascular cells.12,13,18 The integrin expression density is dependent on cellular origin and state of activation. Phagocytosis via the PS-lactadherin-αv integrin pathway is implicated in the removal of apoptotic innate cells but not in the elimination of foreign targets such as pathogens. Clearance and subsequent processing of innate cells should initiate an immunosuppressive effect rather than an immunostimulatory effect, to prevent autoimmune reactions and inflammation.19 Since endothelial cells, like macrophages, are equipped with all elements of the immunosuppressive PS-lactadherin αv-integrin pathway16,20 it seems reasonable to assume that under certain circumstances they actively engage this pathway. Previously, we showed that endothelial cells are capable of lactadherin-dependent phagocytosis of RGD-modified RBC.16,20 In the present study we provide evidence for endothelial cell erythrophagocytosis via this pathway by reporting on the interactions between PS-exposing RBC and endothelium in in vitro models mimicking (patho)physiological conditions. We show that, in the presence of lactadherin, PS-exposing RBC have severely reduced deformability, and are phagocytosed by endothelial cells under both static and flow conditions. This ultimately results in considerable disturbances of the endothelial cell with occasional lethal damage. Our results indicate that the PS-lactadherin-αv-integrin pathway is actively involved in interactions and phagocytosis of RBC and the endothelium. We postulate that this may have important implications for our understanding of vasopathological events seen in a variety of hematologic disorders such as sickle cell disease and malarial infection.
Design and Methods
Red blood cells
All RBC (blood group O, Rhesus D negative) used in this study were acquired from surplus sealed blood bag tubes from banked RBC units that had been used for transfusion. For all experiments, specimens of three individual units were pooled and the storage solution (SAGM; saline, adenine, glucose, mannitol) was removed upon further usage.
Cell culture
Human umbilical vein endothelial cells (HUVEC) were cultured on EGM™-2 endothelial cell growth medium-2 (Lonza, Verviers, Belgium) consisting of EBM-2 medium supplemented with an EGM-2 bullet kit (containing growth factors, 2% fetal bovine serum and antibiotics). HUVEC were used as an αvβ3-expressing model for activated endothelium up to a passage number of 7.
Lactadherin purification
Bovine lactadherin was purified as previously described.21 Purity was checked by sodium dodecylsulfate polyacrylamide gel electrophoresis and N-terminal amino acid sequencing and shown to be 97–98%, in two glycosylation forms. Protein concentrations were determined by amino acid analysis based on o-phthaldialdehyde derivatization.
Induction and measurement of phophatidylserine exposure
Two different stimuli were used to induce exposure of PS by RBC: calcium ionophore A23187 (Calcimycin, Sigma-Aldrich, St Louis, MO, USA), and tert-butyl hydroperoxide (tBHP, Sigma-Aldrich). Incubation with the A23187 ionophore was performed as described by Kuypers et al.22 In brief, prior to incubation with the A23187 ionophore, RBC membrane flippase activity was inhibited by 10 mM N-ethyl maleimide (NEM, Sigma-Aldrich) for 30 min, followed by incubation for 1 h with 4 or 40 μM ionophore in a calcium (1 mM)-containing buffer. RBC were incubated with 1, 2 or 3 mM tBHP for 30 min on a tube rocker (no NEM pretreatment). All incubations were performed at room temperature. PS on the outer surface of the RBC was stained using bovine lactadherin (0.66 mg/mL, followed by a rabbit anti-bovine lactadherin antibody13 (1:200) and a swine anti-rabbit-FITC antibody (1:200, F0205 DAKO, Glostrup, Denmark), and finally measured on a fluorescence activated cell sorter (FACSCanto II™, BD Bioscences, Franklin Lakes, NJ, USA).
Detection of reactive oxygen species
Reactive oxygen species (ROS) were measured to determine the amount of oxidative stress induced by the cellular treatments described above. Control, ionophore and tBHP-treated RBC were incubated with 5 μM 5- (and 6)-chloromethyl-2′,7′-dichlorodihydro-fluorescein diacetate acetyl ester (CM-H2DCFDA, Invitrogen, Carlsbad, CA, USA) at 2% final hematocrit for 30 min at room temperature. Oxidative stress was detected using a FACSCanto II™ (BD Bioscences).
Red blood cell deformability
RBC deformability was analyzed using a laser-assisted optical rotational cell analyzer (LORCA; Mechatronics, Hoorn, The Netherlands) which measures elongation of the cells at increasing shear stress.23 Ionophore-and tBHP-stimulated RBC samples were diluted 200 times in 0.14 mol/L polyvinylpyrrolidone (PVP, Mw 360,000) in PBS (viscosity, 30 mPa/s). One milliliter of the RBC in PVP suspension (37°C) was transferred into the LORCA measuring system and subjected, fully automatically, to a standardized increase of shear stress. Deformation was expressed as an elongation index, as derived from the resulting ellipsoid diffraction pattern. The deformability curve was obtained by plotting the calculated values for the elongation index versus shear stress (Pa).
