Abstract
The spindle assembly checkpoint (SAC) averts aneuploidy by coordinating proper bipolar chromosomal attachment with anaphase-promoting complex/cyclosome (APC/C)-mediated securin and cyclin B1 destruction required for anaphase onset. The generation of a Mad2-based signal at kinetochores is central to current models of SAC-based APC/C inhibition. During mitosis, kinetochores of polar-displaced chromosomes, which are at greatest risk of mis-segregating, recruit the highest levels of Mad2, thereby ensuring that SAC activation is proportionate to aneuploidy risk. Paradoxically, although an SAC operates in mammalian oocytes, meiosis I (MI) is notoriously error prone and polar-displaced chromosomes do not prevent anaphase onset. Here we find that Mad2 is not preferentially recruited to the kinetochores of polar chromosomes of wild-type mouse oocytes, in which polar chromosomes are rare, or of oocytes depleted of the kinesin-7 motor CENP-E, in which polar chromosomes are more abundant. Furthermore, in CENP-E-depleted oocytes, although polar chromosomal displacement intensified during MI and the capacity to form stable end-on attachments was severely compromised, all kinetochores nevertheless became devoid of Mad2. Thus, it is possible that the ability of the SAC to robustly discriminate chromosomal position might be compromised by the propensity of oocyte kinetochores to become saturated with unproductive attachments, thereby predisposing to aneuploidy. Our data also reveal novel functions for CENP-E in oocytes: first, CENP-E stabilises BubR1, thereby impacting MI progression; and second, CENP-E mediates bi-orientation by promoting kinetochore reorientation and preventing chromosomal drift towards the poles.
Keywords: Aneuploidy, CENP-E, Mad2, Meiosis I, Mouse oocytes, Spindle assembly checkpoint
INTRODUCTION
Mad2 recruitment to improperly attached kinetochores is crucial for generating the inhibitory spindle assembly checkpoint (SAC) signal that prevents anaphase-promoting complex/cyclosome (APC/C) activation and anaphase onset (Musacchio and Salmon, 2007). During mitosis, kinetochores of polar chromosomes, which are at greatest risk of mis-segregating, recruit the highest levels of Mad2 (Howell et al., 2000; Waters et al., 1998), thereby tightly coupling SAC activation to aneuploidy risk.
Paradoxically, in spite of possessing an SAC (Hached et al., 2011; Homer et al., 2005b; McGuinness et al., 2009), female meiosis I (MI) remains notoriously error prone (Hassold and Hunt, 2009). An important cause for this vulnerability is the inability of small numbers of polar chromosomes to prevent anaphase onset in oocytes (Nagaoka et al., 2011). Exactly why polar chromosomes should evade the SAC remains unknown, especially as recent data indicate that the oocyte SAC has the capacity to react to even a single unattached chromosome (Hoffmann et al., 2011). Here we address the key issue of how the SAC in oocytes responds to polar chromosomes.
MATERIALS AND METHODS
Oocyte collection and drug treatment
Oocytes were isolated from 4- to 6-week-old pregnant mare serum gonadotropin (PMSG)-primed MF1 mice and cultured as described (Homer et al., 2009). Nocodazole (Sigma) was used at 5 μM (Homer et al., 2005a). Experiments involving animals conformed to the relevant regulatory standards.
Morpholino and cRNA injection
For CENP-E depletion, germinal vesicle stage oocytes were microinjected with a morpholino designed to target mouse Cenpe (NM_173762) designated CENPEMO (5′-CAGCCACTGAAGCCTCCTCGGCCAT-3′; Gene Tools) and maintained for 20-24 hours in isobutylmethylxanthine (IBMX)-treated medium. Mad2MO, ControlMO (for mock depletions) and human BUBR1 cRNA were described previously (Homer et al., 2009; Homer et al., 2005b). Morpholinos were microinjected at 2 mM. For double depletions, combinations of morpholinos (4 mM stock) were microinjected.
