Abstract
The 19-transmembrane multi-subunit γ-secretase complex generates the amyloid β-peptide (Aβ) of Alzheimer’s disease (AD) by intramembrane proteolysis of the β-amyloid precursor protein (APP). Despite substantial advances in elucidating how this protein complex functions, the effect of the local membrane lipid microenvironment on γ-secretase cleavage of substrates is still poorly understood. Using detergent-free proteoliposomes to reconstitute purified human γ-secretase, we examined the effects of fatty acyl (FA) chain length, saturation and double-bond isomerisation, and membrane lipid polar head groups on γ-secretase function. We analyzed γ-secretase activity and processivity (i.e., sequential cleavages of the APP transmembrane domain that convert longer Aβ species (e.g., Aβ46) into shorter ones (e.g, Aβ40)) by quantifying the APP intracellular domain (AICD) and various Aβ peptides, including via a bicine/urea gel system that detects multiple Aβ lengths. These assays revealed several trends: (1) switching from a cis to a trans isomer of a monounsaturated FA chain in phosphatidylcholine (PC) increased γ-activity, did not affect Aβ42/40 ratios, but decreased the ratio of long (≥42) vs. short (≤41) Aβ peptides; (2) increasing FA carbon chain length (14<16<18<20) increased γ-activity, reduced longer Aβ species and reduced Aβ42/40; (3) shifting the position of the double bond in 18:1(Δ9-cis) PC to the Δ6 position substantially reduced activity; (4) gangliosides increased γ-activity but decreased processivity, thus elevating Aβ42/40; (5) phosphatidylserine decreased γ-activity but increased processivity; and (6) phosphatidylinositol strongly inhibited γ-activity. Overall, our results show that subtle changes in membrane lipid composition can greatly influence γ-secretase activity and processivity, suggesting that relatively small changes in lipid membrane composition may affect the risk of AD at least as much as do presenilin or APP mutations.
Alzheimer’s disease (AD) is a neurodegenerative disorder defined by abundant intracellular neurofibrillary tangles of the tau protein and extracellular plaques of the amyloid β-protein (Aβ), leading to progressive loss of memory and cognition (1). The β-amyloid precursor protein (APP) can be processed through either an amyloidogenic or a non-amyloidogenic pathway. The former is initiated by β-secretase cleavage of holoAPP, leading to shedding of the ectodomain (APPs-β) and a 99-residue C-terminal fragment (C99) remaining embedded within the membrane (2, 3). In contrast, non-amyloidogenic processing occurs when the initial ectodomain shedding is caused by α-secretase, leading to release of a slightly longer ectodomain (APPs-α) and leaving a membrane-bound 83-residue stub (C83) (4). Subsequent intramembrane proteolysis of C99 or C83 by γ-secretase leads to the release of the APP intracellular domain (AICD) and either Aβ peptides (5) or p3 peptides (6), respectively. γ-secretase is 19-transmembrane domain aspartyl protease comprised of presenilin (PS1 or PS2 isoform), nicastrin (Nct), anterior pharynx defective-1 (Aph1αL, Aph1αS, or Aph1β isoform), and presenilin enhancer-2 (Pen-2), which are necessary and sufficient for γ-activity (7-9). γ-secretase is responsible for the second and final step in regulated intramembrane proteolysis (RIP) of a large and increasing number of substrates, including APP and Notch (10). A further complexity of γ-secretase function is that cleavage of the membrane-anchored C99 occurs at multiple sequential peptide bonds, starting with ε-cleavage to release AICD from the membrane, leaving a 49- or 48- residue long Aβ. Sequential cleavages of Aβ48/49 every 3-4 residues moving N-terminally occurs first at the so-called ζ-site to produce Aβ45/46, then at the γ-site to produce Aβ42/43 and finally at the γ’ site to produce Aβ38/40 peptides (11). Aβ42 is generally considered the most pathogenic Aβ species, with an elevated Aβ42/Aβ40 ratio used as a marker of pathogenicity, although recently, Aβ43 has also been shown to be pathogenically relevant in vivo (12). The degree to which the initial ε-cleavage products Aβ48 and Aβ49 are trimmed by γ-secretase to shorter Aβ peptides is termed processivity (13). Much work has focused on the understanding of how mutations (13-16), protein cofactors (17, 18) and small organic compounds (19, 20) can modify γ-secretase processing of APP, but far less on how the local membrane lipid environment could also affect function.
Evidence suggests that lipid composition is altered in AD brain tissue but whether this is a cause or effect or both is unclear (21, 22). Most studies of lipid effects on γ-secretase function have focused on cholesterol, including on its high concentration in detergent-resistant membrane microdomains (DRMs) (23, 24), where APP, BACE1 and γ-secretase can all be found [reviewed in (25)]. Moreover, epidemiological evidence suggests that cholesterol-lowering drugs (statins) may reduce AD risk, but whether statins can be used to prevent or treat AD is controversial (26). In addition to cholesterol, there are a large number of other lipid types present in membranes (27).
Bilayer-forming lipids can differ in various attributes, including fatty acyl (FA) chain length, level, position and type of unsaturation, as well as membrane lipid polar head group type. FA chain length has direct effects on membrane fluidity and thickness, the latter of which could affect the Aβ40/Aβ42 ratio (28). With the advent of food processing over the last century, the modern human diet now contains elevated levels of trans isomer fatty acids, which have been linked with an increased risk of coronary heart disease (29). Whether such “trans fats” may also increase the risk of AD is not clear. Trans fats have been reported to increase amyloidogenic and decrease nonamyloidogenic processing of APP in vitro and ex vivo (30), and some evidence suggests an elevated risk of AD with high dietary saturated and trans-unsaturated fats (31). Another report suggested no association between high intake of trans unsaturated fats, cholesterol or other fats and an increased risk of developing AD (32).
The direct effects of membrane lipid composition on the processing of APP by γ-secretase have been studied very little, and clarifying this issue may suggest the involvement of particular lipids in AD pathogenesis as well as guide dietary and therapeutic strategies for reducing AD risk. We recently developed a method to reconstitute purified human γ-secretase complexes into lipid vesicles of defined composition (33). Using this system, we have systematically tested the effects of membrane bilayer composition on Aβ generation, varying certain specific features, including FA chain length, the degree, position and type of FA chain unsaturation, and the membrane lipid polar head group. We report here that bilayer composition can have profound effects on the production of Aβ, the Aβ42/40 ratio, and the processive trimming of long Aβ peptides to shorter, secreted forms.
Experimental procedures
Reagents
All lipids were from Avanti Polar Lipids. POPC, SOPC and specific FA chain-containing PC are semi-synthetic. L-α-phosphatidylcholine (PC), L-α-phosphatidylethanolamine (PE), L-α-phosphatidylserine (PS), total ganglioside extract (GS), sphingomyelin (SM), and whole brain lipid extract (WB) are from porcine brain, L-α-phosphatidylinositol (PI) from bovine liver, and L-α-phosphatidic acid (PA) from chicken egg.
Purification of γ-secretase and recombinant substrate
High grade purification of human γ-secretase from our S20 cell line (co-expressing untagged human PS1 and human Nct-V5/His, Aph1αL-HA and FLAG-Pen-2) was performed by a multi-step protocol (34). Purification of recombinant C100-FLAG was performed as described (35).
