Abstract
Clathrin coats vesicles in all eukaryotic cells and has a well-defined role in endocytosis, moving molecules away from the plasma membrane. Its function on routes towards the plasma membrane was only recently appreciated and is thought to be limited to basolateral transport. Here, an unbiased RNAi-based tubulogenesis screen identifies a role of clathrin (CHC-1) and its AP-1 adaptor in apical polarity during de novo lumenal membrane biogenesis in the C. elegans intestine. We show that CHC-1/AP-1-mediated polarized transport intersects with a sphingolipid-dependent apical sorting process. Depleting each presumed trafficking component mislocalizes the same set of apical membrane molecules basolaterally, including the polarity regulator PAR-6, and generates ectopic lateral lumens. GFP::CHC-1 and BODIPY-ceramide vesicles associate perinuclearly and assemble asymmetrically at polarized plasma membrane domains in a co-dependent and AP-1-dependent manner. Based on these findings, we propose a trafficking pathway for apical membrane polarity and lumen morphogenesis that implies: (1) a clathrin/AP-1 function on an apically directed transport route; and (2) the convergence of this route with a sphingolipid-dependent apical trafficking path.
Keywords: Caenorhabditis elegans, Polarity, Tubulogenesis, Clathrin, AP-1, Sphingolipids
INTRODUCTION
Biological tubes are composed of polarized epithelial cells with their apical sides generating the lumenal surface and their basolateral sides contacting adjacent cells or the extracellular matrix. Polarizing cues come from inside the cell (e.g. through polarized trafficking), from the extracellular environment, or from the plasma membrane itself (Mellman and Nelson, 2008). Many such cues, which are highly conserved between species, have been identified, but their integration during the complex process of tissue morphogenesis is not well understood. It is assumed that plasma membrane-associated polarity determinants, such as the apical partitioning-defective (PAR) complex PAR-3/PAR-6/aPKC, define membrane domain identities, whereas polarized trafficking directs specific membrane components to these domains. Although there is little evidence for the intrinsic ability of vesicular trafficking to define polarized membrane domains, recent analysis of tubulogenesis has demonstrated that it may determine such domains by recruiting the polarity complex components themselves. RAB-11–RAB-8-mediated vesicular delivery of CDC-42, for instance, was shown to be required for recruiting the apical PAR complex to promote apical domain and lumen biogenesis in MDCK 3D tissue culture (Bryant et al., 2010).
Membrane lipids, such as phosphoinositides, are well-characterized sorting molecules that have also been implicated in the asymmetric placement of polarity complex components. Membrane lipids assume a specific place in vesicular sorting, as they themselves may be asymmetrically assorted on plasma membranes (van Meer et al., 2008). For example, phosphoinositides determine both polarized trafficking and polarized domain identities when inserted into the plasma membrane (Di Paolo and De Camilli, 2006; Rodriguez-Boulan et al., 2005). PtdIns(4,5)P2 (PIP2) enrichment at apical membranes by the lipid phosphatase PTEN is also required for CDC-42 and PAR-6 recruitment in MDCK lumen morphogenesis (Martin-Belmonte et al., 2007). Similarly, glycosphingolipids (GSLs), which are saturated obligate membrane sphingolipids (SLs) that are thought to laterally assemble into membrane microdomains (lipid rafts), are enriched on both apical plasma membranes and endomembranes, and apically sort lipids and proteins (Simons and Gerl, 2010). In C. elegans, GSLs define apical membrane domain identities in the expanding intestine and are also required to recruit PAR-6 to the lumen (Zhang et al., 2011).
Clathrin, the prototypical post-Golgi vesicle coat, is primarily studied for its roles in endocytosis and signaling at the plasma membrane. Recently, however, clathrin was shown to regulate basolateral sorting through the epithelial cell-specific AP-1B adaptor, revealing its additional role in membrane-directed trafficking (Deborde et al., 2008; Folsch et al., 1999). Vesicle coat formation, in turn, depends on vesicle membrane lipid composition. PIP2, for instance, functions at several steps in clathrin coat formation, possibly in an AP-2 adaptor-dependent manner (Antonescu et al., 2011). Thus, vesicle lipids, coats, adaptors and their interaction might play a crucial role in the generation of polarized plasma membrane domains.
In a systematic screen for apicobasal polarity and tubulogenesis defects in the C. elegans intestine, we identified clathrin and several subunits of its AP-1 adaptor as being required for apical polarity and lumen formation. Clathrin/AP-1 depletion caused defects similar to those caused by the depletion of GSL-biosynthetic enzymes (also identified in this screen). Further analysis revealed that both trafficking components cooperate in apical sorting.
MATERIALS AND METHODS
Strains and culture conditions
C. elegans strains were maintained, cultured and crossed using standard techniques (Brenner, 1974). See supplementary material Table S1 for strain list. chc-1(tm2866)III/+ was balanced with hT2[qIs48] (Miskowski et al., 2001). aps-1(tm935)V/+ was balanced with nT1[qIs51] (Belfiore et al., 2002). The temperature-sensitive strain chc-1(b1025) was maintained at 16°C unless indicated otherwise.
RNAi and screens
A systematic C. elegans tubulogenesis RNAi screen was designed and carried out as previously described, using animals carrying an erm-1::gfp transgene, outlining the lumens of the intestine, the excretory canal and the gonad (Zhang et al., 2011). RNAi was performed by feeding (Timmons et al., 2001).
Standard RNAi conditions (used in the screen) were defined as dsRNA induction by 2 mM IPTG. Mild RNAi conditions were empirically determined for specific genes after testing serial concentrations of IPTG and/or dilutions with mock RNAi bacteria: for chc-1, IPTG was titrated down to 2 nM; for aps-1, RNAi bacteria were diluted 1:10 with mock RNAi bacteria. For double RNAi, equal amounts of RNAi bacteria of two clones were mixed. RNAi initiated after completion of embryogenesis involved placing eggs or larvae on RNAi plates for evaluating the same generation.
DsRed feeding
chc-1(b1025ts) animals were fed on plates containing DsRed RNAi bacteria for at least 12 hours. The DsRed bacterial feeding strain contains a DsRed plasmid in HT115 bacteria that constitutively produces a faint red color.
Phenotype reversal
chc-1(b1025ts) mutant hermaphrodites were allowed to lay eggs for 1 hour (at 16°C) and subsequently removed. The plates with eggs were transferred to 22°C for 5 hours, then returned to 16°C. Animals were singled the next day and phenotype development and reversal were observed for 6 days.
Lipid labeling and assessment of vesicle association
For lipid labeling, 150 μl E. coli OP50 or HT115 were spiked with 2 μl 5 mM labeled lipid stock solutions (NBD-C6-glucosylceramide stock was 100 μM), for a feeding period of ∼8 hours. The same amounts were used for double labeling. Lipids used were: BODIPY-FL-labeled C5-ceramide (N-[4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl]-sphingosine), BODIPY-TR-labeled C5-ceramide (N-[(4-(4,4-difluoro-5-(2-thienyl)-4-bora-3a,4a-diaza-s-indacene-3-yl)phenoxy)acetyl]sphingosine), BODIPY-FL-labeled C5-sphingomyelin (N-[4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl]sphingosylphosphocholine) (all from Invitrogen) and NBD-C6-glucosylceramide (N-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-d-glucosyl-β1-1′-sphingosine) (Avanti Polar Lipids).
Vesicle colocalization was quantified by counting the number of either overlapping or associated (defined as overlapping or in contact) vesicles in a 800 × 400 pixel grid area of thin confocal sections, unless otherwise indicated.
Genetic interactions
To examine genetic interactions between clathrin, the AP-1 adaptor and SL-biosynthetic enzymes, eggs or L4 stage larvae of wild type, chc-1(b1025ts), let-767(s2716) and let-767(s2819) mutants, all carrying the erm-1::gfp transgene, were placed on RNAi plates containing aps-1, apb-1, let-767, sptl-1, apa-2 or mock RNAi bacteria. Polarity phenotypes, lethality and arrest stages were evaluated in the same or the next generation.
Plasmids and DNA transformation
Translational GFP fusion proteins were generated by in-frame joining of genomic DNA of the gene of interest with GFP by PCR, using the stitching method (Hobert, 2002). The ERM-1::mCherry plasmid was generated by replacing the GFP with mCherry coding sequences in a plasmid expressing an ERM-1::GFP fusion protein (Gobel et al., 2004). Briefly, mCherry DNA was PCR amplified, digested with SmaI and KpnI and ligated to the 3′ end of the erm-1 full-length genomic DNA. DNA was prepared from multiple independent isolates, verified by restriction digestion and sequencing, and a mixture was used for germline transformation of animals by microinjection (Mello et al., 1991). Constructs were injected at 10-100 ng/ml, along with the dominant transgene marker rol-6(su1006).
Antibodies and immunofluorescent staining
Animals were collected in M9 on poly-l-lysine (Sigma)-coated slides, permeabilized by freeze cracking and fixed by sequential incubation in methanol and acetone (Miller and Shakes, 1995). Immunofluorescent staining was performed as described (Zhang et al., 2011). Antibodies were diluted with blocking solution at the following concentrations: MH27 (anti-AJM-1), 1:20; MH33 (anti-IFB-2), 1:20; anti-DLG-1, 1:10 (all from the Developmental Studies Hybridoma Bank, University of Iowa); ICB4, 1:500 (gift from M. de Bono, Laboratory of Molecular Biology, Cambridge University, UK); Alexa Fluor 568 phalloidin (actin), 1:20 (Molecular Probes); TRITC-conjugated goat anti-mouse IgG, 1:100 (Sigma); Cy5-conjugated goat anti-mouse IgG, 1:250 (Jackson ImmunoResearch).