Osmotic fragility
RBC samples were diluted 100 times using a range of NaCl concentrations (0.1–12%) and incubated for 30 min at room temperature. After incubation, cells were centrifuged for 5 min at 1000 x g. To calculate the amount of free hemoglobin resulting from lysis of the cells, the supernatant was measured at a wavelength of 450 nm on a Versamax™ microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Quantification of erythrophagocytosis
Erythrophagocytosis was quantified using a colorimetric assay described by Gebran et al.24 This method is based on the pseudoperoxidase activity of hemoglobin. RBC were initially stimulated with ionophore or tBHP to induce PS exposure. Next, 5 μg lactadherin were added to 15 μL (approximately 1.5×108 cells) of packed RBC, and incubated for 30 min at room temperature. Unbound lactadherin was removed by centrifugation, and 2 μL of packed RBC (approximately 2×107 cells) were subsequently added to HUVEC. HUVEC were seeded in 12-well plates (7×104 cells/well) 1 day prior to sample addition. At different time points after incubation with HUVEC at 37°C in 5% CO2, RBC samples were removed, and 500 μL trypsin/EDTA were added immediately to the wells to detach HUVEC. When detached, 1 mL of PBS containing 5% fetal calf serum was added to inactivate the trypsin. Samples were collected into 1.5 mL Eppendorf tubes and spun down (1500 x g, 5 min, room temperature). Supernatant was removed and ery-shock buffer (0.1% NaCl) was added to the pellets for 15 min at room temperature to lyse remaining non-phagocytosed erythrocytes. After centrifugation (1500 x g, 5 min, room temperature), buffer was removed and the pellets remaining were washed three times by resuspending the cells in 1 mL PBS. The HUVEC were lysed, to release their hemoglobin content, by adding 100 μL 0.2 M Tris-HCl buffer containing 6 M urea to the cell pellets, followed by rigorous vortexing and overnight incubation at 4ºC. Three freeze-thaw steps were performed to ensure complete lysis of HUVEC. Next, 100 μL 2,7-diaminofluorene (DAF, Sigma-Aldrich) working solution (containing H2O2 and the Tris-HCl/urea buffer) were added to the samples, which were transferred to a 96-well plate. Finally, absorbance was measured at a wavelength of 620 nm. A hemoglobin standard curve was included to calculate hemoglobin concentrations.
Light microscopy
One day before sample addition HUVEC (7×103 cells/well) were seeded in pre-coated 16-well chamber slides (LAB-TEK; Nunc, Rochester, NY, USA). The wells were pre-coated by incubation with 1% gelatin at 37ºC for 45 min followed by glutaraldehyde 0.05% at 37ºC for 30 min. After incubation with 0.5 μL packed RBC (approximately 5×106 RBC) which had been pretreated with lactadherin (1 μg/3 μL packed RBC), HUVEC were fixed with 2% paraformaldehyde in PBS. Cells were stained with hematoxylin and eosinand mounted in depex:xylene (3:1). Images were taken using a Nikon TE2000 microscope equipped with a Nikon Digital Sight DS-2Mv camera.
Transmission electron microscopy
HUVEC (25 cm2 Petri dish) were incubated for 4 h at 37ºC in 5% CO2, with 12 μL tBHP-treated, packed RBC (approximately 1.2×108 RBC) which had been pre-incubated with bovine lactadherin. Cells were fixed directly after incubation by adding an equal volume of 4% paraformaldehyde/0.4% glutaraldehyde to the culture flask. After 15 min the fixative/medium was replaced by a 2% paraformaldehyde/0.2% glutaraldehyde solution and stored at 4ºC. After washing with PBS, cells were collected in 2% agarose in PBS solution by scraping, followed by centrifugation. Samples were post-fixed for 2 h in 1% OsO4 in 0.1 M cacodylate buffer (pH 7.4), dehydrated by increasing concentrations of ethanol/propylene oxide and embedded in Epon 812. Sections (70 nm) were prepared using a Reichert Ultracut E (Leica, Wetzlar, Germany) and post-stained with uranyl acetate and lead citrate. Sections were viewed on a FEI Tecnai T12 transmission electron microscope (FEI, Hillsboro, Oregon, USA), and digital images were taken using FEI version 3.2 TEM Imaging and Analysis Software.
Perfusion
HUVEC were grown on glass coverslips that were coated overnight with chromosulfuric acid 2%, post-fixed with glutardialdehyde 1% and washed with ethanol 70% and PBS. RBC stimulated with tBHP 3 mM (in the presence or absence of lactadherin) were perfused at 37ºC in a 5% hematocrit suspension over the HUVEC, at a shear rate of 100 s−1. The association of the RBC with HUVEC during perfusion was visualized by differential interference contrast (DIC) microscopy using an Axio Observer microscope (Carl Zeiss, Oberkochen, Germany). The perfused coverslips were fixed under flow (4% paraformaldehyde for 5 min, 100 s−1), followed by static fixation in paraformaldehyde (1 h at room temperature, then overnight at 4ºC). For confocal imaging, the actin cytoskeleton was stained using phalloidin Alexa 488 (1:100) and the RBC were stained for band 3 (mouse anti-band 3, 1:100, Sigma) with secondary donkey anti-mouse-Cy3 antibody (1:100, Jackson Laboratories, Suffolk, UK). Fluorescent signals were visualized using a confocal system (Leica TCS-SP2, Leica, Wetzlar, Germany). Phalloidin 488-labeled actin and Cy3-labeled band 3 were excited by a confocal argon laser. Samples were viewed using an APO 100× oil objective (zoom 2) and images were acquired using Leica Confocal software (LAS-AF).
Cytotoxicity
HUVEC were seeded in 96-well plates (1×104 cells/well) 24 h prior to the addition of the RBC sample. tBHP-stimulated RBC samples were prepared as described above (see ‘Quantification of erythrophagocytosis’) and incubated with HUVEC at 37°C in 5% CO2. After 4 h, RBC samples were gently removed and replaced by plain EGM-2 medium. Twenty-four hours after the start of incubation, HUVEC were detached using trypsin/EDTA. Cells were stained to detect (early) apoptosis with annexin V-FITC (BioVision) and propidium iodide (PI, Sigma) in a buffer containing calcium (2.5 mM) and 5% fetal calf serum. Finally, (double) staining of HUVEC was detected using a FACSCanto™ II flow cytometer (BD Biosciences).