Reconstitution of γ-secretase to form proteoliposomes
Proteoliposome preparation was performed as described (33) with the following modifications: 1) Hydrated lipids and lipid mixtures were diluted to a total concentration of 1.5 mM in 50 mM HEPES pH 7.2, 150 mM NaCl, 4 mM CHAPSO and purified γ-secretase; 2) Detergent removal (and proteoliposome formation) was carried out by incubating with SM-2 BioBeads (Bio-Rad) at a concentration of 10 mg/1 mg CHAPSO for 2 hr at 4°C, mixing with a HulaMixer (Life Technologies). Vesicle formation was checked by negative-stain electron microscopy as described (33).
In vitro γ-secretase activity assays
Detergent-free γ-secretase proteoliposomes were incubated with 1 μM C100-FLAG at 37°C for 4 hr. Samples were assayed by Western blot for AICD, for Aβ40 and Aβ42 by ELISA (Life Technologies), or by bicine/urea Western blot for Aβ peptides, as described (13).
Cell treatment experiments
CHO-derived S20 cells were seeded at a density of 5×105 cells/well in 6 well plates (Falcon) growing in DMEM/10% FBS (Gibco). 24 hours after initial seeding, hydrated/sonicated lipids (or HEPES buffer alone as a blank control) were added to a final concentration of 10 or 50 μM. 24 hours later, cells were washed twice with 2 ml PBS, then fresh media and lipids were added. A further 24 hours later, media were harvested and cells lysed with RIPA buffer + protease inhibitor cocktail (Roche). Protein concentrations of the clarified lysates were measured by BCA assay (Thermo Scientific).
Western blotting, ELISAs and antibodies
AICD Western blot analysis was performed by electrophoresing activity assay samples on 4-12% Bis/Tris polyacrylamide gel, transferring to polyvinylidene difluoride membrane and probing with anti-human Nicastrin (1:1,000; BD Transduction Laboratories), anti-PS1-NTF (1:1,000; Calbiochem) and anti-FLAG M2 (1:2,000; Sigma). All Western blots were scanned on an Odyssey Infrared Imaging System (Li-Cor), and densitometry analysis used Odyssey software. Aβ40 and Aβ42 from in vitro activity assays were measured by ELISA (Invitrogen). Lipid-treated conditioned media were assayed for Aβ38, Aβ40 and Aβ42 using triplex ELISA (Aβ capture by antibody 4G8) read on a Sector Imager 2400 (Mesoscale Discovery).
Statistical analysis
All quantified data represent an average of at least three independent experiments. Error bars represent mean ± standard error. Comparison between samples was analyzed by one-way ANOVA with a post-test Bonferroni correction.
Results
Because cleavage of substrates by the γ-secretase complex occurs within their transmembrane domains (TMDs) and γ-secretase itself has 19 TMDs and a membrane-embedded active site, the composition of the lipid bilayer intimately surrounding these proteins would be expected to have significant effects on proteolytic processing. With this in mind, we performed activity assays on purified γ-secretase (34) reconstituted into detergent-free lipid vesicles having systematic changes in membrane composition. Using methods modified from our previous membrane lipid study (33), we generated unilamellar proteoliposomes with a mean diameter of 230 nm (95 % confidence interval 207-252 nm; n = 94) as measured by negative-stain electron microscopy (Fig. 1B). Without this detergent removal step, we observed layers of lipid/detergent in irregular shapes (Fig. 1A), with γ-secretase activity unaffected by different FA chain lengths and degrees of saturation (data not shown).
Figure 1. The effect of detergent removal on proteoliposome morphology.
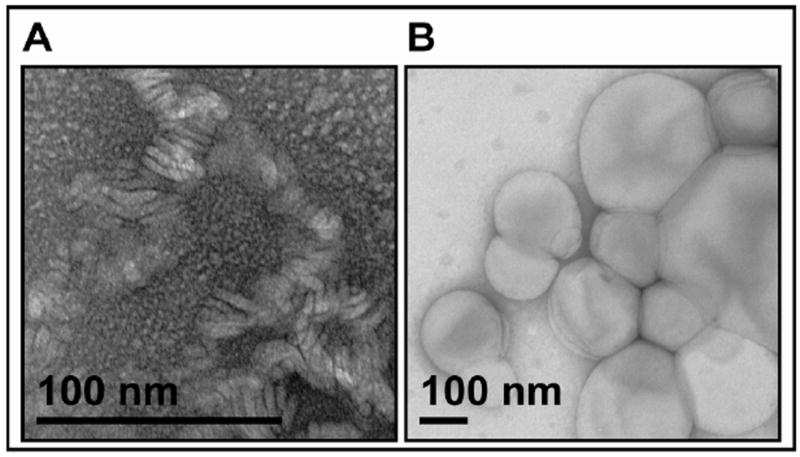
Lipid, CHAPSO and purified γ-secretase complexes were incubated together to form proteoliposomes, then observed by negative staining electron microscopy A) before or B) after detergent removal with SM2 BioBead treatment at 4°C for 2 hr. Note difference in magnification between the two images due to the smaller size of the structures without detergent removal.
Previous studies on effects of the membrane lipid microenvironment have looked at certain polar headgroups (33, 36, 37) but there has been only very limited analysis of the effects of FA chains (30). By changing from cis to trans isomers of mono-unsaturated FA chains, the membrane density and fluidity should be altered due to changes in hydrophobic tail packing (27, 38). With a cis isomer double bond, the FA chain has a kinked orientation, resulting in a less densely packed bilayer and consequently increased fluidity, whereas a trans isomer of the same FA chain length will be more densely packed and have a higher phase transition temperature (i.e. the membrane is less fluid) (39). Given that the 19-TMD γ-secretase complex and its substrates are always embedded in membranes, they need to move within the lipid bilayer to allow substrate docking, lateral gating, cleavage and release of products. As a result, membrane fluidity could affect substrate recognition, handling and proteolysis. In the first half of this report, we describe the effects of FA chain length and isomerisation (cis and trans) with phosphatidylcholine head groups on γ-secretase activity and processivity (see Table 1 for a summary of fatty acyl chains examined). In the second half, we investigate the effects of varying polar headgroups of membrane lipids.