Epifluorescence and confocal microscopy
For large-scale screens, animals were directly observed on plates under an Olympus SZX12 dissecting microscope (Olympus America Center Valley, PA, USA) equipped with a high-power stereo fluorescence attachment (Kramer Scientific Corporation, Amesbury, MA). For detailed characterization, live worms were mounted in M9 buffer on glass slides, immobilized with 10 mM sodium azide (Sigma), and visualized by confocal and Nomarski microscopy. Confocal images were acquired on a TCS SL laser-scanning microscope (Leica Microsystems, Bannockburn, IL, USA). Single-plane images were taken as 6-50 sections along the z-axis at 0.2 μm intervals, and projection images generated by merging. Multi-channel images were taken with minimal laser settings unless indicated otherwise. Laser settings with increased sensitivity were defined as laser power at 60%, 488 nm beam path at 50%, pinhole (airy) at 1.755. Identical laser and confocal settings were used when comparing experimental animals with controls.
To separate fluorescently labeled from autofluorescent intestinal vesicles, empirical scanner settings were established by restricting the wavelengths of the fluorescence filters (green filter spectrum to 500-515 nm, red filter spectrum to 630-700 nm). A considerable decrease in the sensitivity of detecting fluorescently labeled vesicles was accepted. For all overlay experiments, animals were sequentially scanned to exclude false positives caused by channel bleed-through. Images were arranged using Adobe Photoshop with occasional small adjustments for contrast and brightness, and fluorescence intensity was quantified using ImageJ software.
Transmission electron microscopy (TEM)
TEM procedures were carried out according to protocols previously described (Hall, 1995; Zhang et al., 2011). chc-1(b1025ts) L1 larvae were obtained by placing isolated eggs at 16°C for 6 hours, then at 22°C overnight. Thin sections were cut on a Reichert Ultracut E ultramicrotome, collected on formvar-coated gold grids, contrasted with uranyl acetate and lead citrate and viewed in a JEOL 1011 electron microscope equipped with a digital imaging system (Advanced Microscopy Techniques, Danvers, MA, USA) at 80 kV.
Statistics
Data are expressed as mean ± s.d. Statistical significance was determined at the *P<0.05, **P<0.01 and ***P<0.001 levels by Student’s two-tailed t-test using Microsoft Excel software.
RESULTS
Clathrin (CHC-1) depletion disrupts apical membrane polarity and generates lateral lumens
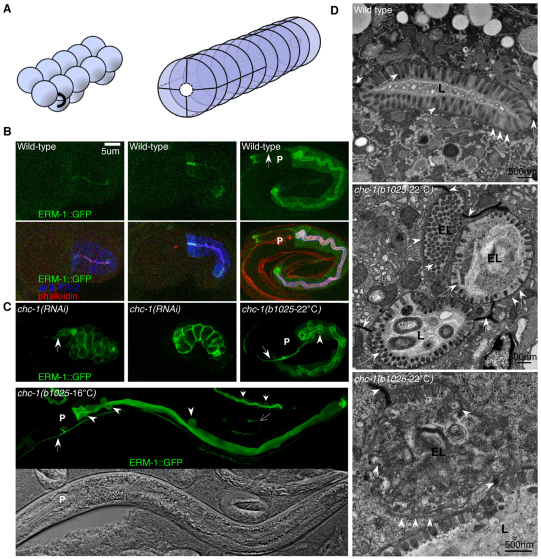
A chromosome III and genome-wide RNAi screen of all lethal genes tracking the asymmetric distribution of apical ERM-1::GFP in the C. elegans intestine identified several distinct classes of polarity phenotypes, most involving the cytoplasmic mislocalization of this apical membrane marker (Zhang et al., 2011). Two classes were distinguished by basolateral membranous ERM-1::GFP misplacement in: (1) the embryonic pre- to early post-intercalation intestine with absent or incomplete lumen formation (Fig. 1B,C, left and middle; 1A for anatomy); and (2) the embryonic or early larval fully intercalated intestine, accompanied by the formation of multiple lateral ectopic lumens (Fig. 1B,C, right; 1D; Fig. 2A,B). Both phenotypic classes also arrested at these respective stages. RNAi with chc-1, the C. elegans clathrin heavy chain ortholog, generated the former phenotype, whereas RNAi with aps-1, the clathrin AP-1 adaptor complex sigma subunit, and apb-1, the AP-1 or AP-2 adaptor complex beta subunit, generated the latter phenotype.
Fig. 1.
Apicobasal polarity alteration and ectopic lumen formation in CHC-1-depleted intestines. (A) Schematic of the pre- and post-intercalation wild-type C. elegans intestine. (Left) E16 stage, with ten dorsal and six ventral cells (three ventral cells obscured). Arrow shows direction of intercalation. (Right) E20 stage, cells arranged into nine intestinal (INT) rings in bilateral symmetry (four cells in first INT). (B) (Top) Apical/lumenal ERM-1::GFP in wild-type (WT) at pre-comma (left, beginning of intercalation; image obtained with increased laser settings, see Materials and methods), comma (middle, intercalation almost complete) and 3-fold embryo (right, fully intercalated intestine) stages. (Bottom) Images combined with staining of actin (phalloidin) and intermediate filaments (anti-IFB2) to outline intestinal tube and lumen. (C) ERM-1::GFP displacement and lumen defects in chc-1(RNAi) and chc-1(b1025ts) animals. (Top) Non-polarized ERM-1::GFP in embryos arrested at pre-comma and comma stage at mid- to late intercalation (left and middle); ectopic lateral lumen formation in fully intercalated intestine in 3-fold embryo (right, arrowhead). (Bottom) Ectopic lumens in L4 (arrowheads) and L1 (small arrows) chc-1(b1025-16°C) larvae (compare with TEM images in D). Note the wild-type 2.5-fold embryo (thin arrow). P, pharynx. Large arrows, excretory canals. Confocal images are shown, with and without corresponding Nomarski images; anterior is left and dorsal up. (D) TEM micrographs of L1 intestines. (Top) Wild-type, showing oval lumen (L), tightly adjacent terminal web (arrowheads) and dense microvilli (long arrows). (Middle, bottom) chc-1(b1025-22°C), showing deformed main lumen (L) with stunted microvilli, ectopic lateral lumens (EL) with terminal web (arrowheads) and short or almost normal microvilli (long arrows). Intact apical junctions (short arrows) are seen in both wild type and chc-1(b1025); note excess junctions between ectopic lumens.
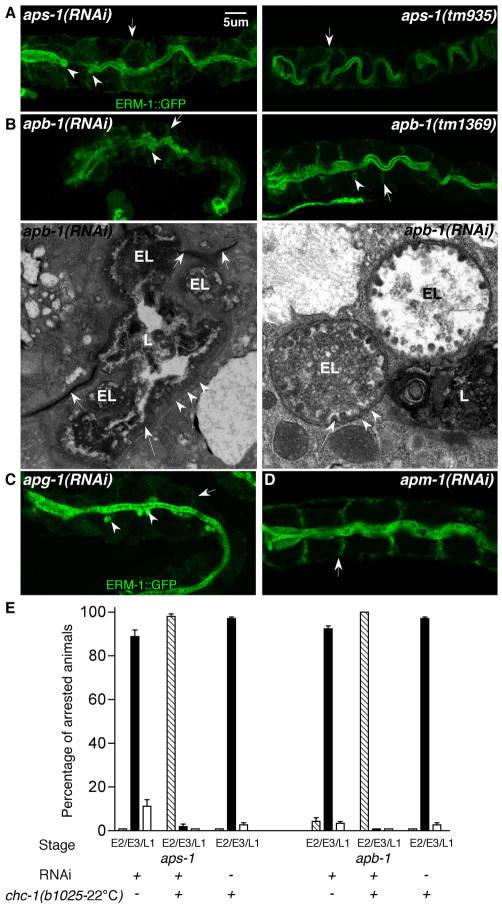
Fig. 2.
Depletion of four different AP-1 adaptor subunits phenocopies the polarity and ectopic lumen defect induced by CHC-1 depletion. (A,B) aps-1 and apb-1 RNAi phenotypes (left) with phenotypes of corresponding presumed null alleles (right) showing basolateral ERM-1::GFP displacement (arrows) and ectopic lumen formation (arrowheads). TEM micrographs of APB-1-depleted L1 intestines (B, bottom) show severely deformed main lumen (L) and ectopic lumens (EL), both with sparse and stunted microvilli (long arrow), terminal web (arrowheads) dissociated from main lumen, and intact apical junctions (short arrows). (C,D) apg-1(RNAi) and apm-1(RNAi) phenotypes (compare with A,B). (E) Genetic interactions between chc-1(b1025), aps-1 RNAi (left) and apb-1 RNAi (right). Double mutant/RNAi animals show enhancement, with appearance of more severe phenotypes (compare with supplementary material Fig. S4D) and earlier arrest. E2, E3 and L1 indicate arrest at 2-fold embryo, 3-fold embryo and L1 larval stages. Mean ± s.d.; n=3 (N>200 animals per experiment).