Statistical analysis
Data were analyzed by non-parametric two-way ANOVA with Bonferroni’s post-test using GraphPad Prism 5 for Mac OS X (GraphPad Software, San Diego, CA, USA).
Results
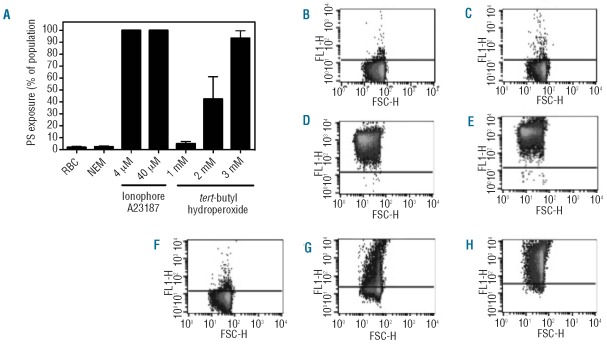
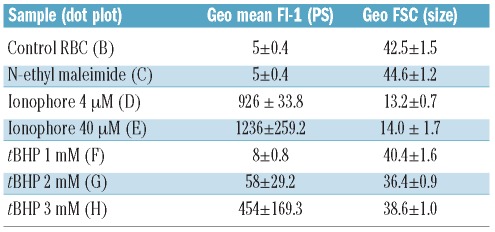
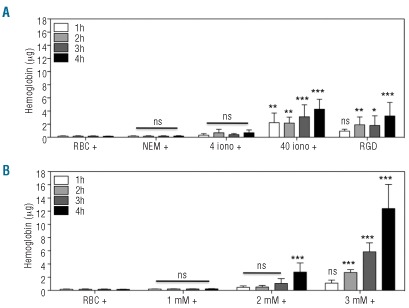
A critical first step for cellular phagocytosis is externalization of PS. RBC were stimulated with ionophore, after pre-treatment with NEM, or with tBHP to induce PS externalization. PS exposure was detected by annexin V-staining using flow cytometry. Untreated control RBC and NEM-treated RBC showed marginal PS exposure (Figure 1A). In contrast, incubation with 4 or 40 μM of the calcium ionophore A23187 resulted in 99.9±0.1% and 99.9±0.0% of the total population of RBC exposing PS. Incubation of RBC with the oxidative damage-inducing compound tBHP25 caused dose-dependent PS exposure, ranging from 4.9±1.9% for 1 mM tBHP, 42.2±18.7% for 2 mM, to 93.4±6.2% for 3 mM tBHP. Calcium ionophore treatment, particularly 40 μM, showed considerably higher geo mean fluorescence intensity (geo MFI) values, indicating a higher number of PS molecules per RBC (Figure 1B-H and Table 1). Furthermore, calcium ionophore-treated RBC demonstrated reduced forward scatter (geo FSC) with a crenated, dehydrated appearance indicating reduced RBC size (Table 1 and Online Supplementary Figure S1).
Figure 1.
Phosphatidylserine (PS) exposure of RBC induced by the calcium ionophore A23187 and oxidative stress (tBHP). (A) Overview of the percentage of PS-exposing RBC after stimulation with the calcium ionophore A23187 or tBHP. Results are the mean ± SD of three independently performed experiments. Typical examples of FACS plots (dot plots) after lactadherin-Alexa 488 staining of (B) control RBC, (C) N-ethyl maleimide (NEM), (D) 4 μM ionophore, (E) 40 μM ionophore, (F) 1 mM tBHP, (G) 2 mM tBHP, and (H) 3 mM tBHP.
Table 1.
Overview of flow cytometry parameters. The geo mean fluorescent intensity (PS exposure of whole population) and the geo forward scatter (FSC; indication for size) value for all treated red blood cell samples.
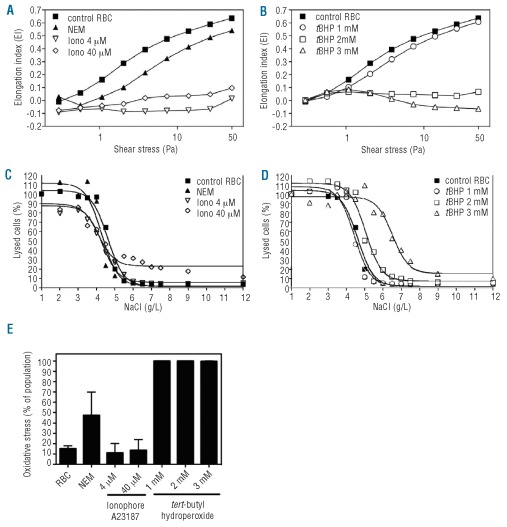
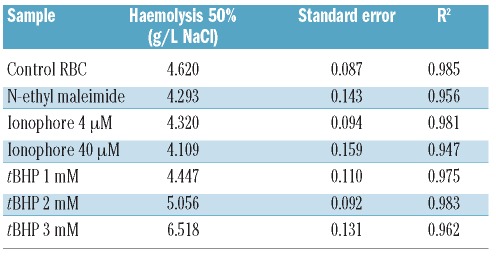
Besides PS exposure, membrane rigidity is a critical determinant of cell phagocytosis20. RBC deformability was determined using a LORCA, which measures elongation of the cells at increasing shear stress. The lower the elongation index, the more rigid and less deformable RBC are. Compared to untreated control RBC, 1 mM tBHP-treated RBC were only slightly less deformable. In contrast, ionophore-, and 2 and 3 mM tBHP-treated RBC showed a severe reduction in RBC deformability (Figure 2A and B). NEM-treated RBC showed only marginally reduced deformability. Another indicator of RBC membrane integrity is the membrane’s ability to withstand osmotic shock. When RBC were stimulated with 40 μM calcium ionophore they showed some increased sensitivity to hypertonic salt concentrations as determined by the osmotic fragility test (Figure 2C, D and Table 2). At hypotonic NaCl concentrations, 2 and 3 mM tBHP-treated RBC showed decreased lysis (50% lysis at 5.1 and 6.5 g/L NaCl, respectively, versus 4.6 g/L for control RBC), indicating that the induced membrane changes altered the osmotic response of these cells. Altogether, these results indicate that both ionophore and tBHP induce PS externalization and affect the RBC membrane integrity. To determine the fraction of RBC that showed oxidative stress upon treatment with either calcium ionophore or tBHP, we measured the amount of ROS using a membrane-permeant fluorescent probe (CM-H2DCFDA), as previously described.26 One hundred percent of the RBC treated with 1, 2 or 3 mM tBHP were positive for ROS. Instead, ROS levels in cells treated with both 4 and 40 μM ionophore were lower (approximately 10%), being similar to those in untreated control cells (Figure 2E).