Table 1. Descriptive and common names for the fatty acyl (FA) sidechains used with a phosphocholine head group.
x:y (Δz-cis/trans): x = FA chain length; y = number of double bonds; z = position of double bond relative to carboxyl terminus; cis/trans = isomer of the double bond. In cases where only one name is given, both FA sidechains of PC are the same. For POPC/SOPC, the FA chains are given in the order of carbons of the glycerol backbone (where the phosphocholine head group is bonded to the 3rd carbon).
| Descriptive name | Common name |
|---|---|
| 14:1 (Δ9-cis) | Myristeleic acid |
| 14:1 (Δ9-trans) | Myristelaidic acid |
| 16:0/18:1 (Δ9-cis) | Palmitic/Oleic acid (POPC) |
| 16:1 (Δ9-cis) | Palmitoleic acid |
| 16:1 (Δ9-trans) | Palmitelaidic acid |
| 18:0/18:1 (Δ9-cis) | Stearic/Oleic acid (SOPC) |
| 18:1 (Δ6-cis) | Petroselenic acid |
| 18:1 (Δ9-cis) | Oleic acid |
| 18:1 (Δ9-trans) | Elaidic acid |
| 18:2 (Δ9,12-cis) | Linoleic acid |
| 20:1 (Δ11-cis) | Eicosenoic acid |
| 20:4 (Δ5,8,11,14-cis) | Arachidonic acid (AA) |
| 22:1 (Δ13-cis) | Erucic acid |
| 22:6 (Δ4,7,10,13,16,19-cis) | Docosahexanoic acid (DHA) |
Shifting from a cis to a trans isomer of a FA chain increases γ-secretase activity and can increase Aβ processivity
To address this issue, we chose to study 16- and 18-carbon FA chains, as these are the most common lengths found in natural lipid bilayers. The mean γ-activity observed in the presence of each FA chain type, as determined by in vitro processing of recombinant APP substrate C100-FLAG to AICD-FLAG and Aβ, was compared to the mean activity of a simultaneously analyzed standard lipid condition, namely 100% POPC (palmitic/oleic acid (16:0/18:1 (Δ9-cis) phosphatidylcholine) or else 100% SOPC (stearic/oleic acid (18:0/18:1 (Δ9-cis) phosphatidylcholine).
Phospholipid mixtures of 90% POPC plus 10% of one of four different FA isomers we tested [PC 16:1 (Δ9-cis), 16:1 (Δ9-trans), 18:1 (Δ9-cis) or 18:1 (Δ9-trans)] had little effect on both AICD and Aβ production vs. the 100% POPC standard (Fig. 2A-C, red bars). Increasing the proportion of 3 of these 4 test isomers to 20% and then 50% led to progressive declines in AICD and Aβ40 production, while Aβ42 production was unchanged (Figs. 2A-B, blue and green bars). The one exception to these declines occurred with PC 18:1 (Δ9-trans), mixtures of which actually increased Aß40 relative to control (90:10 p<0.05, 80:20 p<0.001) and to PC 18:1 (Δ9-cis) (p<0.001). However, there were no significant increases in AICD and Aβ42 levels (Fig. 2A-C, right-most sets of bars). When we increased to 100% concentration of each of the four test isomers (yellow bars), the first 3 isomers showed steep declines in AICD and Aβ40 and a ~50-100% fall in Aβ42 (P<0.001). However, the PC 18:1 (Δ9-trans) isomer was again exceptional: at a 100% concentration, it actually increased Aβ40 production by 50% (P<0.05) and left Aβ42 unchanged. In the absence of POPC, shifting from a cis to trans isomer in both PC 16:1 (Δ9) and PC 18:1 (Δ9) led to a significant increase in Aβ42 generation (P<0.001) (Fig. 2C, yellow bars). Generally similar results were obtained when SOPC rather than POPC served as the standard phospholipid. The same relative changes in Aβ40 and Aβ42 levels were seen in an SOPC background, with the exception that at all concentrations tested, PC 18:1 (Δ9-trans) had no difference in Aβ40 production vs. the 100% SOPC control (data not shown). Taken together, these initial results indicate that the isomer of a double bond in a phospholipid FA chain can directly and significantly influence γ-secretase activity.
Figure 2. The effects of cis vs. trans isomers of FA sidechains on γ-secretase activity.

Activity assays using purified γ-secretase and C100-FLAG substrate were performed in detergent-free vesicles comprising different ratios of various FA-containing PCs at a total lipid concentration of 1.5 mM. Assay solutions were analyzed by A) AICD-FLAG Western blot densitometry as a measure of total γ-activity, B) Aβ40 ELISA, and C) Aβ42 ELISA,,enabling the calculation of D) Aβ42/40 ratios for the POPC background, and E) for the SOPC background. Ratios given in the key denote the percentage of POPC (first number) and of specific FA being tested (second number). Error bars, standard errors. Data were analyzed by one-way ANOVA with Bonferroni post-test correction. a/b/c (comparing to 100% control lipid) and */**/*** (comparing between lipids) denote statistical significances of p<0.05, p<0.01, and p<0.001, respectively (n = 3-12).
We used the above Aβ ELISA data to calculate Aβ42/40 ratios, elevations of which are observed in AD patients with inherited presenilin mutations. Rising concentrations of PC 16:1 (Δ9-cis) and PC 16:1 (Δ9-trans) increased Aβ42/40 over the 100% POPC control, so that 50:50 mixtures of either of these with POPC increased the ratio by ~2-3-fold (Fig. 2D, green bars). In the case of PC 18:1 (Δ9-trans), its enhancement of γ-cleavage (both AICD and Aβ40 rose) led to a ~20% fall in Aβ42/40. When SOPC rather than POPC was the standard lipid (Fig. 2E), an elevated Aβ42/40 ratio was observed at 50% of PC 16:1 (Δ9-cis) but not PC 16:1 (Δ9-trans), and PC 18:1 (Δ9-trans) again caused a small decrease. At 100% PC 16:1 (Δ9-trans), Aβ40 and Aβ42 were very low, but the Aβ42/40 was 6-fold (p<0.001) and 3.5-fold (p<0.001) higher than the 100% POPC and SOPC standards, respectively (Fig. 2D-E, yellow bars); that is, although overall Aβ production was reduced, the Aβ42/40 ratio was shifted towards a more pathogenic one. Another interesting finding ensued from shifting the position of the double bond in PC 18:1 (Δ9-cis) further from the center of the membrane bilayer (to the 6th carbon atom from the carboxyl group). In this PC 18:1 (Δ6-cis) environment, there was very limited γ-secretase activity at either 100% or 50%:50% mixtures with POPC (Supp. Fig. 1), demonstrating that the position of the double bond-induced kink can influence activity. This could be due to a decrease in membrane fluidity, as predicted because the longer stretch of acyl chain is uninterrupted by a double bond (39). When compared with PC 18:1 (Δ9-cis), the Δ6-cis isomer led to significant reductions at 80:20 in Aβ40 (P<0.001), at 50:50 in AICD, Aβ40 and Aβ42 (P<0.001), and at 0:100 in Aβ40 (P<0.01) and Aβ42 (P<0.001) (Supplemental Fig. 1).