To confirm the RNAi results and examine whether the two classes of phenotypes affected the same polarization process, different chc-1 RNAi and mutant conditions were characterized. In contrast to the pre-comma/comma stage embryonic arrest of chc-1(RNAi) animals (penetrance, 100%), the majority of chc-1(b1025ts) animals grew into fertile adults at 16°C [with CHC-1 at 4.5% of wild-type levels (Sato et al., 2009)], but ∼90% displayed the late multiple lumen phenotype (Fig. 1C, bottom; ∼20% as late embryos). Shifting early chc-1(b1025ts) embryos to 22°C or 25°C [CHC-1 at 2% of wild-type levels and unstable (Sato et al., 2009)], caused ∼100% 3-fold embryonic arrest with ectopic lateral lumens in the intercalated intestine, similar to the predominant arrest stage of homozygous progeny of balanced chc-1(tm2866) mutants (carrying an 847 bp chc-1 deletion; not shown). The earlier and more severe nature of the chc-1(RNAi) phenotype as compared with the chc-1(b1025ts and tm2866) mutant phenotype suggests a maternal chc-1 requirement (Fig. 1C). In less severely affected mutants, basolateral ERM-1::GFP was observed prior to ectopic lumen development (supplementary material Fig. S1A, right). Conversely, mild chc-1 RNAi conditions, allowing ∼20% of animals to reach the L2-L3 larval stage, developed ectopic lumens subsequent to basolateral ERM-1::GFP displacement (supplementary material Fig. S1A, left). Initiating chc-1 RNAi from the L1 stage (supplementary material Fig. S1B; see below) enhanced the mild chc-1(b1025) ectopic lumen phenotype at the permissive temperature, inducing fully penetrant L1-L2 larval arrest while increasing the number of ectopic lumens per animal (not shown). Thus, maternal and zygotic chc-1 products dose-dependently regulate apical polarity and lumen morphogenesis in the C. elegans intestine, and the two classes of phenotypes appear to disrupt the same process of membrane polarization, with the basolateral displacement of apical membrane components preceding ectopic lumen formation.
chc-1(RNAi) embryos displayed non-polarized, pan-membranous ERM-1::GFP from the time of its appearance during late intestinal intercalation, suggesting an early CHC-1 requirement. To determine whether CHC-1 was required for the establishment of membrane polarity, we examined ERM-1::GFP during early intestinal polarization in chc-1(RNAi) and chc-1(b1025-22°C,RNAi) embryos, using increased laser settings for confocal analysis (see Materials and methods). Under these conditions, wild-type apical ERM-1::GFP is detected at approximately the start of intercalation, when nuclei have moved to the future apical membrane and cytoplasmic vesicles towards the future basolateral membrane, and apical junctions are at the spot-junction stage (Leung et al., 1999). At this stage, chc-1(RNAi) embryos displayed either apical ERM-1::GFP or both apical and partial basolateral ERM-1::GFP (supplementary material Fig. S2). ERM-1::GFP subsequently increased pan-membranously in the arrested, non-expanding embryonic intestine, with persistently higher apical signal intensity (supplementary material Fig. S2) (in contrast to the decreasing apical signal intensity during larval intestinal expansion in animals with milder phenotypes, see below). This did not suggest the loss of an initially intact polarity, particularly given the possibilities of basolateral ERM-1::GFP being at subdetection levels at early stages and incomplete RNAi phenotypes. Consistent with the latter possibility, basolateral ERM-1::GFP displacement was stronger in chc-1(b1025-22°C,RNAi) than in chc-1(RNAi) embryos (supplementary material Fig. S2). Moreover, the polarization of nuclei and cytoplasmic vesicles was also impaired in chc-1(RNAi) embryos, even in those with exclusive apical ERM-1::GFP (supplementary material Fig. S2). Furthermore, from an early stage on, excess junction material was detected on lateral membranes, suggesting that it could be a part of the process of apical membrane biogenesis at the lateral side (supplementary material Fig. S3). Nevertheless, junction assembly appeared remarkably intact and included the formation of apicolateral junctions (compare with the analysis of junctions in larvae below).
To determine whether CHC-1 is required for membrane polarity maintenance, ERM-1::GFP was examined in animals in which chc-1 RNAi had been initiated after completion of embryogenesis. Late chc-1 RNAi was sufficient to induce ERM-1::GFP displacement, small ectopic lumens and late larval/adult lethality (supplementary material Fig. S1B). Adult induced chc-1 RNAi, which was capable of reducing CHC-1::GFP, had no detectable effect (supplementary material Fig. S1C; data not shown). To test whether the polarity defects were reversible, chc-1(b1025ts-16°C) embryos were shifted to 22°C for 5 hours and then returned to the permissive temperature. This downshift decreased the number and size of ectopic lumens and altered their character (supplementary material Fig. S1D; compare with Fig. S6 for TEM studies of different ectopic lumens).
We conclude that CHC-1 is required to maintain and possibly also to establish apical membrane polarity and a single lumen in the C. elegans intestine in an apparently junction-independent manner. CHC-1 function in polarity maintenance appears to be restricted to embryonic and larval epithelia, where polarity defects and even ectopic lumens remain reversible. These findings are compatible with a CHC-1 function in apical sorting of membrane components and/or polarity regulators during de novo membrane biogenesis.
The AP-1 adaptor functions together with clathrin in the regulation of apical polarity
The similarity between the phenotype of chc-1(b1025-22°C) and that of aps-1(RNAi) and apb-1(RNAi) suggested that these genes act in the same polarity process. To test this and to determine whether the aps-1(RNAi) and apb-1(RNAi) phenotypes were adaptor- and possibly subunit-specific, we characterized different RNAi and mutant conditions. The C. elegans AP-1 adaptor complex contains four subunits: APB-1/beta, APS-1/sigma, UNC-101 or APM-1/mu, and APG-1/gamma. aps-1(RNAi) and apb-1(RNAi) animals arrested as 2- to 3-fold embryos or L1 larvae with basolateral ERM-1::GFP mislocalization and/or small ectopic lumens at the apicolateral angle of the intercalated intestine (penetrance, 90-95%; Fig. 2A,B; supplementary material Fig. S4B). Homozygous progeny of heterozygous aps-1(tm935) and apb-1(tm1369) alleles (carrying 1100 bp and 500 bp deletions, respectively; supplementary material Fig. S4A) arrested as early larvae and copied the RNAi polarity defects, but with a less severe phenotype of predominantly lateral ERM-1::GFP displacement, also suggesting a maternal effect (Fig. 2A,B, right). apg-1(RNAi) fully recapitulated the aps-1(RNAi) and apb-1(RNAi) phenotype, including ectopic lumen formation (penetrance, 99%; Fig. 2C), and apm-1(RNAi) caused predominantly basolateral ERM-1::GFP misplacement (penetrance, 90%) and L2-L3 arrest (Fig. 2D), whereas unc-101(RNAi) did not show obvious defects in the intestine (not shown). Likewise, interference with the endocytic AP-2 clathrin adaptor alpha subunit apa-2 failed to show obvious polarity defects (supplementary material Fig. S4B). Thus, the clathrin AP-1 adaptor is required for apical polarity, whereas its AP-2 adaptor may be dispensable. All AP-1 subunits are required for function, with the possible exception of mu/UNC-101. APB-1 appears to be the C. elegans AP-1 beta subunit.
In further agreement with a common CHC-1/AP-1 function in apical membrane polarity, aps-1 and apb-1, but not apa-2, RNAi initiated after completion of embryogenesis induced a polarity defect in the mature larval intestine that resembled the chc-1(larval RNAi) phenotype (supplementary material Fig. S1B), and aps-1(RNAi), apb-1(RNAi), apg-1(RNAi) and chc-1(b1025) mutant animals displayed an excretory canal apical membrane and lumen biogenesis defect (supplementary material Fig. S4C). To examine genetic interactions, we analyzed chc-1(b1025ts);aps-1(RNAi) and chc-1(b1025ts);apb-1(RNAi) double mutant/RNAi animals. At 16°C, chc-1(b1025) grow to fertile adults with a mild ectopic lumen phenotype (see above; Fig. 1C, bottom), whereas aps-1(RNAi), apb-1(RNAi) and chc-1(b1025) at 22°C arrest as 3-fold embryos or L1 larvae with multiple ectopic lumens (Fig. 2A,B). chc-1(b1025);aps-1(RNAi) and chc-1(b1025);apb-1(RNAi) double mutant/RNAi animals (at either 16°C or 22°C) revealed strong enhancement by arresting earlier than either single mutant/RNAi animal and resembled the severe chc-1(RNAi) phenotype with only partially intercalated intestines, pan-membranous ERM-1::GFP, cytoplasmic ERM-1::GFP inclusions and incomplete apical lumen formation (Fig. 2E; supplementary material Fig. S4D). L1-initiated aps-1 and apb-1 RNAi, which induce only very mild polarity defects on their own (supplementary material Fig. S1B), increased the number of ectopic lumens in chc-1(b1025-16°C) animals and caused an earlier growth arrest (not shown).
We conclude that the similar CHC-1 and AP-1 reduction-of-function phenotypes result from different degrees of interference with the same, or with different aspects of the same, process of membrane polarization and lumen formation.