Immunocytochemistry
Immunofluorescence and cold treatment (4°C for 10 minutes) were performed as described previously (Homer et al., 2009). Primary antibodies included β-tubulin (Sigma); ACA (ImmunoVision); γ-tubulin (Abcam); CENP-E (Dr T. Yen, Fox Chase Cancer Center, USA); BubR1 (Dr Stephen Taylor, University of Manchester, UK) and Mad2 (Dr K. Wassmann, CNRS UMR7622, France). Secondary antibodies (Invitrogen) included Alexa Fluor 488- or 546-labelled goat anti-human; Alexa Fluor 633- or 488-labelled goat anti-mouse; Alexa Fluor 488- or 546-labelled goat anti-rabbit; and Alexa Fluor 488-labelled goat anti-sheep. DNA was stained using Hoechst 33342 (10 μg/ml; Sigma). Images were captured using a Zeiss LSM510 META confocal microscope configured as follows: for Hoechst 33342, 364 nm UV laser excitation combined with a 385-470 nm band-pass emission filter; for Alexa Fluor 488, 488 nm argon laser line combined with a 505-550 nm band-pass emission filter; for Alexa Fluor 546, 543 nm helium/neon1 laser combined with a 560-615 nm band-pass emission filter; and for Alexa Fluor 633, a 633 nm helium/neon2 laser with a 650 nm long-pass emission filter.
Western blotting
Pre-cast 3-8% Tris-acetate gels (Invitrogen) and a mouse monoclonal anti-CENP-E antibody (Abcam) were used for CENP-E. BubR1, securin, GAPDH and actin immunoblotting were performed as described (Homer et al., 2009; Homer, 2011). HRP-conjugated antibodies were detected using ECL Plus (GE Healthcare) and protein bands were semi-quantitatively assayed (Homer et al., 2009).
RESULTS AND DISCUSSION
Mad2 is not overtly enriched at the kinetochores of polar bivalents in wild-type oocytes
In mouse oocytes, MI lasts ∼8-10 hours, beginning with germinal vesicle breakdown (GVBD) and concluding with first polar body extrusion (PBE), when recombined homologous chromosomes (bivalents) segregate (Homer et al., 2009; Homer et al., 2005b). During this time, numerous microtubule-organising centres nucleate a spindle, which is gradually remodelled into a bipolar form (Schuh and Ellenberg, 2007). As polar chromosomes constitute an important focus of the SAC, we first determined when bipolarity was established. Using strict criteria, we found that bipolarity was not established until ∼6 hours post-GVBD (supplementary material Fig. S1A,B). Also, consistent with recent data (Kitajima et al., 2011), we found that kinetochores reoriented to face in opposite directions by 6 hours post-GVBD (supplementary material Fig. S1C-E), beyond which time less than 5% of oocytes (n>150) possessed severely displaced polar bivalents, entirely in keeping with previous results (Kitajima et al., 2011; Yang et al., 2010).
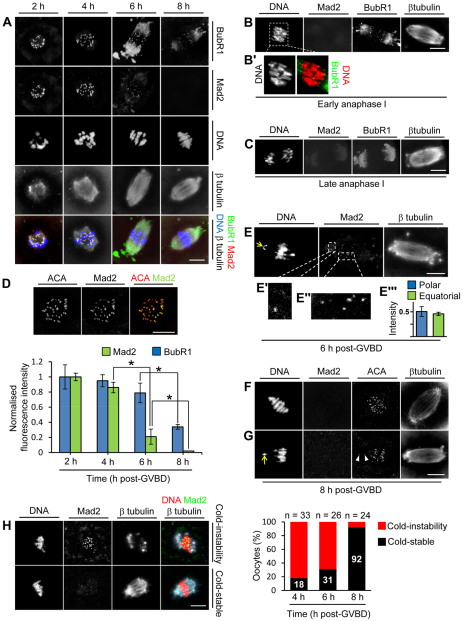
Consistent with previous results (Kitajima et al., 2011), we found that kinetochore levels of Mad2 (Mad2l1 – Mouse Genome Informatics) declined during MI (Fig. 1A,D). Additionally, by focusing on the stage during which the spindle was bipolar, we were now able to compare Mad2 recruitment to equatorial and polar bivalents. Significantly, low levels of Mad2 were retained at many equatorial bivalents by 6 hours post-GVBD (when the spindle first becomes bipolar), and there was an additional ∼2 hours before complete Mad2 displacement (Fig. 1A,D). Strikingly, among the few oocytes with severely polar-displaced bivalents at 6 hours post-GVBD, Mad2 decorated kinetochores of both equatorial and polar bivalents to a similar degree (Fig. 1E-E″′). Furthermore, by 8 hours post-GVBD, all kinetochores completely lacked Mad2 (Fig. 1A,F), even when polar bivalents were present (Fig. 1G). Collectively, this represented a marked departure from the mitotic template in which polar-displaced kinetochores retain high levels of Mad2 and the attainment of an equatorial chromosomal position is promptly followed by complete loss of Mad2 (Hoffman et al., 2001; Howell et al., 2000; Waters et al., 1998).