There is growing evidence that quantifying only Aβ40 and Aβ42 over-simplifies Aß production (13), as exemplified by recent reports regarding the potential pathogenicity of Aβ43 (12). To examine the production of Aβ species of various C-terminal lengths, we employed a bicine/urea PAGE system (13, 40). A representative gel (Fig. 3A), shows that a 50:50 mixture of PC 18:1 (Δ9-cis) with POPC decreased Aβ40, while longer species including Aβ42 and Aβ43 do not appear changed, in agreement with our ELISA data (Fig. 2B-C). PC 18:1 (Δ9-trans) induced no change in Aβ42 but a slight increase in Aβ40 and a corresponding decrease in Aβ43 (Fig. 3A). Using many such gels, we performed densitometry and estimated the relative levels of 3 groups of Aβ peptides: Aβ40, Aβ42+Aβ43, and Aβ45 and longer (Fig. 3B-C). This semi-quantitative method revealed a relative decrease in the production of longer Aβ species (both 42/43 and 45+) and a relative increase in Aβ40 as the percentage of PC 18:1 (Δ9-trans) rose, compared to the effects of POPC/PC 18:1 (Δ9-cis) mixtures or the standard POPC alone (Fig. 3B-C). This effect of the trans isomer of PC 18:1 (Δ9) should be anti-amyloidogenic, because the relative levels of longer, more hydrophobic Aß species are diminished. In the case of PC 16:1 (Δ9), the ratios of longer Aβ peptides to Aβ40 were high (>1.0) with both the cis and trans isomers, suggesting decreased processivity of γ-secretase in the presence of this phospholipid (Fig. 3C). Unlike with the ELISA-calculated ratios, these data reveal a difference between cis and trans isomer at 50:50 mixtures with POPC. Under these conditions, the trans form of both 16:1 (Δ9) and 18:1 (Δ9) result in a significant reduction in Aβ(42+43)/40 relative to the cis isoform (P<0.05 and P<0.001, respectively) (Fig 3C). Thus, the orientation of a FA chain double-bond as well as chain length can substantially alter the relative production of longer vs. shorter Aβ peptides by purified γ-secretase.
Figure 3. The effect of cis vs. trans isomer FA sidechains on γ-secretase processivity.
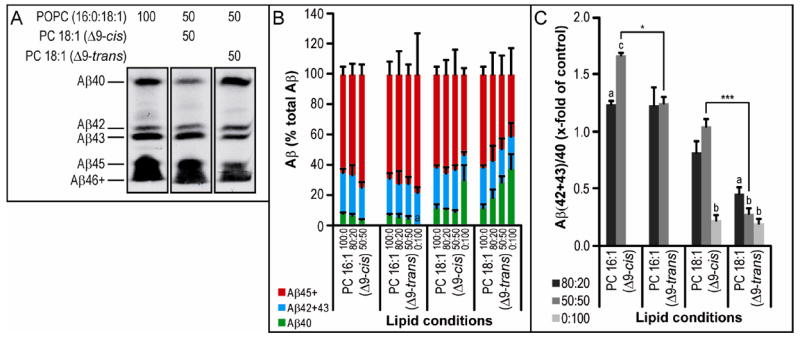
Activity assay samples were run on bicine/urea gels, transferred to PVDF, then probed for human Aβ (using 6E10 antibody). A) Part of one blot is shown as an example. B) All blots were analyzed by densitometry and the results plotted as a percentage of the sum of all Aβ bands in a lane in groups of Aβ40 (green bars), Aβ42+Aβ43 (blue bars), and Aβ45+Aβ46+ (red bars). C) Ratios of Aβ(42+43)/Aβ40 were calculated from the densitometry data. Ratios below denote the percentage of POPC and specific FA chain being tested. Where no bar is shown, band intensity was too low for detection by Western blot. Statistical analysis was performed as in Figure 2 (n = 3).
Increasing FA chain length enhances γ-secretase activity and processivity, and lowers Aβ42/40
In addition to FA isomer differences altering membrane density and fluidity, FA chain lengths have effects on fluidity as well as membrane thickness. The latter parameter would be expected to have a profound effect on an intramembrane protease that cleaves sequentially at different points along the substrate’s membrane-spanning domain. In the previous section, we saw that in a membrane of 100% PC 16:1 (Δ9-cis), there was no detectable γ-secretase activity, and a 50:50 mixture with POPC still left activity at less than half of that in pure POPC (Fig. 2A). When we examined the effects of the Δ9-cis FA chain being extended serially by 2 carbons (i.e., 16 vs. 18 vs. 20 vs. 22 carbons), we observed a stepwise increase in the production of Aβ40 (p<0.001), even when PC 20:1 (Δ11-cis) phospholipids constituted 100% of the membrane (Fig. 4A). In the latter condition, the sole availability of the Δ11-cis form of PC 20:1 may mean that some of the observed effect is due to the change in double bond position. Going to 22 carbons, there was a 40% decrease in activity (as measured by AICD-FLAG Western blot) with 50:50 mixtures containing PC 22:1 (Δ13-cis) relative to those containing PC 20:1 (Δ11-cis) (p<0.001), probably due to the high phase transition temperature of PC 22:1 (data not shown). At just 14 carbons, production fell to two-thirds of control levels in 80:20 mixtures containing PC 14:1(Δ9-cis) and almost to zero at 50:50 mixtures (p<0.001), with a significant reduction in levels of Aβ40 (p<0.01) and Aβ42 (p<0.001) and an increase in Aβ42/40 ratio (P<0.001) relative to PC 16:1 (Δ9-cis) (Supp. Fig. 2). Production of Aβ42 was essentially unchanged in mixtures containing FA chains of increasing length, but at 100% of the test FA chain, Aβ42 levels did rise (p<0.001 for PC 18:1 (Δ9-cis) versus PC 16:1 (Δ9-cis) and P<0.01 for PC 20:1 (Δ11-cis) versus PC 18:1 (Δ9- cis)) (Fig. 4B, yellow bars). With a smaller increase in Aβ42 relative to Aβ40, the Aβ42/40 ratio decreased to half the control level in the presence of the longer 20:1 (Δ11-cis) (P<0.001) (Fig. 4C). Conversely, reducing FA chain length from 18 to 16 carbons led to an increase in Aβ42/40 by 2.5-fold (P<0.01), primarily due to a decrease in Aβ40 production with no change in Aβ42 levels (Fig. 4A-C). Further reducing FA chain length to 14:1 (Δ9-cis) raised Aβ42/40 even more to ~4.5 × control levels at 50:50 mixtures (P<0.001) (Supp. Fig. 2).
Figure 4. The effect of FA chain length on γ-secretase activity.
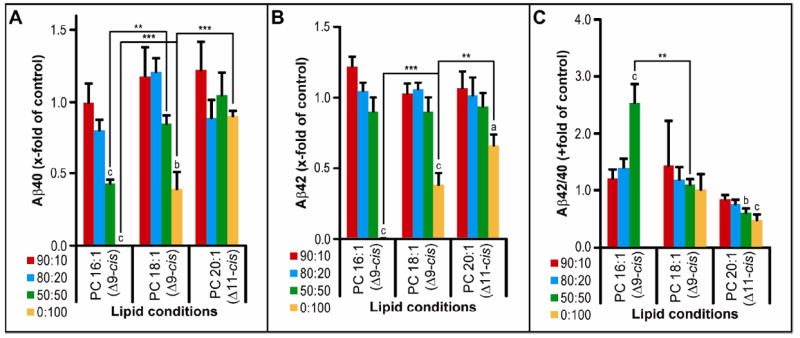
Activity assays were analyzed by the same method as in Figure 2B-D.
Using the bicine/urea gel system to examine longer Aβ peptides, we found that a membrane composed of 50% PC 20:1(Δ11-cis) and 50% POPC led to a striking reduction in longer Aβ species (Aβ42+) with no change in Aβ40 levels (Fig. 5A-B). In accord with the ELISA data, the Aβ(42+43)/40 ratio fell stepwise with increasing chain length (P<0.001), reaching low (physiological) levels in a membrane of 100% PC 20:1 (Δ11-cis) (Fig. 5B).