Clathrin/AP-1 depletion mislocalizes multiple apical molecules, including PAR-6, and reduces RAB-11+ apical membrane-associated vesicles
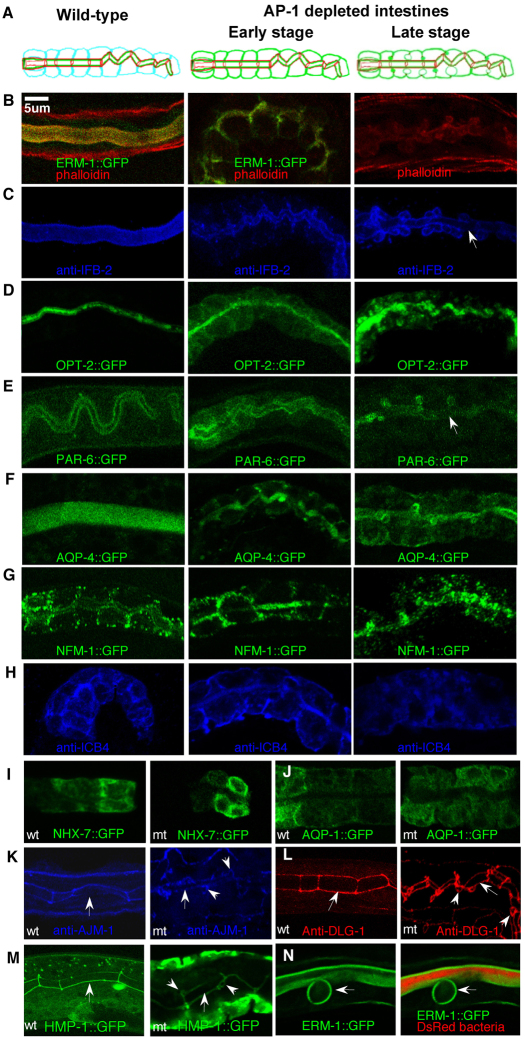
To determine whether CHC-1/AP-1 depletion caused a general polarity defect, additional submembranous and integral membrane proteins were analyzed in chc-1, aps-1 and apb-1 RNAi/mutant animals. All apical molecules examined, i.e. actin filaments (via phalloidin), intermediate filaments (via IFB-2), the Par polarity complex component PAR-6, the oligopeptide transporter OPT-2 and the water channel AQP-4, were displaced laterally and/or cytoplasmically, prior to accumulating around ectopic lateral lumens (Fig. 3B-F; 3A for anatomy). By contrast, the basolaterally enriched molecules ICB4 (an unidentified pan-membranous molecule), the ERM-1 family member NFM-1, the Na+/H+ transporter NHX-7 and the aquaporin AQP-1, were less affected, albeit partially cytoplasmically displaced (Fig. 3G-J). Over time, molecules were lost from the apical, while being gained at the basolateral, membrane, which is compatible with an underlying apical sorting defect during membrane expansion (Fig. 3C,E, arrows; supplementary material Fig. S5).
Fig. 3.
Placement of polarized transmembranous, submembranous and junction molecules in AP-1-depleted intestines. (A) Schematics of wild-type and AP-1-depleted intestines showing apical membranes (green), basolateral membranes (blue) and apical junctions (red). (B-J) Apicobasal membrane components in wild-type (wt, left) and APS-1- or APB-1-depleted L1 larval intestines (mt, middle and right column in B-H, right column in I,J). aps-1, apb-1 and chc-1 mutant/RNAi animals show similar displacement of each marker (N>30 each). All apical markers are displaced basolaterally and/or cytoplasmically before being displaced to ectopic lumens (EL): (B) actin (phalloidin), ERM-1 overlay; (C) intermediate filaments (IFB-2); (D) OPT-2; (E) PAR-6; (F) AQP-4. Note concomitant decrease of markers at apical membrane (arrows in C,E, see supplementary material Fig. S5 for quantification) and the presence of sealed ectopic lumens at the main lumen. Pan-membranous and basolateral markers are partially cytoplasmically displaced: (G) NFM-1 (pan-membranous punctate expression construct shown here); (H) pan-membranous ICB4; (I,J) basolateral NHX-7 and AQP-1, not visibly affected. (K-M) The apical junction components AJM-1, HMP-1/alpha-catenin and DLG-1/Discs large are contiguous (arrows) at apicolateral boundaries in both wild type and mutant. Note additional junctions surrounding ectopic lumens (arrowheads). (N) Intestinal section of CHC-1-depleted larva fed with DsRed bacteria. DsRed is seen in the main lumen but not ectopic lumen (arrow), indicating a sealed junction. Confocal images shown throughout.
Transmission electron microscopy (TEM) revealed a corresponding coincidental structural conversion of apical and basolateral domains: microvilli were shortened and the terminal web dissociated from the apical lumen, while both appeared de novo at lateral circularized membranes, confirming them as ectopic lumenal membranes (Fig. 1D; Fig. 2B; supplementary material Fig. S6A-D). TEM and confocal analysis of several apical junction components in animals depleted of CHC-1 or AP-1 subunits, as well as feeding experiments with fluorescently labeled bacteria, revealed intact junctions at their wild-type apicolateral sealing positions, indicating that the membrane polarity defects are unlikely to be a consequence of junction assembly defects (e.g. membrane equilibration) (Fig. 1D; Fig. 3K-N; supplementary material Fig. S6; compare with supplementary material Fig. S3). Instead, excess junctions formed around ectopic lumens along lateral membranes during their acquisition of apical membrane characteristics (Fig. 1D; Fig. 3K-M; supplementary material Fig. S6). However, membrane pockets that lacked apical junctions were also observed in larvae with mild phenotypes and might represent the reversion of milder polarity defects during epithelial expansion (supplementary material Fig. S6E; Fig. S1D) (Zhang et al., 2011).
We conclude that the loss of CHC-1 and its AP-1 adaptor complex results in a general apicobasal polarity conversion, without apparent preceding junction assembly defects. The lateral displacement of apical membrane components corresponds to the structural transformation of the lateral into an apical membrane with ectopic lateral lumen formation.
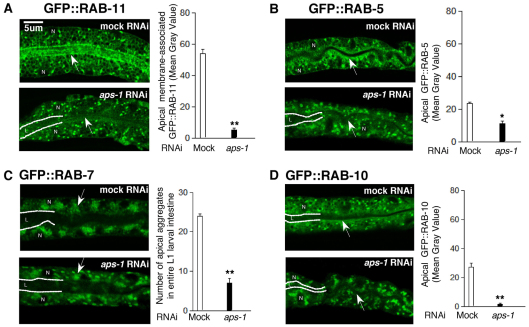
To investigate the requirement of clathrin/AP-1 for post-Golgi trafficking, we examined RAB-5 early, RAB-7 late, RAB-10 basolateral and RAB-11 apical recycling endosomes in wild-type and mutant/RNAi intestines (Chen et al., 2006). Most conspicuously, GFP::RAB-11 vesicles were lost from the lumen of aps-1(RNAi) and apb-1(RNAi) larval intestines at early stages of polarity conversion. Furthermore, L1-specific apical GFP::RAB-7 aggregates were reduced in number and apical GFP::RAB-5 and GFP::RAB-10 vesicle subfractions were also depleted (Fig. 4). In larvae with fully developed phenotypes, only a residual string of vesicles remained along basal membranes (supplementary material Fig. S7; data not shown).
Fig. 4.
Effect of clathrin/AP-1 depletion on post-Golgi vesicles. Selective decrease of apical endosomal subfractions in aps-1(RNAi) L1 larval intestines at early stages of polarity conversion [lower panels; similar results were obtained for apb-1- and chc-1(RNAi) animals], as compared with wild type (upper panels; compare with supplementary material Fig. S7 for later stages of polarity conversion). Confocal images (left) and quantification of vesicles or fluorescence intensity (right) are shown. (A) Decrease of GFP::RAB-11 vesicles from the apical membrane (arrows). (B) Reduced subapical GFP::RAB-5 enrichment (arrows). (C) Decrease of L1-specific GFP::RAB-7 apical aggregates (arrows). (D) Decrease of subapical GFP::RAB-10 (arrows); note the perinuclear GFP::RAB-10 assembly. L, lumen (also partially outlined); N, nucleus. Mean ± s.e.m. (n=3); *P<0.05, **P<0.01, two-tailed t-test. N>50 animals for each marker.
We conclude that CHC-1/AP-1 are required for the apical localization of RAB-11-associated, presumably lumenal membrane-forming endosomes, and also affect apical subfractions of other post-Golgi vesicles in the C. elegans intestine.
Clathrin/AP-1 genetically interact with sphingolipid-biosynthetic enzymes in apical sorting
The polarity phenotype induced by clathrin/AP-1 depletion closely resembles that induced by interference with specific fatty acid- and SL-biosynthetic enzymes that affect polarity through the biosynthesis of GSLs (Zhang et al., 2011). To determine whether clathrin/AP-1 and SLs function together in apical sorting, genetic interactions between clathrin, its adaptors and SL-biosynthetic enzyme genes were investigated. Since both GSLs and clathrin are required for oocyte viability, partial loss-of-function conditions were examined (Grant and Hirsh, 1999; Nomura et al., 2011). Simultaneously decreasing both SL biosynthesis and clathrin or AP-1 enhanced the polarity phenotype of either and generated novel phenotypes.