Fig. 1.
Equatorial location and K-fibre formation are not prerequisites for Mad2 displacement. (A-C) z-projections of mouse oocytes immunostained for BubR1, Mad2 and β-tubulin. DNA was stained using Hoechst 33342. (D) Quantification of kinetochore Mad2 and BubR1. Oocytes were double labelled for Mad2 or BubR1 plus anti-centromere antibody (ACA) at all four time points on the same day and z-stacks were acquired using identical settings within a subvolume spanning the entire kinetochore-containing region as illustrated. Background-corrected total integrated fluorescence intensity for a region of interest (ROI) was determined at ACA foci and for the corresponding ROI in the Mad2 and BubR1 channels. Mad2:ACA and BubR1:ACA ratios were determined for more than 200 kinetochores per time point (three experiments) and normalised to the intensity at 2 hours post-GVBD. Data are mean ± s.e.m.; *P<0.05 by Student’s t-test. (E-E″′) An oocyte at 6 hours post-GVBD shows a polar-displaced bivalent (arrow) with levels of Mad2 (E′) that are comparable to those at equatorial bivalents (E″). (E″′) Mad2 fluorescence intensities at polar kinetochores (n=8) and equatorial kinetochores (n=73) from four oocytes at 6 hours post-GVBD. Data were normalised to the maximal intensity within each oocyte. Data are mean ± s.e.m. (F,G) Mad2 is undetectable by 8 hours post-GVBD regardless of chromosomal position. Note the absence of Mad2 at kinetochores (arrowheads, G) of a polar-displaced bivalent (arrow, G). (H) Cold-stable microtubule content. Immunostained images depict two phenotypes after cold treatment: the ‘cold-instability phenotype’ (microtubule depolymerisation and high Mad2) and the ‘cold-stable phenotype’ (stable microtubules and low Mad2). Scale bars: 10 μm.
Displacement of the bulk of Mad2 occurs in spite of low K-fibre content
We next investigated why such a protracted interval elapsed before Mad2 became completely displaced from bivalents that were equatorial and bi-oriented. During mitosis, microtubules that form stable end-on attachments with kinetochores (K-fibres) are crucial for displacing Mad2 from bi-oriented chromosomes (Hoffman et al., 2001; Putkey et al., 2002). We therefore asked whether K-fibre content differed between 6 and 8 hours post-GVBD. K-fibres impart tension across kinetochores (Deluca et al., 2002) and are differentially stable to cold treatment (Rieder, 1981). We found that cold treatment induced rudimentary spindles and Mad2 re-recruitment to kinetochores in ∼60% and ∼10% of oocytes at 6 and 8 hours post-GVBD, respectively (Fig. 1H). Next, by measuring inter-kinetochore distances, we found that tension became maximal at 8 hours post-GVBD (supplementary material Fig. S2), coincident with which, kinetochore BubR1 (Bub1b – Mouse Genome Informatics) levels, which are well known to become depleted in response to tension (Skoufias et al., 2001; Uchida et al., 2009), declined significantly (Fig. 1A,D). As with mitotic BubR1, which declines but does not disappear at metaphase (Hoffman et al., 2001), BubR1 persisted at kinetochores by early anaphase I (Fig. 1B,B′) before disappearing by late anaphase I (Fig. 1C). Overall, these data are consistent with previous analyses showing late K-fibre formation in oocytes (Brunet et al., 1999). Importantly, however, by using two independent measures for K-fibres we could now quantify K-fibre content and correlate this with kinetochore Mad2 levels specifically during the bipolar stage.