Figure 5. The effect of FA chain length on γ-secretase processivity.
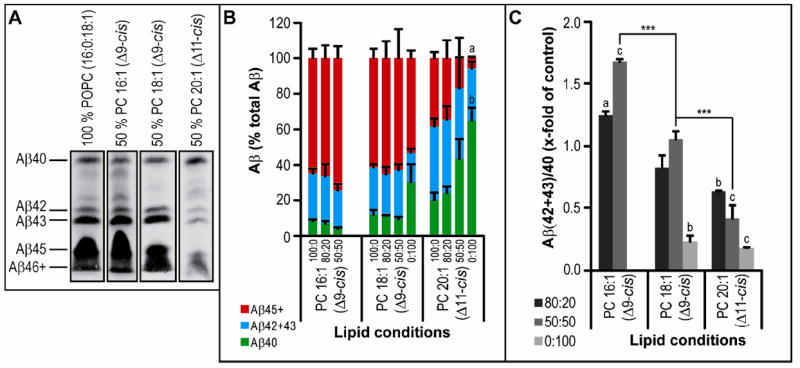
Activity assays were analyzed by the same method as in Figure 3A-C.
Variations in phospholipid head groups alter γ-secretase activity
Another important feature of membrane lipids is the identity of polar head groups, which can vary from the simple choline in phosphatidylcholine (PC), to a complex oligosaccharide in gangliosides (GS). These lipid head groups are known to play roles in the interaction of proteins with the membrane, but how they affect intramembrane proteolysis by γ-secretase is not well understood. Previous work from our group (33) showed that mixtures of different lipid head group types can significantly alter the degree of proteolysis of C100 to AICD and Aβ40 by γ-secretase, but we did not examine relative changes among different Aβ peptide species. We have therefore revisited some of the conditions explored earlier by quantifying AICD-FLAG via Western blotting, Aβ40 and Aβ42 via ELISA, and longer Aβ species via bicine/urea gels, all by supplementing whole brain PC with each of the test lipids described.
Densitometry of AICD-FLAG production from C100-FLAG by γ-secretase in various differently composed membranes revealed that only in a 90% PC:10% GS lipid mixture was an increase in total γ-activity observed (P<0.05) (Supp. Fig. 3A). At other ratios of PC:GS and with all other lipid types tested [phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphoinositol (PI), phosphotidic acid (PA), sphingomyelin (SM) and cerebrosides (CS)], γ-activity was unaffected until the test lipid comprised more than 50% of the membrane, at which point a marked reduction in all γ-products was observed (Supp. Fig. 3A). As was previously reported by Osawa et al. (36), phosphatidylinositol (PI) markedly inhibited γ-activity, with as little as a 10% portion in the membrane causing a complete loss of AICD generation (Supp. Fig. 3A). We found a similar but less potent negative effect from phosphatidic acid (PA): γ-activity was completely abrogated at a 50% membrane composition (Supp. Fig. 3A). Analyzing the same samples by Aβ ELISA revealed similar patterns of effect, except that in the cases of phosphatidylserine (PS) and PA, we saw an increase in Aβ40 production (vs. control, p<0.01) with a 75% PC:25% PS or 25% PA ratio (Fig. 6A and Supp. Fig. 3B). Interestingly, GS was the only lipid type to increase Aβ42 production at a wide range of lipid ratios, while all other lipid head groups (PE, PS, SM, PI and PA) decreased it (Fig. 6B and Supp. Fig. 3C).
Figure 6. The effect of various lipid head groups on γ-secretase activity.
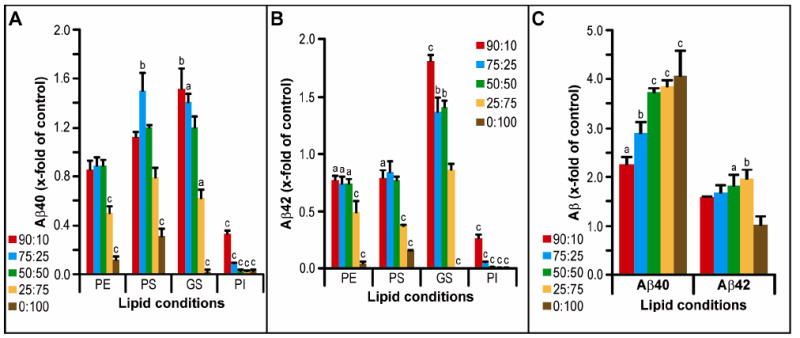
Activity assays using purified γ-secretase and C100-FLAG substrate were performed in detergent-free vesicles comprising different ratios of various lipid types (A, B) mixed with whole brain PC extract at a total lipid concentration of 1.5 mM. Activity assay samples were analyzed by the same method as in Figure 2B-C. C) Aβ40 and Aβ42 ELISA data for whole brain lipid extract is given on a separate graph due to the different y-axis scale required. Statistical analyses were performed as in Figure 2 (n = 3-6).
To examine a potentially more physiological lipid condition for γ-secretase activity, we also performed in vitro activity assays with increasing percentages (again within a PC membrane) of whole porcine brain (WB) lipid extract, which is a complex mixture of lipids containing all of the lipids we have tested individually above. By both AICD and Aβ40 measures, we found a stepwise increase in γ-activity with each increase in percentage of WB present in the PC membrane, peaking at 4 times control levels (Fig. 6C; AICD data not shown). Aβ42 ELISA revealed a smaller increase (to ~2-fold) at 25:75 mixtures of PC to WB lipids, whereas going to 100% WB lipids dropped Aβ42 production down to control (i.e., 100% PC) levels (Fig. 6C).
Calculating Aβ42/40 ratios based on all of the ELISA data shown in Fig. 6 revealed that PE and SM did not change this ratio from controls, while GS increased it as much as 2-fold (when GS was 75% of the mixture with PC; p<0.001) (Fig. 7B and Supp. Fig. 4). With increasing amounts of whole brain lipids, we observed significant stepwise decreases in Aβ42/40 ratios down to a final level ~20 % of the PC control at 100% WB lipid (p<0.001). This pattern was similar with PS, PI and PA, although the latter two head groups gave low levels of total activity (Fig. 7B and Supp. Fig. 4). Samples were further analyzed on bicine/urea gels (Figs. 7a,d), which likewise showed that PE and SM did not significantly alter Aβ42+43/40, and both PS and WB significantly (P<0.001) reduced the ratio (Fig. 7C). Unexpectedly, the densitometry data from these blots did not agree with the ELISA data, largely due to a high level of Aβ43 production (a species that is not detected by the ELISA) in both 75% GS and control membranes (Fig. 7D). The WB lipid condition gave a pattern of Aβ species much closer to physiological, with a ~10% ratio of Aβ42/40 and trace levels of the longer Aβ species (Fig. 7A,C,D). The only individual lipid head group giving a similar pattern to WB lipids was a high level of PS (Fig. 7A,C,D), suggesting that PS could be an important lipid type for correct γ-secretase processing of substrate, although the levels of Aβ production in pure PS were much reduced compared to WB and the 100% PC control.