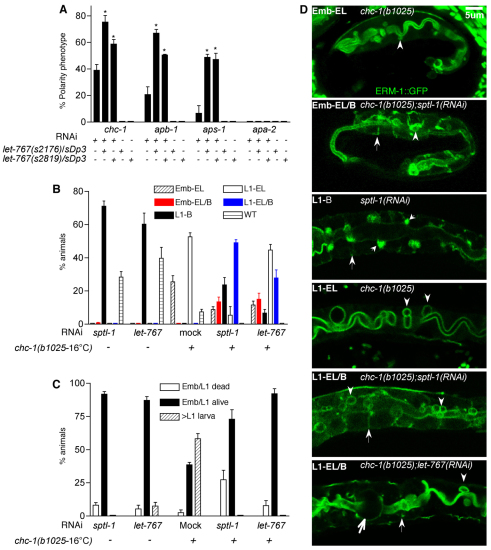
let-767(s2819) and let-767(s2176) are moderate and severe loss-of-function alleles, respectively, of the SL-biosynthetic enzyme steroid dehydrogenase/3-ketoacyl-CoA reductase (Entchev et al., 2008; Kuervers et al., 2003). Progeny of mutants balanced with a duplication (sDp3) that have lost sDp3 die as larvae after transitioning from the basolateral polarity to the ectopic lumen phenotype (Kuervers et al., 2003; Zhang et al., 2011). Standard chc-1, aps-1 and apb-1, but not apa-2, RNAi caused sterility in both let-767 alleles, suggesting an interaction as early as during oocyte development, but precluding further analysis. To bypass this early requirement, RNAi was introduced after embryogenesis was complete. L1-initiated chc-1, aps-1 and apb-1, but not apa-2, RNAi causes a mild phenotype with basolateral ERM-1::GFP misplacement and/or ectopic lumens in ∼5-40% of animals (supplementary material Fig. S1B). This phenotype was dominantly enhanced in let-767(s2176);sDp3 and let-767(s2819);sDp3 animals carrying the duplication (themselves wild type in appearance) (Fig. 5A).
Fig. 5.
Genetic interactions between clathrin/AP-1 and SL-biosynthetic enzymes. (A) Dominant enhancement of chc-1, apb-1 and aps-1(larval RNAi) polarity phenotypes by two let-767 alleles (s2176 and s2819). Only let-767;sDp3 animals carrying the duplication are shown. Note absence of interaction with apa-2. Mean ± s.d. is shown; n=3 (N>200 animals per experiment); *P<0.05, two-tailed t-test. (B,C) Enhancement of let-767(RNAi) and sptl-1(RNAi) polarity defects (B) and lethality (C) in chc-1(b1025-16°C) mutants. Progeny were evaluated prior to full phenotype development [80 hours (B) and 104 hours (C) after egg laying]. Note the absence of wild-type polarity and the appearance of novel phenotypes in double mutant/RNAi animals (B) and the increased lethality in chc-1(b1025);sptl-1(RNAi) animals (C). Emb, embryo; L1, L1 stage larva; EL, ectopic lumen; B, basolateral ERM-1::GFP displacement; Emb-EL/B and L1-EL/B, novel enhanced phenotypes (indicated by color). (D) Confocal images of representative phenotypes shown in B. Large arrows, basolateral ERM-1::GFP displacement; small arrows, apicolateral ERM-1::GFP accumulation; arrowheads, ectopic lumens; thin arrow, large ectopic lumen.
The converse scenario of chc-1(b1025);sptl-1(RNAi) and chc-1(b1025);let-767(RNAi) double mutants also demonstrated enhancement. sptl-1 encodes serine palmitoyltransferase, which catalyzes the first step in SL biosynthesis, and has a more severe RNAi polarity phenotype than let-767(RNAi) (Zhang et al., 2011). At the permissive temperature, chc-1(b1025) displays a mild polarity phenotype with ectopic lumens in ∼90% of late embryos or larvae (see above; Fig. 5B,D, Emb-EL, L1-EL), whereas sptl-1(RNAi) and let-767(RNAi) animals arrest as L1 larvae with ERM-1::GFP basolateral mislocalization, followed by its enrichment at apicolateral angles where ectopic lumens subsequently emerge (Fig. 5B,D, L1-B). In chc-1(b1025);sptl-1(RNAi) and chc-1(b1025);let-767(RNAi) animals, 10-20% embryos displayed basolateral ERM-1::GFP displacement (in addition to ectopic lumens) and 30-50% of L1 larvae (∼80 hours after hatching) displayed enlarged ectopic lumens or multiple small ectopic lumens with coincident basolateral ERM-1::GFP displacement, all rarely seen in either single mutant/RNAi condition (Fig. 5B,D, Emb-EL/B, L1-EL/B). The ability of mild chc-1 loss to accelerate the development of the SL loss-mediated polarity phenotype was also reflected in the earlier arrest of chc-1(b1025);sptl-1(RNAi) double mutants (embryonic versus larval lethality; Fig. 5C).
We conclude that CHC-1/AP-1 and SLs contribute to the same or a parallel apical sorting function during polarized membrane biogenesis, supporting a role of clathrin/AP-1 on an apical trafficking route.
GFP::CHC-1 vesicles assemble underneath the apical membrane cytoskeleton and associate with BODIPY-Cer vesicles near Golgi membranes
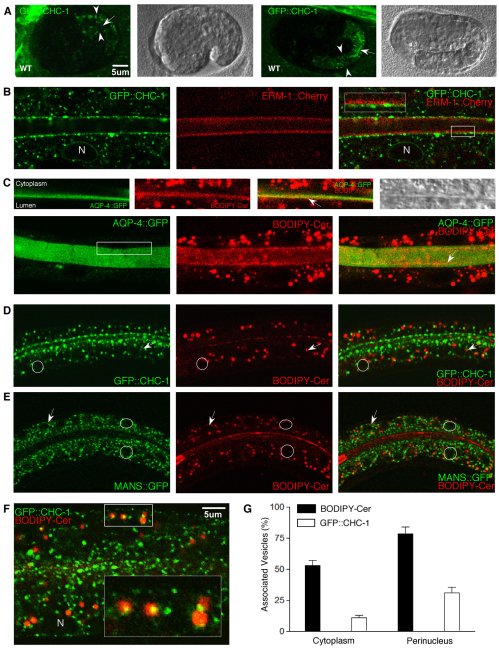
CHC-1/AP-1 and SLs could act sequentially or concomitantly on the same vesicle population or on different vesicles traveling along the same or an associated route. To assess their potential physical interaction in polarized trafficking, the subcellular distribution of vesicle- and plasma membrane-associated clathrin and SLs was examined during wild-type intestinal development. An intestine-specific vha-6p-gfp::chc-1 transgene generates pan-cytoplasmic GFP puncta, which overlay a CHC-1 antibody and a chc-1p-gfp::chc-1 transgene that partially rescues clathrin function-defective dnj-25(RNAi) animals (Greener et al., 2001; Sato et al., 2009). From approximately the time of intestinal lumen formation, GFP::CHC-1 puncta asymmetrically assembled in a linear fashion along the apical membrane, assuming their adult intestinal expression pattern (Fig. 6A,B). Unexpectedly, these clathrin puncta were found to collect underneath the ERM-1-associated submembranous cytoskeleton, suggesting that they are not coated pits and might serve other than endocytic functions (Fig. 6B; supplementary material Fig. S8A).
Fig. 6.
Subcellular localization and association of GFP::CHC-1 and BODIPY-Cer in wild-type intestines. (A) Confocal and Nomarski images of comma (left) and 2-fold (right) embryo with GFP::CHC-1 vesicles assembling at the intestinal lumen (arrows). Intestinal width is indicated by arrowheads. (B) Punctate and linear GFP::CHC-1 assembles underneath the lumenal cytoskeleton, adjacent to, but not overlapping with, ERM-1::Cherry (higher magnification in inset), and assembles perinuclearly (vha-6-gfp::chc-1 shown). N, nucleus. (C) BODIPY-Cer colocalizes with transmembranous AQP-4::GFP at the apical/lumenal membrane. (Top) Isolated single lumenal membrane sleeve (equivalent to boxed area, lower left; compare with supplementary material Fig. S8B). Note that BODIPY-Cer extends into the lumen (arrows). (Bottom) Both lumenal membrane sleeves. Note the irregular BODIPY-Cer puncta and patches in the lumen, adjacent to the apical membrane (arrows). (D) GFP::CHC-1 assembles with BODIPY-Cer perinuclearly (for clarity, one nucleus is circled and one arrowed). Note the weak perinuclear GFP::CHC-1 rings decorated with BODIPY-Cer vesicles (arrows, right). (E) BODIPY-Cer vesicles close to, but not overlapping, MANS::GFP-labeled Golgi membranes. Note the identical perinuclear pattern as in D (examples of nuclei indicated as in D). (F) A subset of BODIPY-Cer vesicles partially overlaps GFP::CHC-1 (yellow). Boxed region is shown at higher magnification in the inset. (G) Quantification of cytoplasmic and perinuclear vesicle association. Mean ± s.e.m. (n=3, N>200 vesicles per counting).