Intriguingly, these data now show that by 6 hours post-GVBD, when the majority of kinetochore Mad2 was displaced, most oocytes were deficient in K-fibres, pointing to an unusually high propensity for kinetochores to acquire microtubule interactions – that is, even unstable interactions sufficed for displacing Mad2. We hypothesized that this unusual propensity could account for the lack of preferential Mad2 recruitment to the small numbers of polar bivalents in wild-type oocytes, thereby compromising biased SAC activation at polar bivalents.
CENP-E depletion induces polar bivalents and unstable kinetochore-microtubule interactions
In order to test our hypothesis, we sought conditions under which unstable kinetochore-microtubule interactions and polar chromosomes were prevalent, two features that characterise mitotic cells deficient for the plus-end-directed kinesin-7 motor CENP-E (Putkey et al., 2002). We found that CENP-E undergoes net synthesis and localises to kinetochores until metaphase I, before relocating to the spindle midzone at anaphase I (supplementary material Fig. S3).
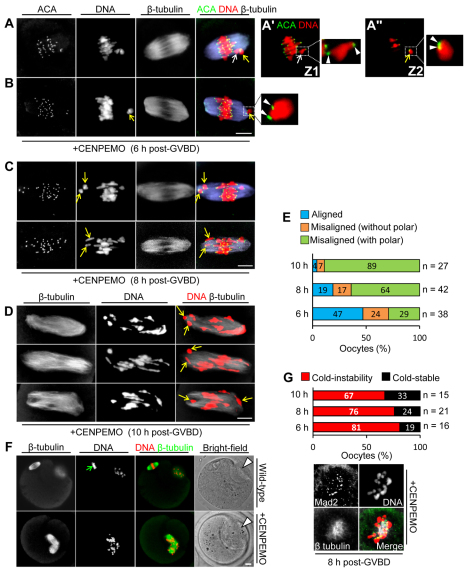
To evaluate CENP-E function, we depleted CENP-E using a morpholino antisense approach (supplementary material Fig. S4). We found that 93% (n=73) of CENP-E-depleted oocytes assembled a bipolar spindle by 6 hours post-GVBD. During the delayed MI transit observed after CENP-E depletion (discussed below), the proportion of oocytes exhibiting severely polar-displaced chromosomes almost trebled (Fig. 2A-E), suggesting that CENP-E-depleted oocytes were unable to restrain bivalents at the equator. Also, 37% (n=38) and 40% (n=42) of bivalents at 6 and 8 hours post-GVBD, respectively, possessed juxtaposed kinetochores following CENP-E depletion (Fig. 2A,B), indicating that CENP-E was required for kinetochore reorientation and that failure to do so contributed to misalignment. Predictably, among CENP-E-depleted oocytes that exited MI, gross misalignment persisted at meiosis II (MII) (Fig. 2F). Notably, ∼70-80% of CENP-E-depleted oocytes chronically lacked prominent cold-stable microtubules (Fig. 2G), consistent with the known role of CENP-E in stabilising kinetochore-microtubule interactions (Putkey et al., 2002). Overall, therefore, following CENP-E depletion, unstable kinetochore-microtubule interactions predominate and polar bivalents are frequent.
Fig. 2.
Polar chromosomal displacement and unstable kinetochore-microtubule interactions following CENP-E depletion. (A-D) z-projections of immunostained CENP-E-depleted mouse oocytes. In the oocyte shown in A, compact (yellow arrow) and extended (white arrow) bivalents are displaced from the midline, as more clearly seen in magnified views of separate z-sections (Z1, A′ and Z2, A″) (see also supplementary material Fig. S1C-E). In B, a polar compact bivalent with juxtaposed kinetochores (arrowheads) is magnified. By 8 hours (C) and 10 hours (D) post-GVBD, polewards bivalent displacement becomes increasingly severe (arrows). (E) Proportions of oocytes bearing (1) all aligned chromosomes, (2) chromosomes displaced from the main equatorial group but not severely polar-displaced (e.g. A), and (3) severely displaced polar bivalents (e.g. B-D) were determined at 6, 8 and 10 hours post-GVBD. (F) MII-arrested oocytes. Note the polar bodies (arrowheads) and tight chromosomal alignment in the wild-type oocyte (green arrow). (G) Cold-stable microtubule content after CENP-E depletion. Percentages of ‘cold-stable’ and ‘cold-instability’ phenotypes (see Fig. 1H) at 6, 8 and 10 hours post-GVBD. Depicted is a typical cold-treated CENP-E-depleted oocyte exhibiting spindle collapse and strong Mad2 recruitment. Scale bars: 10 μm.