Figure 7. The effect of lipid head groups on γ-secretase processivity.
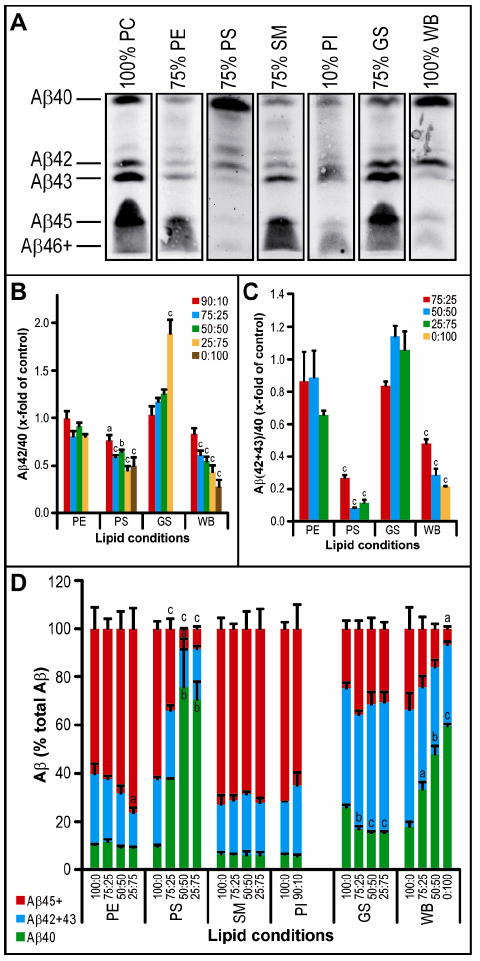
Activity assay samples were analyzed by the same method as in Figures 2D and 3A-C.
Exposing living cells to polyunsaturated fatty acyl side chains decreases Aβ production and increases Aβ42/40
The above experiments sought to determine the effects of lipid variation directly on the activity of purified γ-secretase. We also examined the effect of certain fatty acyl side chains on the γ-secretase processing of APP in the context of living cells. Chinese hamster ovary (CHO) cells stably overexpressing all 4 components of human γ-secretase as well as human APP (34) were treated for 48 hr with different phospholipids (final concentrations of 10 μM or 50 μM) in vesicles prepared by hydration of the dried lipid and sonication, or else just with vehicle (the buffer used for hydrating the lipids). The resultant conditioned media (CM) were quantified for Aβ40 and Aβ42. We found that treatment of the cells with PC 16:1 (Δ9-cis) or (Δ9-trans) in vesicles had no major effect on Aβ40 and Aβ42 levels compared to vehicle alone (Fig. 8A) but did slightly increase Aβ42/40 by 30% (p<0.001) and 15% (p<0.05), respectively (Fig. 8B). However, treatment with the longer PC 18:1 (Δ9-cis) elevated both Aβ40 and Aβ42 levels by 30-40% (Aβ40 p<0.001; Aβ42 p<0.01), while its trans isomer produced no change from control levels (Fig. 8A), with neither isomere significantly altering Aβ42/40 ratios relative to control (Fig. 8B). Interestingly, the earlier in vitro observation that decreasing FA chain length increased Aβ42/40 ratio was also found when comparing intact cells treated with PC 18:1 (Δ9-cis) versus PC 16:1 (Δ9-cis) (p<0.01) (Fig. 8B). Exposing the cells to the polyunsaturated fatty acyl side chain arachidonic acid (AA) (i.e., PC 20:4 (Δ5,8,11,14-cis)) at 50 μM (but not 10 μM) reduced Aβ40 by 30% (p<0.001) while not changing Aβ42 significantly (Fig. 8A). Treatment with polyunsaturated FA side chain docosahexaenoic acid (DHA) (i.e., PC 22:6 (Δ4,7,10,13,16,19-cis)) at 50 μM (but not 10 μM) decreased both Aβ40 and Aβ42. In accord with these data, the Aβ42/40 ratio was elevated by ~40% with AA (p<0.001) but only slightly elevated with DHA (p<0.05), showing a significant difference between these two PUFA exposures (p<0.05) (Fig. 8B).
Figure 8. The effect of FA chain length and saturation on γ-secretase activity in living cells.
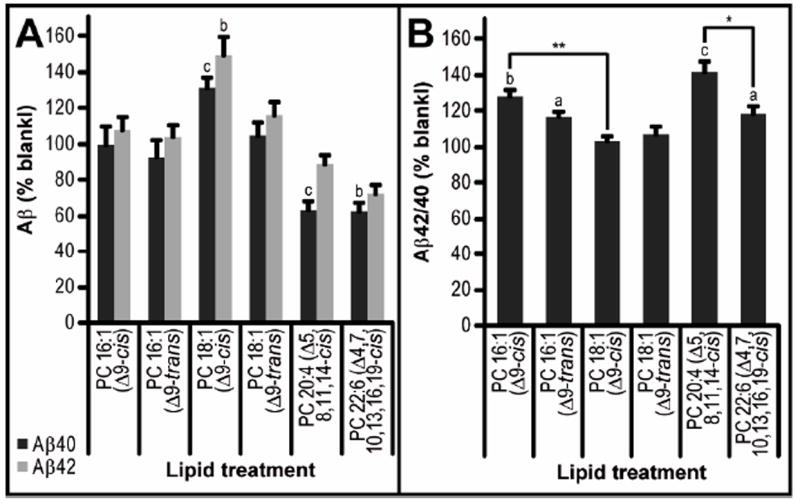
S20 CHO cells were treated with 50 μM lipid vesicles in standard growth media for 48 hr. Conditioned media were analyzed by 4G8 triplex Aβ ELISA (Mesoscale Discovery) and values normalized to the BCA assays of the cell lysates. A) Aβ levels are given as a percentage of normalized Aβ readings from vehicle-treated controls from the same plate. B) Aβ42/40 ratio is calculated from non-normalized readings, then given as a percentage of vehicle-treated controls from the same plate. Statistical analysis was performed as in Figure 2 (n = 5-12).
Discussion
Using a modification of a detergent-free γ-activity assay devised in our lab (33), we find that the lipid microenvironment surrounding γ-secretase has a very important and complicated role in the activity of this intramembrane protease. This study shows that both the fatty acyl chains and the polar head groups of lipids can critically regulate the proteolysis. There have been relatively few studies on the in vivo effects of FA chains (with DHA being the most studied) or lipid head groups in animals and humans, largely due to the longitudinal nature of such studies and the difficulty of fully controlling dietary lipid intake. In the current study, we applied a reductionist approach by studying purified γ-secretase activity in a controlled system with specific pure lipids and purified enzyme. This approach avoids many of the confounding effects found in vivo such as altered protein expression and trafficking, interaction with other proteins, and cell toxicity. Most in vitro studies on γ-secretase activity have used proteins in the partially purified state in isolated cellular membranes, which contain many other membrane-bound proteins and an abundance of lipids, all of which make it difficult to discern any effects of specific lipids on protease activity. Furthermore, without removing the invariably used detergents from the system, the assay actually measures the effect of lipid-detergent mixtures on γ-secretase activity and not solely the effects of the lipids.