BODIPY-labeled ceramide [BODIPY-Cer; Cer is the immediate precursor of glucosylceramide (GlcCer), the GSL backbone] and NBD-labeled GlcCer, when fed to C. elegans, also localize to intestinal puncta and additionally label the lumenal membrane (Zhang et al., 2011). GSLs are enriched at, and function on, lumenal leaflets of Golgi, vesicle and plasma membranes (Simons and Gerl, 2010). Several lines of evidence indicated that exogenous BODIPY-Cer at least partially reflects the endogenous location and function of GSLs: red and green fluorescent BODIPY-Cer colocalized at apical membranes and formed fully overlapping puncta and ring structures with reciprocal bleaching of fluorescence, suggesting that they share endomembranes and plasma membranes (supplementary material Fig. S8D, left column); BODIPY-Cer puncta overlapped NBD-GlcCer and BODIPY-sphingomyelin (SM) puncta (supplementary material Fig. S8D, middle columns) and colocalized with endosomal markers (see below); BODIPY-Cer and NBD-GlcCer partially rescue SL-dependent polarity defects and are themselves displaced during polarity conversion (Zhang et al., 2011).
The plasma membrane-associated position of BODIPY-Cer was found to be apical of ERM-1, overlapping the integral membrane protein AQP-4, and slightly extending on the lumenal side (where it collected in small puncta; Fig. 6C; supplementary material Fig. S8B). Cytoplasmic BODIPY-Cer vesicles, like GFP::CHC-1 vesicles, formed perinuclear patterns juxtaposed to MANS+ Golgi membranes (Fig. 6D-E). BODIPY-Cer vesicle subfractions partially or fully overlapped RAB-11 puncta and were surrounded by RAB-7 rings, but did not colocalize with RAB-5+ or RAB-10+ endosomes or LMP-1+ lysosomes (supplementary material Fig. S8D; Fig. S9A,B).
We assessed BODIPY-Cer and GFP::CHC-1 colocalization by sequential confocal scanning of thin sections (to exclude false-positive overlay) and tightening of channel spectra to reduce autofluorescence (small vesicles are preferentially lost with this approach; see Materials and methods). Under these conditions, more than 50% of cytoplasmic BODIPY-Cer vesicles associated (partially overlapped or were in contact) with GFP::CHC-1 vesicles (versus ∼10% of GFP::CHC-1 vesicles associating with BODIPY-Cer vesicles; Fig. 6F,G). Perinuclearly, the association increased to over 70% for BODIPY-Cer vesicles (and ∼30% for GFP::CHC-1 vesicles).
We conclude that the subcellular localization and partial association of BODIPY-Cer and GFP::CHC-1 are compatible with the possibility that clathrin and vesicle SLs interact at one or several steps on an apically directed post-Golgi vesicular trafficking route during lumenal membrane biogenesis.
The asymmetric distribution of GFP::CHC-1 and BODIPY-Cer vesicles to polarized membrane domains is dependent on each other and on AP-1
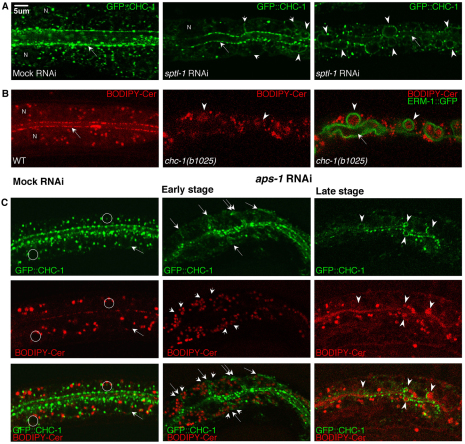
To examine whether the distribution and partial association of GFP::CHC-1 and BODIPY-Cer vesicles was specific and relevant to polarized membrane biogenesis, we followed their subcellular localization during polarity conversion subsequent to perturbing clathrin/AP-1 or SL biosynthesis. Interference with SL biosynthesis displaced GFP::CHC-1 vesicles to lateral membranes during early stages, and to ectopic lumenal membranes during later stages of polarity conversion, and it decreased the submembranous apical clathrin population and the overall number of clathrin vesicles (Fig. 7A; supplementary material Fig. S10A). Conversely, clathrin depletion basolaterally misassembled Cer vesicles and decreased their overall number (Fig. 7B). BODIPY-Cer became displaced to ectopic lateral lumens, lateral to the lateralized ERM-1::GFP, during late stage polarity conversion.
Fig. 7.
Interdependent and AP-1-dependent polarized distribution of GFP::CHC-1 and BODIPY-Cer vesicles. Confocal images of control (left) versus early (middle) and late (right) stage mutant/RNAi L1 intestinal polarity phenotypes (except middle image in B, which shows a late stage phenotype). (A) GFP::CHC-1 misplacement to basolateral (short arrows) and ectopic lumenal membranes (arrowheads) by sptl-1 RNAi. Note the concomitant GFP::CHC-1 reduction at apical membrane (long arrows) and nuclei (N) (obscured by ectopic lumens in late stage phenotype). (B) BODIPY-Cer misplacement to ectopic lateral lumens (arrowheads) in chc-1(b1025) on the lateral/lumenal side of ERM-1::GFP. Note the concomitant apical BODIPY-Cer loss (long arrows; wild-type lumenal membrane staining is obscured by intralumenal BODIPY-Cer patches; compare with Fig. 6C). (C) GFP::CHC-1 and BODIPY-Cer vesicle biogenesis, localization and association require APS-1. (Left column) Wild-type GFP::CHC-1 and BODIPY-Cer vesicles assemble perinuclearly (examples indicated by circles, arrows) and at the lumenal membrane. (Middle column) Basolateral displacement of GFP::CHC-1 (long arrows; note placement on both sides of lateral membrane indicated by double arrow) and BODIPY-Cer vesicles (short arrows) during early polarity conversion. (Right column) GFP::CHC-1 and BODIPY-Cer assemble at ectopic lumens (arrowheads) during later stages of polarity conversion; GFP::CHC-1 is placed at the cytoplasmic side and BODIPY-Cer at the lumenal side of ectopic lumens.
We next asked whether the association and co-dependent polarized distribution of GFP::CHC-1 and BODIPY-Cer vesicles was AP-1 dependent. aps-1 but not apa-2 RNAi displaced GFP::CHC-1 and BODIPY-Cer to basolateral membranes and to ectopic lateral lumens (Fig. 7C). aps-1 RNAi also reduced the apical membrane-associated GFP::CHC-1 population and the number of GFP::CHC-1 vesicles (Fig. 7C; supplementary material Fig. S10A), and it abolished the perinuclear assembly of GFP::CHC-1 and BODIPY-Cer vesicles, eliminating their Golgi-proximal association (Fig. 7C).
To determine whether GFP::CHC-1 and BODIPY-Cer vesicles might be secondarily recruited to an apically transformed lateral membrane domain during polarity conversion, the temporal relationship between vesicle misrouting and the displacement of apical membrane components was examined. The lateral displacement of BODIPY-Cer upon aps-1 RNAi was found to occur independently of ERM-1::GFP basolateral displacement (supplementary material Fig. S10B,C). Moreover, laterally assembled BODIPY-Cer vesicles occasionally overlapped with transient ERM-1::GFP vesicles that formed prior to the basolateral displacement of ERM-1::GFP at the initial stage of polarity conversion (supplementary material Fig. S10B,C) (Zhang et al., 2011).
We conclude that the polarized membrane association of GFP::CHC-1 and BODIPY-Cer vesicles is dependent on each other and on AP-1, raising the possibility that SL-rich vesicle membranes, at least transiently, recruit clathrin through AP-1 at Golgi and/or post-Golgi endosomal membranes to generate an apically directed vesicle population. The displacement of these vesicles early during polarity conversion is compatible with their initial and direct contribution to membrane polarization and lumen biogenesis.
DISCUSSION
Clathrin/AP-1 regulate apical polarity and lumen formation in the developing C. elegans intestine
Clathrin functions on many, particularly endocytic, trafficking routes, but has not been implicated on biosynthetic routes towards the apical membrane (McMahon and Boucrot, 2011). Recently, however, clathrin was shown to regulate basolateral polarity in mammalian epithelial cell lines, largely dependent on its epithelial cell-specific adaptor AP-1B, characterized by its mu1B subunit (Deborde et al., 2008; Folsch et al., 1999). Our findings now reveal a role for clathrin/AP-1 in the regulation of apical polarity in the expanding C. elegans intestinal epithelium. The requirement of several AP-1 subunits for apical polarity suggests a sorting function that cannot be exclusively attributed to subunit specificity. We and others (Shafaq-Zadah et al., 2012) note, however, that RNAi with APM-1/mu but not UNC-101/mu [which are both ubiquitously expressed and equally similar to mu1A and mu1B (Shim et al., 2000)] generates apical polarity defects [as confirmed in a presumed unc-101 null mutant (Shafaq-Zadah et al., 2012)]. UNC-101 directs polarized vesicular transport along dendrites, which, although anterograde, might involve a basolateral component (Dwyer et al., 2001). A different mu/UNC-101-specific sorting function, perhaps one that is tissue specific, is thus not excluded. Basolateral sorting appears to be also affected by loss of CHC-1/AP-1, albeit to a lesser degree in our hands. Although apical mis-sorting might secondarily affect basolateral membrane components, a role of CHC-1/AP-1 in both apical and basolateral transport could be envisioned as an early sorting step requiring additional signals, or as a directional switch of apical and basolateral cargo or vesicles.