Figure 2.
Mechanical alterations of RBC induced by calcium ionophore and oxidative stress (tBHP). Changes in RBC deformability analyzed by LORCA measurements. The elongation patterns of RBC stimulated with 4 μM and 40 μM calcium ionophore showed severe loss of deformability (A) while N-ethyl maleimide (NEM)-treated RBC were only slightly less deformable than untreated RBC. RBC treated with 2 mM or 3 mM tBHP also showed severe loss of deformability, whereas 1 mM tBHP showed normal deformability (B). Osmotic fragility studies demonstrated increased hemolysis of 40 μM calcium ionophore-stimulated RBC at hypertonic salt concentrations (C). At hypotonic NaCl concentrations RBC treated with 2 and 3 mM tBHP showed decreased hemolysis, indicating that membrane changes altered the osmotic response of these cells (D). Oxidative stress was measured using a probe for intracellular reactive oxygen species (ROS), CM-H2DCFDA. For each group the percentage of cells that stained positive for oxidative stress is depicted in the graph (E). Red blood cells treated with either 1, 2 or 3 mM tBHP stained 100% of the total population positive for oxidative stress, whereas cells treated with 4 and 40 μM ionophore only showed levels of oxidative stress comparable to those of untreated control cells.
Table 2.
Overview of the degree of hemolysis. All osmotic fragility data were fitted to a sigmoid curve in order to calculate the NaCl concentration at which 50% of RBC lysed.
Subsequently, we investigated whether lactadherin could form a bridge between RBC and the endothelium to facilitate the phagocytosis of the cells. Cultured HUVEC were used to phagocytose calcium ionophore- and tBHP-stimulated RBC in the presence or absence of lactadherin. Lactadherin was added in excess of exposed PS molecules (Online Supplementary Figure S2). Erythrophagocytosis was quantified by measuring the (pseudo)peroxidase activity of internalized hemoglobin. Ionophore-treated RBC pre-incubated with lactadherin showed erythrophagocytosis that increased with dose and time. In absence of lactadherin no intracellular hemoglobin could be detected (Figure 3A). The higher concentration of ionophore (i.e. 40 μM) led to levels of erythrophagocytosis comparable to those of the previously studied RGD-modified RBC.16 Induction of oxidative damage, induced by tBHP treatment, led to a similar dose- and time-dependent increase in erythrophagocytosis as that occurring in the presence of lactadherin (Figure 3B). RBC treated with the highest concentration of tBHP (3 mM) were phagocytosed to a larger extent after 3 or 4 h incubation, compared to RBC treated with 40 μM calcium ionophore (2.5- and 4-fold, respectively). RBC stimulated with 1 mM tBHP were not phagocytosed, even in the presence of lactadherin. Taken together, these results show that both ionophore and tBHP predispose RBC to uptake by endothelial cells via the PS-lactadherin αv-integrin pathway. Since oxidative stress is a well-known (patho)physio-logical condition we used tBHP-treated RBC in subsequent experiments as a model of erythrocytes subject to oxidative stress.
Figure 3.
Erythrophagocytosis by endothelial cells (HUVEC) of PS-exposing RBC induced by the calcium ionophore A23187 and oxidative stress (tBHP). (A) Quantification of erythrophagocytosis by HUVEC after 1, 2, 3, and 4 h of incubation with RBC treated with 4 and 40 μM calcium ionophore in the presence (+) of lactadherin, and RGD-modified RBC16. (B) Quantification of erythrophagocytosis by HUVEC after 1, 2, 3, and 4 h of incubation with tBHP in the presence (+) of lactadherin. Each experiment was independently performed three times. Graphs depict the mean ± SD of three independently performed pseudoperoxidase assays. Statistical analysis was performed in which all groups were compared to the ‘RBC +’ group at the same time point. *, P<0.05; **, P<0.01; ***, P<0.001; ns: not significant.