Mad2 is not enriched at polar bivalents and becomes completely displaced by unstable attachments after CENP-E depletion
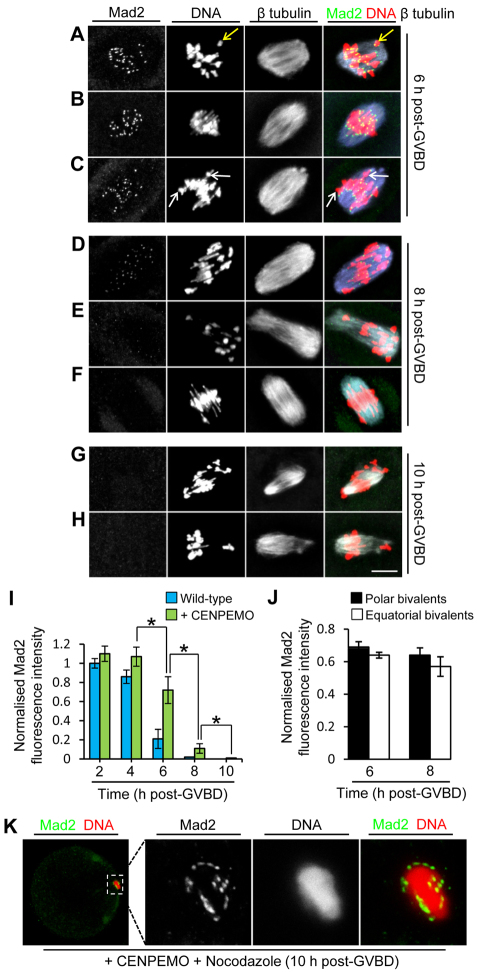
Although mean Mad2 intensity declined between 2 and 8 hours post-GVBD following CENP-E depletion, Mad2 displacement was delayed by ∼2 hours compared with wild-type oocytes (Fig. 3A-I), and this was likely to reflect the reduced efficiency of kinetochore-microtubule interactions. Surprisingly, even with marked misalignment, there was no discernible difference in Mad2 fluorescence ascribable to chromosomal position at 6 hours (Fig. 3A-C) or 8 hours (Fig. 3D-F) post-GVBD. Instead, as with wild-type oocytes, Mad2 declined uniformly across kinetochores without any evidence of selective retention at polar chromosomes (Fig. 3J). Strikingly, although only about one-third of CENP-E-depleted oocytes exhibited cold-stable microtubules, kinetochores still became completely devoid of Mad2 by 10 hours post-GVBD (Fig. 3G-I). Mad2 dissociation was dependent upon kinetochore-microtubule interactions, as Mad2 was re-recruited following spindle depolymerisation with nocodazole (n=12; Fig. 3K). Thus, polar kinetochores in oocytes can become saturated with non-K-fibre attachments, contrasting sharply with CENP-E-deficient mitotic cells in which compromised K-fibre formation culminates in chronic and biased Mad2 recruitment to polar-displaced kinetochores (Putkey et al., 2002). Collectively, these data strongly support the contention that oocyte kinetochores bind microtubules relatively easily, and that this promotes Mad2 dissociation and severely compromises the ability to selectively direct SAC components to polar bivalents.
Fig. 3.