As membrane lipids consist of a polar head group and a hydrophobic tail, we asked whether one or both of these constituents could play a role in the activity of an intramembrane protease such as γ-secretase. An example of this approach was reported by Contreras et al., who found a transmembrane domain sequence of p24 (involved in coat protein [COPI] vesicle biogenesis) to which the polar head group and FA chain of SM-18:5 directly and specifically bind, leading to efficient retrograde COPI-dependent transport (41). In order to similarly investigate the role of membrane lipids in γ-secretase function, we separated our experiments into two parts, first systematically varying the FA chain isomerisation and length with a constant head group (PC), and then second, examining various different lipid head groups.
We found that changing a monounsaturated FA isomer from cis to trans with a constant chain length led to an increase in γ-secretase cleavage of C100-FLAG substrate, measured by AICD Western blotting and Aβ40 and Aβ42 ELISAs, without significantly affecting Aβ42/40 ratio. However, upon closer examination using a bicine/urea gel system in which a range of Aβ peptide lengths can be resolved, we observed that shifting from cis to trans FA isomer reduces Aβ43 production and, in the case of PC 18:1 (Δ9), also reduces Aβ species of 45 or more residues. Following the Ihara model of sequential, N-terminally directed cleavage of the APP TMD starting at Aβ48 or Aβ49 (40), our results can be explained as the trans isomer increasing processivity, that is, increasing the degree of the Aβ45/48 to Aβ42 and Aβ43/46/49 to Aβ40 conversions. An alternative explanation not implicating a processive cleavage mechanism could be that alterations in the lipid micro-environment affect the precise site of γ-cleavage through local changes in membrane thickness, leading to differential alignments of the catalytic aspartate residues with the substrate’s transmembrane domain. However, given the clear evidence of tri- and tetra-peptide release from the C99 transmembrane domain during γ-secretase cleavage (11) and the changes in Aβ species seen on our bicine/urea gels, we favor the model of a sequential processing of substrate.
When analyzing our reconstituted γ-secretase system for effects of FA chain length, we observed a bell shape of γ-activity peaking at 18- or 20-carbon lengths, with very low activity at 14 carbons and decreased activity at 22 carbons. The most common length for FA chains in humans is in the range of 16-18 carbons. We also observed a decrease in Aβ42/40 (measured by ELISA) and reduced Aβ(42+43)/40 and loss of Aβ45+ peptides (measured by bicine/urea PAGE) as FA chain length increased, suggesting longer FA chains allow more physiological processing of the C99 APP substrate. The discrepancy between this higher processivity to shorter Aβ peptides and natural FA chain length patterns in cells may be due to the localization of γ-secretase and substrate to detergent resistant membranes (DRMs), which are less fluid and contain greater levels of the longer 20 and 22 FA chains (42). A mechanistic explanation of our data could be that with a thicker hydrophobic region and reduced fluidity of a membrane, the longer Aβ species (Aβ45+) may be better retained in the γ-secretase complex and therefore undergo subsequent γ-cleavage to the shorter (and less amyloidogenic) species, Aβ40 and Aβ38. In terms of establishing a general model of how the lipid microenvironment affects γ-secretase function, it will be necessary to perform these assays with additional substrates, such as Notch, which is implicated in several types of cancer. Our study has focused on the role of γ-secretase relevant to Alzheimer’s disease, but we assume that the processivity model may turn out to be universal for most if not all substrates, based on the observations of Fukumori et al (43) that even the autoproteolysis of PS1 occurs in a stepwise manner with cleavages every three residues.
Another principal thrust of our study was to expand on previous work (33) examining the effects of various polar head groups of membrane lipids on γ-secretase activity. Our previous work largely focused on the effect of cholesterol in conjunction with various different lipid types, showing that cholesterol causes a very significant increase (then decrease at higher concentrations in a bell shaped response) in γ-secretase activity. The data shown here look at cholesterol-free proteoliposomes to investigate how lipid head groups per se can influence γ-secretase activity and processivity. We have shown that when combined with PC, increasing proportions of PS, GS or PA elevate Aβ40 production in a bell-shaped fashion, while only GS increased Aβ42. Correspondingly, the pathogenically important Aβ42/40 ratio was increased by high GS concentrations and decreased by PS and PA. These findings on the direct effects of GS on γ-secretase activity support studies in mice in which various enzymes of the ganglioside synthesis pathways have been knocked out. In particular, in vivo prevention of the generation of disialogangliosides (e.g. GD3, GD2 and GD1b), thereby elevating GM3 concentrations, can decrease Aβ production and aggregation in APP/PS1 transgenic mice (44, 45). It may now be useful to investigate γ-secretase activity and processivity in proteoliposomes comprising specific ganglioside subtypes in order to reveal potential differences.
Intriguingly, PS was the only lipid head group tested here that reduced relative levels of the very long Aβ species, Aβ45+, to the same low levels as did a whole brain lipid extract, which mimics the physiological lipid environment of γ-secretase. This finding may be due to the tendency of PS to locate to the inner leaflet of membranes, providing asymmetry to the bilayer (46), and its anionic head-group locking to C99 in the membrane more strongly due to the greater polarity between uncharged hydrophobic FA chains and the negatively charged head group, thus allowing further processing by γ-secretase. We also found that another anionic lipid type, PI, completely abrogates γ-secretase activity even at very low concentrations. This finding is in agreement with Osawa et al. (36), who discovered that phosphatidylinositol(3,4)/(4,5)-diphosphate (PIP2) and PIP3 directly inhibit γ-secretase activity in a competitive manner and that the inhibitory potency increases with the level of PIP phosphorylation and requires the FA side chains. Perhaps a reduction in PI levels, which has been reported in the anterior temporal cortex of AD brains (47), would reduce PI-mediated inhibition of γ-secretase, thus leading to an increase in Aβ production. Moreovoer, a relative loss of PS, as observed in AD mouse models (48, 49), may elevate the proportion of longer Aβ species, resulting in a more pathogenic Aβ profile. This hypothetical formulation mentions just two types of lipid head groups. Taking into account the numerous lipid head group types (e.g. PC, PE, GS, SM, etc.), each containing various different subtypes and with two FA sidechains that can vary in length, saturation and isomerisation, the complexity of the role of membrane lipids in γ-secretase activity and processivity becomes apparent. Recently, Nesic et al (37) used siRNA-mediated knockdown of PE synthesis in cells and D. melanogaster to reduce Aβ generation via increased α-secretase cleavage and decreased γ-secretase processing of APP. This demonstrates that lipid modulation in vivo can have positive effects on Aβ production and potentially on AD pathogenesis.