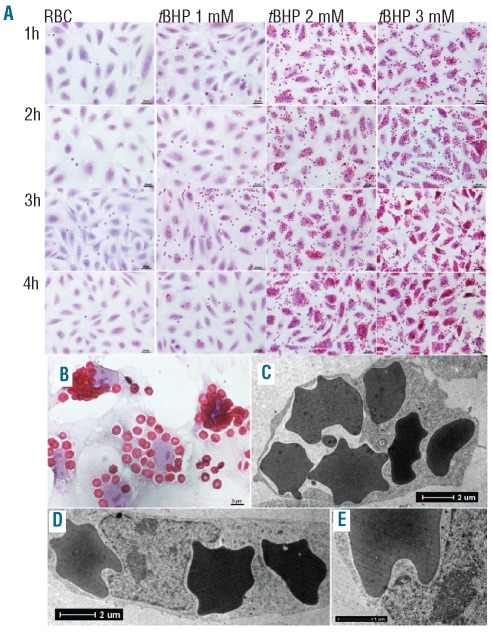
Erythrophagocytosis of tBHP-treated RBC by HUVEC, in the presence of lactadherin, was visualized by microscopy. Control RBC and RBC stimulated with 1 mM tBHP were not internalized by HUVEC (Figure 4A). In contrast, HUVEC show marked phagocytosis of RBC treated with 2 mM or 3 mM tBHP. This uptake increased with time (Figure 4A). As in our RGD-modified RBC model,16,20 multiple RBC were phagocytosed per endothelial cell. Indeed, after treatment with 3 mM tBHP between 5–30 RBC could be seen per HUVEC (Figure 4B). Notably, some of the RBC had an altered, echinocyte-like morphology. Transmission electron microscopy was used to study erythrophagocytosis by HUVEC in more detail. Multiple intracellular RBC could be detected after erythrophagocytosis of 3 mM tBHP-treated RBC. This internalization was accompanied by a change in the shape (‘rounding up’) of HUVEC (Figure 4C) and indentations of their nuclei (Figure 4D and E).
Figure 4.
Imaging of erythrophagocytosis by HUVEC of oxidative stress (3 mM tBHP)-stimulated RBC. (A) Overview of erythrophagocytosis of lactadherin-incubated tBHP-stimulated RBC in time. Large numbers of RBC are phagocytosed, increasing in time, particularly for the RBC stimulated by 2 and 3 mM tBHP. Hematoxylin & eosin (H&E) stained; scale bars represent 20 μm. (B) Close up of HUVEC incubated for 4 h with 3 mM tBHP-stimulated RBC pre-incubated with lactadherin showing large numbers of RBC in each HUVEC. H&E stained; scale bar represents 5 μm. (C) Transmission electron microscopy micrograph showing the intracellular presence of multiple phagocytosed RBC making the HUVEC ‘round up’. Scale bar represents 2 μm. (D) Transmission electron microscopy micrograph showing the intracellular presence of multiple phagocytosed RBC making the nucleus indented. Scale bar represents 2 μm. (E) Close up of image D. Scale bar represents 1 μm.
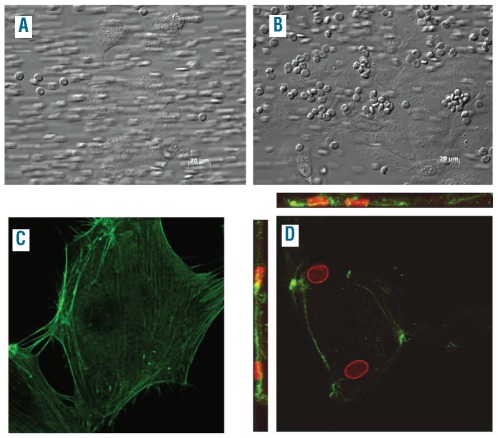
We next investigated whether erythrophagocytosis by the endothelium also occurs under flow conditions. In order to do this, we perfused 3 mM tBHP-treated RBC over a HUVEC monolayer. The flow rate was set at 100 s−1, which is comparable to the velocity of blood in capillaries and small venules. During this experiment, differential interference contrast microscopy images, were taken throughout the perfused area (Figure 5). In absence of lactadherin, the association between tBHP-treated RBC and HUVEC was mostly unstable (Figure 5A). In contrast, pre-incubation with lactadherin caused increased and stable association of RBC with HUVEC (Figure 5B). Similar results were obtained at a higher shear rate (300 s−1), comparable to the velocity of blood in large venules (Online Supplementary Figure S3). Confocal microscopy was used to investigate whether the adherence was followed by internalization. As shown in Figure 5, RBC were not phagocytosed in the absence of lactadherin (Figure 5C), whereas in the presence of lactadherin several intracellular RBC could be detected (Figure 5D).
Figure 5.
Perfusion of oxidative stress (3 mM tBHP)-stimulated RBC over HUVEC. Images taken during and after perfusion (shear rate 100 s−1) of 3 mM tBHP-stimulated RBC over HUVEC, (A) in the absence of lactadherin no association was seen whereas (B) in the presence of lactadherin marked endothelial cell association was seen. Differential interference contrast images; scale bars represent 20 μm. Confocal images taken after perfusion of 3 mM-tBHP stimulated RBC over HUVEC, (C) in the absence of lactadherin no association was seen and (D) in the presence of lactadherin RBC were taken up by HUVEC. Z-stack scans through HUVEC were made to show RBC are internalized by HUVEC. Actin cytoskeleton = green (phalloidin), RBC = red (band 3), magnification 100× (zoom 2).
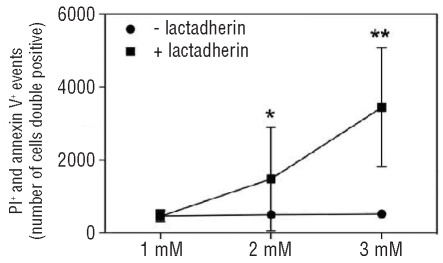
To investigate whether erythrophagocytosis had cytotoxic effects on HUVEC we double stained the endothelial cells with propidium iodide (a cell death marker) and annexin V-FITC (revealing PS exposure, an apoptosis marker), after 24 h incubation with tBHP-treated RBC. HUVEC incubated with 2 mM tBHP-treated RBC, and more so those incubated with 3 mM tBHP-treated RBC, in presence of lactadherin, showed significant cytotoxicity (Figure 6).