CENP-E-depleted kinetochores become devoid of Mad2 in spite of polar displacement. (A-C) By 6 hours post-GVBD, most kinetochores retain Mad2 that is equally prominent at equatorially located bivalents (B) as at polar-displaced bivalents regardless of whether bivalents are compact (yellow arrow, A) or extended (white arrows, C). (D-F) By 8 hours post-GVBD, low Mad2 levels are detectable in some oocytes (D), but Mad2 is undetectable in others (E,F). Where Mad2 is detectable, there is no discernible difference between equatorial and polar bivalents (D). (G,H) By 10 hours post-GVBD, Mad2 is completely undetectable, including at severely poleward-displaced bivalents. (I,J) Kinetochore Mad2 levels in wild-type and CENP-E-depleted oocytes (I) and at polar and equatorial bivalents in CENP-E-depleted oocytes (J). Intensities were normalised either to values at 2 hours post-GVBD in wild-type oocytes (I) or to maximal intensities in individual oocytes (J). Data are mean ± s.e.m.; *P<0.05 by Student’s t-test). (K) Mad2 becomes re-recruited in CENP-E-depleted oocytes treated with nocodazole. Scale bars: 10 μm.
The presence of Mad2 at kinetochores correlates with ongoing SAC activation in oocytes
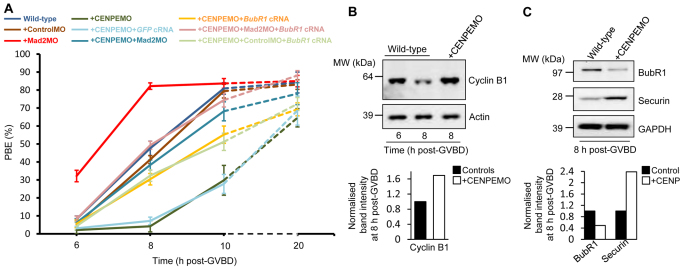
We next examined whether kinetochore Mad2 retention was physiologically relevant to SAC signalling. In mouse oocytes, cyclin B1 destruction, a reporter for SAC inactivation (Clute and Pines, 1999), is observed by 8 hours post-GVBD (Fig. 4B) (Homer et al., 2009) and therefore correlates very closely with Mad2 dissociation (Fig. 1A,D). PBE becomes maximal ∼2 hours later, when cyclin B1 is at a nadir (Homer et al., 2005b), entirely consistent with our current findings showing peak PBE at ∼10 hours post-GVBD (Fig. 4A). By contrast, following CENP-E depletion, delayed Mad2 dissociation results in cyclin B1 stabilisation by 8 hours post-GVBD and delayed PBE (Fig. 4A,B). Following Mad2 displacement by 10 hours post-GVBD, however, PBE increased markedly, so that by 20 hours post-GVBD, PBE approached wild-type levels (Fig. 4A). Furthermore, in both wild-type and CENP-E-depleted oocytes, Mad2 depletion accelerated PBE (Fig. 4A). Altogether, these data indicate that the presence of Mad2 at kinetochores is indicative of continuing SAC activation.
Fig. 4.
Exit from MI in CENP-E-depleted oocytes is mediated by Mad2 and BubR1. (A) PBE in wild-type, mock-depleted (+ControlMO), CENP-E-depleted (+CENPEMO), Mad2-depleted (+Mad2MO), CENP-E and Mad2 co-depleted (+CENPEMO+Mad2MO) oocytes, CENP-E-depleted oocytes co-expressing either BubR1 (+CENPEMO+BubR1 cRNA) or GFP (+CENPEMO+GFP cRNA), CENP-E and Mad2 co-depleted oocytes co-expressing BubR1 (+CENPEMO+Mad2MO+BubR1 cRNA) and CENP-E and mock co-depleted oocytes co-expressing BubR1 (+CENPEMO+ControlMO+BubR1 cRNA). Data are mean ± s.e.m. (B,C) Western blots showing cyclin B1 (B) and BubR1 and securin (C) (30 oocytes per lane). GAPDH and actin served as loading controls. Band intensities at 8 hours post-GVBD were normalised to values in controls.