In vivo interference with CHC-1 and several AP-1 subunits, however, primarily causes an apical polarity defect in the C. elegans intestine, with complete transformation of lateral into apical membrane domains. This phenotype closely resembles the C. elegans intestinal polarity defect caused by the depletion of GSLs, which are membrane lipids with a documented role in apical sorting (Simons and Gerl, 2010; Zhang et al., 2011). Loss of CHC-1/AP-1 revealed a similar, apparently junction-independent, conversion of apicobasal membrane domain identities, with subsequent ectopic lumen formation, likewise suggesting a defect in apical sorting. This phenotype furthermore resembles the microvillus inclusion disease phenotype, which has also been linked to polarized trafficking based on its underlying genetic mutations in the unconventional myosin MYO5B in humans and RAB8 in mice (Cutz et al., 1989; Muller et al., 2008; Sato et al., 2007).
Clathrin/AP-1 cooperate with SLs in the regulation of polarity and may function on an apical route
In principle, two trafficking routes could be perturbed by a clathrin/AP-1- and SL-dependent sorting defect: (1) an apical biosynthetic/exocytic route (direct, transcytotic or recycling), delivering apical cargo to (or back to) the apical membrane (and/or its junctions); or (2) a basolateral endocytic route, removing apical membrane (or junction) components from the basolateral membrane (this argument disregards the possibility of defects in the transport of polarized membrane domain inhibitors).
The following findings support a role of CHC-1/AP-1 on a membrane-directed apical route: CHC-1/AP-1 depletion phenocopies the polarity defect induced by the loss of GSL-biosynthetic enzymes that genetically interact; it induces apical loss and basolateral gain of apical characteristics on expanding membranes, compatible with apical misrouting; it removes apical membrane-forming (such as RAB-11) vesicles from the lumen; AP-1 loss depletes clathrin-coated vesicles and SLs from the lumen and misplaces both to the basolateral membrane; and CHC-1 associates with Cer-rich vesicles near the Golgi and the endocytic recycling compartment, which are documented sorting stations for membrane-directed transport (Rodriguez-Boulan et al., 2005). We have no evidence for a role of the clathrin endocytic AP-2 adaptor in this process, although its function in the C. elegans intestine is unclear and was only tested here by RNAi (of note, apa-2 RNAi was, however, able to enhance clathrin lethality; data not shown). Together, these findings could suggest a scenario in which SL-rich membrane microdomains recruit AP-1/clathrin at Golgi and/or post-Golgi endosomal membranes to regulate apical sorting. A similar process was recently proposed for PtdIns(3,4,5)P3 (PIP3) recruiting AP-1B to recycling endosomes to regulate basolateral sorting (Fields et al., 2009).
This interpretation has several implications. First, it places clathrin/AP-1 on a novel apical biosynthetic route, whereas their role in the removal of apical membrane components from basolateral membranes would place these molecules on previously established endocytic and perhaps endosome-to-lysosome-directed routes. However, the anterograde sorting function of CHC-1/AP-1 at the Golgi, although not apically directed, is well characterized (Sachse et al., 2002). There is also evidence for the presence and function of clathrin/AP-1 on other apically destined vesicle populations: Drosophila AP-1 localizes to both Golgi and endosomal membranes and colocalizes with RAB-11 (Benhra et al., 2011); AP-1 functions in the biogenesis of apically moving secretory granules (Burgess et al., 2011; Lui-Roberts et al., 2005); and a CHC-1/AP-1-dependent plasma membrane-directed secretory endo-lysosomal compartment was recently characterized (Laulagnier et al., 2011).
Second, it implies a convergence of clathrin-dependent and SL/lipid microdomain (raft)-dependent trafficking pathways, which are currently thought of as distinct trafficking machineries in endocytosis (Grant and Donaldson, 2009; Le Roy and Wrana, 2005). However, clathrin/AP-1-dependent sorting functions of SLs have been reported on plasma, Golgi and endosomal membranes that include apical trafficking routes (Masuyama et al., 2009; Puri et al., 2001). Of note, clathrin-independently endocytosed BODIPY-Cer (as used in this study) was found on clathrin-dependently endocytosed vesicles, and Cer-enriched microdomains were returned to the plasma membrane via RAB-11 recycling endosomes, demonstrating that these two components can converge on a single apically directed vesicle (Sharma et al., 2003).
Third, it suggests that the submembranous apical string of GFP::CHC-1 vesicles, recruited to the lumen during its biogenesis, contains apically targeted vesicles. Plasma membrane-associated fluorescently labeled clathrin vesicles are generally interpreted as coated pits involved in AP-2-dependent endocytosis (Greener et al., 2001; Sato et al., 2009). TEM, live-cell imaging and single-molecule tracking are, however, currently redefining clathrin coats and clathrin vesicle populations at plasma membranes, some of which are AP-1 associated (Keyel et al., 2004; Mattheyses et al., 2011; Saffarian et al., 2009).
Clathrin-coated and SL-rich vesicles assemble at polarized domains of expanding plasma membranes in a co-dependent and AP-1-dependent manner
CHC-1/AP-1-mediated sorting could either directly deliver multiple apical membrane components or could secondarily determine apical membrane domains through the direct or indirect recruitment of specific polarity determinants. PAR-6 is an obvious candidate downstream effector for this apical polarity pathway, possibly recruited through CDC-42 (see Shafaq-Zadah et al., 2012). Another candidate effector is ERM-1/ezrin, which is proposed to be sufficient to initiate apical membrane and microvillus biogenesis (ten Klooster et al., 2009; Zhu et al., 2010). Lateral displacement of such molecules could initiate the transformation of lateral into apical domains and generate lateral lumens.
Intriguingly, however, we find that the interference with CHC-1/AP-1 and/or SL biosynthesis not only misdirects polarized membrane components, but also mislocalizes entire vesicle populations during polarized membrane biogenesis. Moreover, the assembly of Cer-labeled vesicles at ectopic sites of apical membrane biogenesis during the initial phase of polarity conversion in the C. elegans intestine raises the possibility that these vesicles are primary effectors of membrane polarization, rather than being secondarily recruited or generated by the apical or apically altered lateral membrane domain. The sorting process itself might thus directly contribute to determining polarized plasma membrane domains, with CHC-1/AP-1 conferring directional cues to the vesicles themselves. AP-1/CHC-1 recruitment by SL-rich vesicle membranes could, for example, choose, set in place, or enable vesicles to use a specific actomyosin or microtubule track for their directional movement to, or back to, the apical membrane. For instance, both clathrin and AP-1 have been implicated in microtubule connections: the N-terminus of CHC binds directly to the spindle, serving the trafficking-independent role of clathrin in mitosis (Royle et al., 2005); and the AP-1 accessory molecule Gadkin associates with the kinesin motor KIF5, directly linking AP-1-associated vesicles and microtubules in mammalian cells (Schmidt et al., 2009). Directional vesicle movements during apical membrane and lumen biogenesis have been observed early on, in vivo and in vitro, and include the shift of the entire trans-Golgi endomembrane system during MDCK polarity conversion towards the new apical membrane (Wang et al., 1990; Rodriguez-Fraticelli et al., 2011).
Supplementary Material
Acknowledgments
We thank D. Baillie (Fraser University, Burnaby, BC, Canada), H. Fares (University of Arizona, Tucson, AZ, USA), K. Kemphues (Cornell University, Ithaca, NY, USA), T. Kurzchalia (Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany), T. Lamitina (University of Pennsylvania, Philadelphia, PA, USA), K. Nehrke (University of Rochester Medical Center, Rochester, NY, USA), G. Ruvkun (Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA), J. Simske (Case Western Reserve University School of Medicine, Cleveland, OH, USA), K. Strange (Vanderbilt University Medical Center, Salisbury Cove, ME, USA), the Caenorhabditis Genetics Center (NIH Center for Research Resources) and S. Mitani (National Bioresource Project, Tokyo University, Japan) for strains, plasmids and antibodies and in particular B. Grant (Rutgers University, Piscataway, NJ, USA) for kindly providing numerous strains pertinent to this study; G. Ruvkun for the lethal RNAi library; M. McKee (MGH Microscopy Core/partially funded by the IBD grant DK43351 and BA DE award DK57521) for TEM work; E. Membreno for C. elegans maintenance; and H. Weinstein and A. Walker (Massachusetts General Hospital) for ongoing support.