Figure 6.
Cytotoxic effect of erythrophagocytosis on HUVEC. Red blood cells (RBC) treated with 3 mM tBHP were pre-incubated with lactadherin and incubated with HUVEC for 4 h. After 24 h cytotoxicity (apoptosis) was measured by staining the HUVEC with both pro-pidium iodide (PI; cell death marker) and annexin V-FITC (PS exposure; apoptosis marker). Generally, cells incubated with RBC that were treated with 3 mM tBHP, in the presence of lactadherin, showed some cytotoxicity. In addition, RBC treated with 2 mM tBHP, also resulting in marked erythrophagocytosis, showed less cytotoxicity; after incubation with 1 mM tBHP-treated RBC no toxicity was seen. The graph depicts mean values and error bars represent ± SD. Statistical analysis: *P<0.05 versus same tBHP concentration without lactadherin; **P<0.001 versus same tBHP concentration without lactadherin.
Discussion
We previously showed that RBC modified with RGD peptides coupled to their outer surface, a model mimicking lactadherin opsonization, led to marked erythrophagocytosis by HUVEC.16,20 To further explore the role of endothelial cells in the removal of lactadherin-opsonized PS-exposing RBC, we induced different pathophysiological changes to the RBC and subsequently examined the interaction with endothelial cells in vitro under both static and flow conditions. In both conditions, RBC that exposed PS and demonstrated loss of membrane integrity and/or deformability, induced by either oxidative damage or a calcium ionophore, were prone to phagocytosis by endothelial cells. Phagocytosis of increasing numbers of RBC caused endothelial cell disturbances and morphological changes. Increased expression of apoptotic markers on endothelial cells indicates that, eventually, this may lead to endothelial cell death.
In order to mimic RBC damage in vivo, RBC were treated with either the calcium ionophore 23187 or the oxidative damage-inducing agent tBHP. Calcium ionophores, widely used to induce PS exposure, lead to a variety of cellular changes, including extensive vesiculation and K+ release. The latter results in cell shrinkage.27 tBHP induces oxidative damage by membrane lipid peroxidation and degradation of hemoglobin28 and represents an established model for oxidative stress.29,30 After incubation with either the calcium ionophore or tBHP, a large fraction of the RBC population exposed PS. This exposure was accompanied by a strong decrease in cellular deformability. Indeed marked changes were observed in the capacity of RBC to withstand osmotic stress, both after calcium ionophore and tBHP treatment. This may be another indication of altered membrane integrity. In particular, RBC exposed to oxidative stress were found to be resistant to hypotonic lysis. This likely reflects increased rigidity of the RBC.
When compared to our model of lactadherin-opsonized RGD-modified RBC,16,20 RBC treated with 40 μM calcium ionophore were internalized by HUVEC to a similar degree. However, when exposed to oxidative stress there was a 4-fold increase in erythrophagocytosis. Since the rigidity of such RBC was substantially increased, these data are in agreement with our previous findings, and those of other groups, that increased rigidity of (PS-exposing) targets is crucial for efficient phagocytosis.20,31
The highest PS exposure and most severe loss of deformability (following treatment with ionophore 4 or 40 μM or tBHP 2 or 3 mM) was associated with the most pronounced erythrophagocytosis, indicating that high levels of PS exposure and a loss of membrane integrity both play important roles. Importantly however, it cannot be excluded that, in addition to PS, other induced RBC membrane changes may play a role in erythrophagocytosis.
Because in vivo RBC likely do not interact with the endothelium only under static conditions we also studied these interactions during flow. Perfusion experiments at a velocity rate comparable to that in microvasculature confirmed the interaction and subsequent erythrophagocytosis by HUVEC of lactadherin-opsonized RBC exposed to oxidative stress. Notably, endothelial cell erythrophagocytosis occurs only for RBC that expose PS. In addition, there appears to be an absolute requirement for lactadherin. Because PS (scavenger)-receptors, described to be involved in the phagocytosis of PS-exposing cells by macrophages, do not require opsonins such as lactadherin,32 these receptors appear not to be involved in endothelial cell erythrophagocytosis.
Rapid clearance of PS-exposing apoptotic cells is crucial to prevent tissue damage resulting from inflammation or autoimmune responses against intracellular antigens released from the dying cells.33–35 In tissues, apoptotic cell recognition, removal and processing usually occurs within a few hours.36 The initial studies on RBC clearance seem to confirm this. A pioneering study by Alan Schroit and coworkers showed that artificial insertion of PS on RBC led to rapid clearance by Kupffer cells and splenic macrophages, with maximum clearance occurring within the first 60 min.37 Importantly, RBC deformability in that study was likely to have been unaltered since PS was artificially inserted into the RBC membranes rather than brought about by physiological mechanisms. This may have delayed clearance. We have shown that PS-exposing RBC with increased rigidity are very efficiently recognized and cleared. This makes it very difficult to detect these cells in the circulation. The numbers of PS-exposing RBC measured in several studies may, therefore, be an underestimation of the true amount of PS-exposing cells. In addition, it is also possible that, before being removed and eliminated, PS is shielded by molecules such as lactadherin.4 For these reasons it is very difficult to detect PS-exposing RBC and study the characteristics required for opsonization and subsequent phagocytosis.