BubR1 instability contributes to delayed MI transit after CENP-E depletion
Notably, following co-depletion of CENP-E and Mad2, the accelerated transit typical of Mad2-depleted oocytes (Homer et al., 2005b) was not observed and indeed transit through MI remained slower than in wild-type oocytes (Fig. 4A), indicating that SAC activation was not solely responsible for MI delays following CENP-E depletion. CENP-E is known to interact with BubR1 (Chan et al., 1998), and in oocytes BubR1 restrains securin (Pttg1 – Mouse Genome Informatics) overaccumulation that would otherwise induce MI arrest (Homer et al., 2009). Interestingly, we found that BubR1 was reduced and securin was stabilised in CENP-E-depleted oocytes (Fig. 4C). Furthermore, PBE in CENP-E-depleted oocytes could be partially restored by expressing BubR1, but not GFP, from exogenous cRNA (Fig. 4A). Thus, delayed MI transit after CENP-E depletion was in part due to BubR1 instability. Together, SAC activation and BubR1 instability accounted for MI delays after CENP-E depletion, as PBE returned to wild-type levels following combined Mad2 depletion and BubR1 expression (Fig. 4A).
These data show that the non-K-fibre-based mode in oocytes enables polar chromosomes to evade the SAC. This reconciles the seemingly contradictory observation that polar chromosomes – for instance in aged oocytes (Pan et al., 2008; Volarcik et al., 1998) and among recombination-deficient oocytes (Nagaoka et al., 2011) – do not prevent anaphase onset even though SAC functionality remains grossly intact with the capacity to respond to minute inhibitory signals (Hoffmann et al., 2011). Conversely, when polar bivalents are rare, as in younger oocytes, the inability to react to polar chromosomes is inconsequential and aneuploidy rates are low (Duncan et al., 2009; Homer et al., 2005b; Pan et al., 2008). We speculate that the exposed location of kinetochores at the very extremities of bivalents (see supplementary material Fig. S1E′) and the high microtubule density of oocyte spindles greatly augment the likelihood of microtubule capture even when stable attachments do not form.
These data also show that CENP-E fulfils two important roles required for stable bi-orientation in oocytes: kinetochore reorientation followed by K-fibre formation, the latter being required for restraining bi-oriented bivalents at the equator. Intriguingly, whereas CENP-E relocates mitotic chromosomes from the pole to equator (congression) (Kapoor et al., 2006), during MI CENP-E prevents chromosomal drift in the opposite direction. These data help to explain the misalignment phenotypes of aged oocytes, which have been found to exhibit reduced levels of CENP-E (Pan et al., 2008; Volarcik et al., 1998). We also reveal an unexpected effect of CENP-E depletion in oocytes, that of BubR1 instability, which impacts MI progression. It is interesting to speculate that oocytes might deploy such SAC-independent delays under conditions that predispose to polar chromosomes.
Supplementary Material
Acknowledgments
We thank Tim Yen, Stephen Taylor and Katja Wassmann for the very generous gifts of reagents; and are grateful to the J. Carroll lab for helpful discussions.
Footnotes
Funding
This work was supported by a Wellcome Trust Fellowship [082587/Z/07/Z to H.H.]. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.078352/-/DC1
References
- Breuer M., Kolano A., Kwon M., Li C. C., Tsai T. F., Pellman D., Brunet S., Verlhac M. H. (2010). HURP permits MTOC sorting for robust meiotic spindle bipolarity, similar to extra centrosome clustering in cancer cells. J. Cell Biol. 191, 1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S., Santa Maria A., Guillaud P., Dujardin D., Kubiak J. Z., Maro B. (1999). Kinetochore fibers are not involved in the formation of the first meiotic spindle of mouse oocytes, but control the exit from the first meiotic M phase. J. Cell Biol. 146, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G. K., Schaar B. T., Yen T. J. (1998). Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. J. Cell Biol. 143, 49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute P., Pines J. (1999). Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1, 82–87 [DOI] [PubMed] [Google Scholar]
- Deluca J. G., Moree B., Hickey J. M., Kilmartin J. V., Salmon E. D. (2002). hNuf2 inhibition blocks stable kinetochore-microtubule attachment and induces mitotic cell death in HeLa cells. J. Cell Biol. 159, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan F. E., Chiang T., Schultz R. M., Lampson M. A. (2009). Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol. Reprod. 81, 768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hached K., Xie S. Z., Buffin E., CladiFre D., Rachez C., Sacras M., Sorger P. K., Wassmann K. (2011). Mps1 at kinetochores is essential for female mouse meiosis I. Development 138, 2261–2271 [DOI] [PubMed] [Google Scholar]