Footnotes
Funding
This work was supported by the National Institutes of Health [grants HD044589 and GM078653] and a Mattina R. Proctor Award to V.G. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.077347/-/DC1
References
- Antonescu C. N., Aguet F., Danuser G., Schmid S. L. (2011). Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol. Biol. Cell 22, 2588–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore M., Mathies L. D., Pugnale P., Moulder G., Barstead R., Kimble J., Puoti A. (2002). The MEP-1 zinc-finger protein acts with MOG DEAH box proteins to control gene expression via the fem-3 3′ untranslated region in Caenorhabditis elegans. RNA 8, 725–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhra N., Lallet S., Cotton M., Le Bras S., Dussert A., Le Borgne R. (2011). AP-1 controls the trafficking of Notch and Sanpodo toward E-cadherin junctions in sensory organ precursors. Curr. Biol. 21, 87–95 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. M., Datta A., Rodriguez-Fraticelli A. E., Peranen J., Martin-Belmonte F., Mostov K. E. (2010). A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 12, 1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess J., Jauregui M., Tan J., Rollins J., Lallet S., Leventis P. A., Boulianne G. L., Chang H. C., Le Borgne R., Kramer H., et al. (2011). AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila. Mol. Biol. Cell 22, 2094–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Schweinsberg P. J., Vashist S., Mareiniss D. P., Lambie E. J., Grant B. D. (2006). RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol. Biol. Cell 17, 1286–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutz E., Rhoads J. M., Drumm B., Sherman P. M., Durie P. R., Forstner G. G. (1989). Microvillus inclusion disease: an inherited defect of brush-border assembly and differentiation. N. Engl. J. Med. 320, 646–651 [DOI] [PubMed] [Google Scholar]
- Deborde S., Perret E., Gravotta D., Deora A., Salvarezza S., Schreiner R., Rodriguez-Boulan E. (2008). Clathrin is a key regulator of basolateral polarity. Nature 452, 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G., De Camilli P. (2006). Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 [DOI] [PubMed] [Google Scholar]
- Dwyer N. D., Adler C. E., Crump J. G., L’Etoile N. D., Bargmann C. I. (2001). Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron 31, 277–287 [DOI] [PubMed] [Google Scholar]
- Entchev E. V., Schwudke D., Zagoriy V., Matyash V., Bogdanova A., Habermann B., Zhu L., Shevchenko A., Kurzchalia T. V. (2008). LET-767 is required for the production of branched chain and long chain fatty acids in Caenorhabditis elegans. J. Biol. Chem. 283, 17550–17560 [DOI] [PubMed] [Google Scholar]
- Fields I. C., King S. M., Shteyn E., Kang R. S., Folsch H. (2009). Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B-dependent sorting in polarized epithelial cells. Mol. Biol. Cell 21, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H., Ohno H., Bonifacino J. S., Mellman I. (1999). A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99, 189–198 [DOI] [PubMed] [Google Scholar]
- Gobel V., Barrett P. L., Hall D. H., Fleming J. T. (2004). Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev. Cell 6, 865–873 [DOI] [PubMed] [Google Scholar]
- Grant B., Hirsh D. (1999). Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. D., Donaldson J. G. (2009). Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener T., Grant B., Zhang Y., Wu X., Greene L. E., Hirsh D., Eisenberg E. (2001). Caenorhabditis elegans auxilin: a J-domain protein essential for clathrin-mediated endocytosis in vivo. Nat. Cell Biol. 3, 215–219 [DOI] [PubMed] [Google Scholar]
- Hall D. H. (1995). Electron microscopy and three-dimensional image reconstruction. Methods Cell Biol. 48, 395–436 [DOI] [PubMed] [Google Scholar]
- Hobert O. (2002). PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32, 728–730 [DOI] [PubMed] [Google Scholar]
- Keyel P. A., Watkins S. C., Traub L. M. (2004). Endocytic adaptor molecules reveal an endosomal population of clathrin by total internal reflection fluorescence microscopy. J. Biol. Chem. 279, 13190–13204 [DOI] [PubMed] [Google Scholar]
- Kuervers L. M., Jones C. L., O’Neil N. J., Baillie D. L. (2003). The sterol modifying enzyme LET-767 is essential for growth, reproduction and development in Caenorhabditis elegans. Mol. Genet. Genomics 270, 121–131 [DOI] [PubMed] [Google Scholar]
- Laulagnier K., Schieber N. L., Maritzen T., Haucke V., Parton R. G., Gruenberg J. (2011). Role of AP1 and Gadkin in the traffic of secretory endo-lysosomes. Mol. Biol. Cell 22, 2068–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy C., Wrana J. L. (2005). Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 6, 112–126 [DOI] [PubMed] [Google Scholar]
- Leung B., Hermann G. J., Priess J. R. (1999). Organogenesis of the Caenorhabditis elegans intestine. Dev. Biol. 216, 114–134 [DOI] [PubMed] [Google Scholar]
- Lui-Roberts W. W., Collinson L. M., Hewlett L. J., Michaux G., Cutler D. F. (2005). An AP-1/clathrin coat plays a novel and essential role in forming the Weibel-Palade bodies of endothelial cells. J. Cell Biol. 170, 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., Mostov K. (2007). PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama N., Kuronita T., Tanaka R., Muto T., Hirota Y., Takigawa A., Fujita H., Aso Y., Amano J., Tanaka Y. (2009). HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J. Biol. Chem. 284, 15927–15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheyses A. L., Atkinson C. E., Simon S. M. (2011). Imaging single endocytic events reveals diversity in clathrin, dynamin and vesicle dynamics. Traffic 12, 1394–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H. T., Boucrot E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 [DOI] [PubMed] [Google Scholar]
- Mellman I., Nelson W. J. (2008). Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 9, 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. (1991). Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Shakes D. C. (1995). Immunofluorescence microscopy. Methods Cell Biol. 48, 365–394 [PubMed] [Google Scholar]
- Miskowski J., Li Y., Kimble J. (2001). The sys-1 gene and sexual dimorphism during gonadogenesis in Caenorhabditis elegans. Dev. Biol. 230, 61–73 [DOI] [PubMed] [Google Scholar]
- Muller T., Hess M. W., Schiefermeier N., Pfaller K., Ebner H. L., Heinz-Erian P., Ponstingl H., Partsch J., Rollinghoff B., Kohler H., et al. (2008). MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 40, 1163–1165 [DOI] [PubMed] [Google Scholar]
- Nomura K. H., Murata D., Hayashi Y., Dejima K., Mizuguchi S., Kage-Nakadai E., Gengyo-Ando K., Mitani S., Hirabayashi Y., Ito M., et al. (2011). Ceramide glucosyltransferase of the nematode Caenorhabditis elegans is involved in oocyte formation and in early embryonic cell division. Glycobiology 21, 834–848 [DOI] [PubMed] [Google Scholar]
- Puri V., Watanabe R., Singh R. D., Dominguez M., Brown J. C., Wheatley C. L., Marks D. L., Pagano R. E. (2001). Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154, 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Kreitzer G., Musch A. (2005). Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6, 233–247 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli A. E., Galvez-Santisteban M., Martin-Belmonte F. (2011). Divide and polarize: recent advances in the molecular mechanism regulating epithelial tubulogenesis. Curr. Opin. Cell Biol. 23, 638–646 [DOI] [PubMed] [Google Scholar]
- Royle S. J., Bright N. A., Lagnado L. (2005). Clathrin is required for the function of the mitotic spindle. Nature 434, 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse M., Urbe S., Oorschot V., Strous G. J., Klumperman J. (2002). Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell 13, 1313–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffarian S., Cocucci E., Kirchhausen T. (2009). Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol. 7, e1000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Ernstrom G. G., Watanabe S., Weimer R. M., Chen C. H., Sato M., Siddiqui A., Jorgensen E. M., Grant B. D. (2009). Differential requirements for clathrin in receptor-mediated endocytosis and maintenance of synaptic vesicle pools. Proc. Natl. Acad. Sci. USA 106, 1139–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Mushiake S., Kato Y., Sato K., Sato M., Takeda N., Ozono K., Miki K., Kubo Y., Tsuji A., et al. (2007). The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 448, 366–369 [DOI] [PubMed] [Google Scholar]
- Schmidt M. R., Maritzen T., Kukhtina V., Higman V. A., Doglio L., Barak N. N., Strauss H., Oschkinat H., Dotti C. G., Haucke V. (2009). Regulation of endosomal membrane traffic by a Gadkin/AP-1/kinesin KIF5 complex. Proc. Natl. Acad. Sci. USA 106, 15344–15349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaq-Zadah M., Brocard L., Solari F., Michaux G. (2012). AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development 139, 2061–2070 [DOI] [PubMed] [Google Scholar]
- Sharma D. K., Choudhury A., Singh R. D., Wheatley C. L., Marks D. L., Pagano R. E. (2003). Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278, 7564–7572 [DOI] [PubMed] [Google Scholar]
- Shim J., Sternberg P. W., Lee J. (2000). Distinct and redundant functions of mu1 medium chains of the AP-1 clathrin-associated protein complex in the nematode Caenorhabditis elegans. Mol. Biol. Cell 11, 2743–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Gerl M. J. (2010). Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 11, 688–699 [DOI] [PubMed] [Google Scholar]
- ten Klooster J. P., Jansen M., Yuan J., Oorschot V., Begthel H., Di Giacomo V., Colland F., de Koning J., Maurice M. M., Hornbeck P., et al. (2009). Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev. Cell 16, 551–562 [DOI] [PubMed] [Google Scholar]
- Timmons L., Court D. L., Fire A. (2001). Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103–112 [DOI] [PubMed] [Google Scholar]
- van Meer G., Voelker D. R., Feigenson G. W. (2008). Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. Z., Ojakian G. K., Nelson W. J. (1990). Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J. Cell Sci. 95, 153–165 [DOI] [PubMed] [Google Scholar]
- Zhang H., Abraham N., Khan L. A., Hall D. H., Fleming J. T., Gobel V. (2011). Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat. Cell Biol. 13, 1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Crothers J., Jr, Zhou R., Forte J. G. (2010). A possible mechanism for ezrin to establish epithelial cell polarity. Am. J. Physiol. Cell Physiol. 299, C431–C443 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.