Endothelial cells harboring RBC exposed to oxidative stress showed no clear immediate signs of degradation. The number of engulfed RBC may be important because large numbers of internalized RBC are likely to disturb intracellular processes (through steric effects) and/or induce intracellular release of oxidized free heme. This leads to cellular dysfunction and, eventually, cell death.38 Our results indicate that after 24 h only a fraction of endothelial cells involved in erythrophagocytosis show signs of apoptosis. This may suggest that, up to a certain level, endothelial cells are well capable of internalizing and subsequently processing damaged or aberrant RBC. As for erythrophagocytosis by macrophages, endothelial cells have also been shown to be capable of recycleing iron upon activation by increasing the expression of key enzymes in this pathway, such as heme oxygenase-1 (HO-1), ferroportin and ferritin.39–42 The characteristics of RBC which may affect the fate and rate of processing could include cellular features such as membrane integrity, rigidity and viscosity.
The phagocytes present in liver and spleen are considered to be the major sites of clearance of defective and senescent RBC. Notably, hepatic sinusoidal endothelial cells were recently shown to play a role in the sequestration of PS-exposing damaged RBC in the liver.43 In particular the phagocytic capacity of Küpffer cells in the liver was found to be significantly enhanced by hepatic sinusoidal endothelial cells, mediated by stabilin-1 and stabilin-2. We show here that endothelial cells themselves also exert phagocytic properties, in both static and flow conditions. It is conceivable that especially under conditions of strongly increased demand for RBC clearance, the endothelium becomes involved. Consistent with this, erythrophagocytosis by sinus endothelial cells of the spleen has been described in several cases of hemolytic anemia.44–46 We, therefore, propose a role for activated endothelium in the removal of PS-exposing lactadherin opsonized RBC under (patho)physiological conditions in vivo, such as sickle cell disease.
Acknowledgments
The authors would like to thank Dr. Max R. Hardeman for excellent assistance with the LORCA deformability experiments and Silvie A.E. Sebastian for excellent technical assistance with the perfusion experiments.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Lang F, Lang KS, Lang PA, Huber SM, Wieder T. Mechanisms and significance of eryptosis. Antioxid Redox Signal. 2006;8(7–8):1183–92. doi: 10.1089/ars.2006.8.1183. [DOI] [PubMed] [Google Scholar]
- 2.Lutz HU. Innate immune and non-immune mediators of erythrocyte clearance. Cell Mol Biol (Noisy-le-grand) 2004;50(2):107–16. [PubMed] [Google Scholar]
- 3.Bosman GJ, Willekens FL, Werre JM. Erythrocyte aging: a more than superficial resemblance to apoptosis? Cell Physiol Biochem. 2005;16(1–3):1–8. doi: 10.1159/000087725. [DOI] [PubMed] [Google Scholar]
- 4.Kuypers FA, de Jong K. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell Mol Biol (Noisy-le-grand) 2004;50(2):147–58. [PubMed] [Google Scholar]
- 5.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci USA. 1998;95(6):3077–81. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasgupta SK, Abdel-Monem H, Guchhait P, Nagata S, Thiagarajan P. Role of lactadherin in the clearance of phosphatidylserine-expressing red blood cells. Transfusion. 2008;48(11):2370–6. doi: 10.1111/j.1537-2995.2008.01841.x. [DOI] [PubMed] [Google Scholar]
- 7.Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8(9):365–72. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16(4):189–97. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Kuypers FA. Phospholipid asymmetry in health and disease. Curr Opin Hematol. 1998;5(2):122–31. doi: 10.1097/00062752-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Kempe DS, Akel A, Lang PA, Hermle T, Biswas R, Muresanu J, et al. Suicidal erythrocyte death in sepsis. J Mol Med. 2007;85(3):273–81. doi: 10.1007/s00109-006-0123-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Inukai T, Hirose K, Akahane K, Nemoto A, Takahashi K, et al. Induction of impaired membrane phospholipid asymmetry in mature erythrocytes after chemotherapy. Int J Hematol. 2005;82(2):132–6. doi: 10.1532/IJH97.05009. [DOI] [PubMed] [Google Scholar]
- 12.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 13.Andersen MH, Berglund L, Rasmussen JT, Petersen TE. Bovine PAS-6/7 binds alpha v beta 5 integrins and anionic phospholipids through two domains. Biochemistry. 1997;36(18):5441–6. doi: 10.1021/bi963119m. [DOI] [PubMed] [Google Scholar]
- 14.Raymond A, Ensslin MA, Shur BD. SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem. 2009;106(6):957–66. doi: 10.1002/jcb.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanayama R, Tanaka M, Miwa K, Nagata S. Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol. 2004;172(6):3876–82. doi: 10.4049/jimmunol.172.6.3876. [DOI] [PubMed] [Google Scholar]
- 16.Fens MH, Mastrobattista E, de Graaff AM, Flesch FM, Ultee A, Rasmussen JT, et al. Angiogenic endothelium shows lactadherin-dependent phagocytosis of aged erythrocytes and apoptotic cells. Blood. 2008;111(9):4542–50. doi: 10.1182/blood-2007-06-094763. [DOI] [PubMed] [Google Scholar]
- 17.Silvestre JS, Thery C, Hamard G, Boddaert J, Aguilar B, Delcayre A, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11(5):499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 18.Byzova TV, Rabbani R, D’Souza SE, Plow EF. Role of integrin alpha(v)beta3 in vascular biology. Thromb Haemost. 1998;80(5):726–34. [PubMed] [Google Scholar]