- Hassold T., Hunt P. (2009). Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr. Opin. Pediatr. 21, 703–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D. B., Pearson C. G., Yen T. J., Howell B. J., Salmon E. D. (2001). Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell 12, 1995–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S., Maro B., Kubiak J. Z., Polanski Z. (2011). A single bivalent efficiently inhibits cyclin B1 degradation and polar body extrusion in mouse oocytes indicating robust SAC during female meiosis I. PLoS ONE 6, e27143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer H. (2011). Evaluating spindle assembly checkpoint competence in mouse oocytes using immunoblotting. Methods Mol. Biol. 782, 33–45 [DOI] [PubMed] [Google Scholar]
- Homer H., McDougall A., Levasseur M., Murdoch A., Herbert M. (2005a). Mad2 is required for inhibiting securin and cyclin B degradation following spindle depolymerisation in meiosis I mouse oocytes. Reproduction 130, 829–843 [DOI] [PubMed] [Google Scholar]
- Homer H., McDougall A., Levasseur M., Yallop K., Murdoch A., Herbert M. (2005b). Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 19, 202–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer H., Gui L., Carroll J. (2009). A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science 326, 991–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B. J., Hoffman D. B., Fang G., Murray A. W., Salmon E. D. (2000). Visualization of Mad2 dynamics at kinetochores, along spindle fibers, and at spindle poles in living cells. J. Cell Biol. 150, 1233–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor T., Lampson M., Hergert P., Cameron L., Cimini D., Salmon E., McEwen B., Khodjakov A. (2006). Chromosomes can congress to the metaphase plate before biorientation. Science 311, 388–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima T. S., Ohsugi M., Ellenberg J. (2011). Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell 146, 568–581 [DOI] [PubMed] [Google Scholar]
- McGuinness B. E., Anger M., Kouznetsova A., Gil-Bernabq A. M., Helmhart W., Kudo N. R., Wuensche A., Taylor S., Hoog C., Novak B., et al. (2009). Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr. Biol. 19, 369–380 [DOI] [PubMed] [Google Scholar]
- Musacchio A., Salmon E. D. (2007). The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8, 379–393 [DOI] [PubMed] [Google Scholar]
- Nagaoka S. I., Hodges C. A., Albertini D. F., Hunt P. A. (2011). Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors. Curr. Biol. 21, 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Ma P., Zhu W., Schultz R. M. (2008). Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev. Biol. 316, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkey F., Cramer T., Morphew M., Silk A., Johnson R., McIntosh J., Cleveland D. (2002). Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev. Cell 3, 351–365 [DOI] [PubMed] [Google Scholar]
- Rieder C. L. (1981). The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma 84, 145–158 [DOI] [PubMed] [Google Scholar]
- Schuh M., Ellenberg J. (2007). Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 130, 484–498 [DOI] [PubMed] [Google Scholar]
- Skoufias D. A., Andreassen P. R., Lacroix F. B., Wilson L., Margolis R. L. (2001). Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl. Acad. Sci. USA 98, 4492–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. S. K., Takagaki K., Kumada K., Hirayama Y., Noda T., Hirota T. (2009). Kinetochore stretching inactivates the spindle assembly checkpoint. J. Cell Biol. 184, 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volarcik K., Sheean L., Goldfarb J., Woods L., Abdul-Karim F., Hunt P. (1998). The meiotic competence of in-vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum. Reprod. 13, 154–160 [DOI] [PubMed] [Google Scholar]
- Waters J. C., Chen R. H., Murray A. W., Salmon E. D. (1998). Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141, 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver B., Bonday Z., Putkey F., Kops G., Silk A., Cleveland D. (2003). Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J. Cell Biol. 162, 551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K. T., Li S. K., Chang C. C., Tang C. J., Lin Y. N., Lee S. C., Tang T. K. (2010). Aurora-C kinase deficiency causes cytokinesis failure in meiosis I and production of large polyploid oocytes in mice. Mol. Biol. Cell 21, 2371–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.