- 19.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–30. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Fens MH, Storm G, Pelgrim RC, Ultee A, Byrne AT, Gaillard CA, et al. Erythrophagocytosis by angiogenic endothelial cells is enhanced by loss of erythrocyte deformability. Exp Hematol. 2010;38(4):282–91. doi: 10.1016/j.exphem.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Hvarregaard J, Andersen MH, Berglund L, Rasmussen JT, Petersen TE. Characterization of glycoprotein PAS-6/7 from membranes of bovine milk fat globules. Eur J Biochem. 1996;240(3):628–36. doi: 10.1111/j.1432-1033.1996.0628h.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD, et al. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87(3):1179–87. [PubMed] [Google Scholar]
- 23.Hardeman MR, Besselink GA, Ebbing I, de Korte D, Ince C, Verhoeven AJ. Laser-assisted optical rotational cell analyzer measurements reveal early changes in human RBC deformability induced by photodynamic treatment. Transfusion. 2003;43(11):1533–7. doi: 10.1046/j.1537-2995.2003.00566.x. [DOI] [PubMed] [Google Scholar]
- 24.Gebran SJ, Romano EL, Pons HA, Cariani L, Soyano AN. A modified colorimetric method for the measurement of phagocytosis and antibody-dependent cell cytotoxicity using 2,7-diaminofluorene. J Immunol Methods. 1992;151(1–2):255–60. doi: 10.1016/0022-1759(92)90125-d. [DOI] [PubMed] [Google Scholar]
- 25.Trotta RJ, Sullivan SG, Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. The relative roles of haem-and glutathione-dependent decomposition of t-butyl hydroperoxide and membrane lipid hydroperoxides in lipid peroxidation and haemolysis. Biochem J. 1983;212(3):759–72. doi: 10.1042/bj2120759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Y, Tilley L, Kenny S, Klonis N. Dual labeling with a far red probe permits analysis of growth and oxidative stress in P. falciparum-infected erythrocytes. Cytometry A. 2010;77(3):253–63. doi: 10.1002/cyto.a.20856. [DOI] [PubMed] [Google Scholar]
- 27.Allan D, Thomas P. Ca2+-induced biochemical changes in human erythrocytes and their relation to microvesiculation. Biochem J. 1981;198(3):433–40. doi: 10.1042/bj1980433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trotta RJ, Sullivan SG, Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. Effects of the hexose monophosphate shunt as mediated by glutathione and ascorbate. Biochem J. 1982;204(2):405–15. doi: 10.1042/bj2040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarang Z, Madi A, Koy C, Varga S, Glocker MO, Ucker DS, et al. Tissue transglutaminase (TG2) facilitates phosphatidylserine exposure and calpain activity in calcium-induced death of erythrocytes. Cell Death Differ. 2007;14(10):1842–4. doi: 10.1038/sj.cdd.4402193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou CG, Agar NS, Jone GL. Oxidative insult in sheep red blood cells induced by t-butyl hydroperoxide: the roles of glutathione and glutathione peroxidase. Free Radic Res. 2001;34(1):45–56. doi: 10.1080/10715760100300051. [DOI] [PubMed] [Google Scholar]
- 31.Beningo KA, Wang YL. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci. 2002;115(Pt 4):849–56. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155(4):649–59. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407(6805):784–8. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 34.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2(12):965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 35.Hengartner MO. Apoptosis: corralling the corpses. Cell. 2001;104(3):325–8. doi: 10.1016/s0092-8674(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 36.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372(6501):100–3. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 37.Schroit AJ, Madsen JW, Tanaka Y. In vivo recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J Biol Chem. 1985;260(8):5131–8. [PubMed] [Google Scholar]
- 38.Cambos M, Scorza T. Robust erythrophagocytosis leads to macrophage apoptosis via a hemin-mediated redox imbalance: role in hemolytic disorders. J Leukoc Biol. 2011;89(1):159–71. doi: 10.1189/jlb.0510249. [DOI] [PubMed] [Google Scholar]
- 39.Delaby C, Pilard N, Hetet G, Driss F, Grandchamp B, Beaumont C, et al. A physiological model to study iron recycling in macrophages. Exp Cell Res. 2005;310(1):43–53. doi: 10.1016/j.yexcr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Balla J, Vercellotti GM, Nath K, Yachie A, Nagy E, Eaton JW, et al. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol Dial Transplant. 2003;18(Suppl 5):v8–12. doi: 10.1093/ndt/gfg1034. [DOI] [PubMed] [Google Scholar]
- 41.Nanami M, Ookawara T, Otaki Y, Ito K, Moriguchi R, Miyagawa K, et al. Tumor necrosis factor-alpha-induced iron sequestration and oxidative stress in human endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25(12):2495–501. doi: 10.1161/01.ATV.0000190610.63878.20. [DOI] [PubMed] [Google Scholar]
- 42.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, et al. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9(12):2119–37. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 43.Lee SJ, Park SY, Jung MY, Bae SM, Kim IS. Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood. 2011;117(19):5215–23. doi: 10.1182/blood-2010-10-313239. [DOI] [PubMed] [Google Scholar]
- 44.Ishihara T, Matsumoto N, Adachi H, Takahashi M, Nakamura H, Uchino F, et al. Erythrophagocytosis by the sinus endothelial cell of the spleen in haemolytic anaemias. Virchows Arch A Pathol Anat Histol. 1979;382(3):261–9. doi: 10.1007/BF00430402. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira JA, Feliu E, Rozman C, Berga L, Bombi JA, Marti M, et al. Morphologic and morphometric light and electron microscopic studies of the spleen in patients with hereditary spherocytosis and autoimmune haemolytic anaemia. Br J Haematol. 1989;72(2):246–53. doi: 10.1111/j.1365-2141.1989.tb07690.x. [DOI] [PubMed] [Google Scholar]
- 46.Jiskoot PM, Halsey C, Rivers R, Bain BJ, Wilkins BS. Unusual splenic sinusoidal iron overload in sickle cell/haemoglobin D-Punjab disease. J Clin Pathol. 2004;57(5):539–40. doi: 10.1136/jcp.2002.004481. [DOI] [PMC free article] [PubMed] [Google Scholar]