In summary, we have shown that the lipid microenvironment in which γ-secretase is situated can have direct and potent effects on overall Aβ generation as well as processivity, the proportion of different length Aβ peptides, both of which factors are implicated in AD pathogenesis. The effects we document involve several aspects of membrane lipids, including the length of FA chains, their degree of unsaturation, their cis vs. trans isomerization and the identity of the polar head groups. The central conclusion of our systematic analyses is that variation in membrane lipid composition could have effects on the pathogenic ratio of longer to shorter Aβ peptides that are equally or more adverse than the effects of presenilin mutations or other protein changes that receive far more study. Accordingly, our data recommend attempting specific manipulations of membrane lipid composition by using genetic and dietary approaches in animals. The findings also suggest specific targets for small molecule inhibitors to modify lipid biosynthesis pathways as well as lipid dietary changes that might similarly delay the onset or slow the progression of Alzheimer’s disease.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant P01 AG015379
Abbreviations
- AD
Alzheimer’s disease
- FA
fatty acyl
- APP
β-amyloid precursor protein
- Aβ
amyloid β-peptide
- AICD
APP intracellular domain
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- GS
gangliosides
- PI
phosphatidylinositol
- WB
whole brain lipid
- SM
sphingomyelin
- PA
phosphatidic acid
- ELISA
enzyme-linked immunosorbent assay
- CHAPSO
3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonic acid
Footnotes
Supporting Information
Supporting information available. Supplemental figure S1 shows the effect of shifting double bond position in PC 18:1 (Δ-cis) on γ-secretase activity. Supplementary figure 2 shows a comparison of the effect of PC 14:1 (Δ9-cis) vs. PC 16:1 (Δ9-cis) on γ-secretase activity. Supplementary figure 3 shows: A) the effect of lipid head groups on γ-secretase activity (AICD production), B) the effect of SM and PA on Aβ40 and Aβ42 generation. Supplementary figure 4 shows the effect of SM, PI and PA on γ-secretase processivity. This material is available free of charge via the internet at http://pubs.acs.org
References
- 1.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Seubert P, Oltersdorf T, Lee MG, Barbour R, Blomquist C, Davis DL, Bryant K, Fritz LC, Galasko D, Thal LJ, et al. Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature. 1993;361:260–263. doi: 10.1038/361260a0. [DOI] [PubMed] [Google Scholar]
- 3.Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 4.Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science (New York, N Y) 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 5.Xia W, Wolfe MS. Intramembrane proteolysis by presenilin and presenilin-like proteases. J Cell Sci. 2003;116:2839–2844. doi: 10.1242/jcs.00651. [DOI] [PubMed] [Google Scholar]
- 6.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 7.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nature cell biology. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 9.Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 10.Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nature reviews. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 11.Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29:13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito T, Suemoto T, Brouwers N, Sleegers K, Funamoto S, Mihira N, Matsuba Y, Yamada K, Nilsson P, Takano J, Nishimura M, Iwata N, Van Broeckhoven C, Ihara Y, Saido TC. Potent amyloidogenicity and pathogenicity of Abeta43. Nat Neurosci. 2011;14:1023–1032. doi: 10.1038/nn.2858. [DOI] [PubMed] [Google Scholar]
- 13.Quintero-Monzon O, Martin MM, Fernandez MA, Cappello CA, Krzysiak AJ, Osenkowski P, Wolfe MS. Dissociation between the processivity and total activity of gamma-secretase: implications for the mechanism of Alzheimer’s disease-causing presenilin mutations. Biochemistry. 2011;50:9023–9035. doi: 10.1021/bi2007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 15.Herl L, Thomas AV, Lill CM, Banks M, Deng A, Jones PB, Spoelgen R, Hyman BT, Berezovska O. Mutations in amyloid precursor protein affect its interactions with presenilin/gamma-secretase. Mol Cell Neurosci. 2009;41:166–174. doi: 10.1016/j.mcn.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berezovska O, Lleo A, Herl LD, Frosch MP, Stern EA, Bacskai BJ, Hyman BT. Familial Alzheimer’s disease presenilin 1 mutations cause alterations in the conformation of presenilin and interactions with amyloid precursor protein. J Neurosci. 2005;25:3009–3017. doi: 10.1523/JNEUROSCI.0364-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, Gu Y, Sanjo N, Glista M, Rogaeva E, Wakutani Y, Pardossi-Piquard R, Ruan X, Tandon A, Checler F, Marambaud P, Hansen K, Westaway D, St George-Hyslop P, Fraser P. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature. 2006;440:1208–1212. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 19.Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 20.Lleo A, Berezovska O, Herl L, Raju S, Deng A, Bacskai BJ, Frosch MP, Irizarry M, Hyman BT. Nonsteroidal anti-inflammatory drugs lower Abeta42 and change presenilin 1 conformation. Nat Med. 2004;10:1065–1066. doi: 10.1038/nm1112. [DOI] [PubMed] [Google Scholar]
- 21.Wells K, Farooqui AA, Liss L, Horrocks LA. Neural membrane phospholipids in Alzheimer disease. Neurochem Res. 1995;20:1329–1333. doi: 10.1007/BF00992508. [DOI] [PubMed] [Google Scholar]
- 22.Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res. 1998;23:81–88. doi: 10.1023/a:1022457605436. [DOI] [PubMed] [Google Scholar]
- 23.Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. The Journal of cell biology. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordy JM, Hooper NM, Turner AJ. The involvement of lipid rafts in Alzheimer’s disease. Mol Membr Biol. 2006;23:111–122. doi: 10.1080/09687860500496417. [DOI] [PubMed] [Google Scholar]
- 26.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol. 2011;68:1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escriba PV, Gonzalez-Ros JM, Goni FM, Kinnunen PK, Vigh L, Sanchez-Magraner L, Fernandez AM, Busquets X, Horvath I, Barcelo-Coblijn G. Membranes: a meeting point for lipids, proteins and therapies. Journal of cellular and molecular medicine. 2008;12:829–875. doi: 10.1111/j.1582-4934.2008.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartmann T, Bieger SC, Bruhl B, Tienari PJ, Ida N, Allsop D, Roberts GW, Masters CL, Dotti CG, Unsicker K, Beyreuther K. Distinct sites of intracellular production for Alzheimer’s disease A beta40/42 amyloid peptides. Nat Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- 29.Teegala SM, Willett WC, Mozaffarian D. Consumption and health effects of trans fatty acids: a review. J AOAC Int. 2009;92:1250–1257. [PubMed] [Google Scholar]
- 30.Grimm MO, Rothhaar TL, Grosgen S, Burg VK, Hundsdorfer B, Haupenthal VJ, Friess P, Kins S, Grimm HS, Hartmann T. Trans fatty acids enhance amyloidogenic processing of the Alzheimer amyloid precursor protein (APP) J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Schneider J, Wilson RS. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 32.Engelhart MJ, Geerlings MI, Ruitenberg A, Van Swieten JC, Hofman A, Witteman JC, Breteler MM. Diet and risk of dementia: Does fat matter?: The Rotterdam Study. Neurology. 2002;59:1915–1921. doi: 10.1212/01.wnl.0000038345.77753.46. [DOI] [PubMed] [Google Scholar]
- 33.Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osenkowski P, Li H, Ye W, Li D, Aeschbach L, Fraering PC, Wolfe MS, Selkoe DJ. Cryoelectron microscopy structure of purified gamma-secretase at 12 A resolution. J Mol Biol. 2009;385:642–652. doi: 10.1016/j.jmb.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimberly WT, Esler WP, Ye W, Ostaszewski BL, Gao J, Diehl T, Selkoe DJ, Wolfe MS. Notch and the amyloid precursor protein are cleaved by similar gamma-secretase(s) Biochemistry. 2003;42:137–144. doi: 10.1021/bi026888g. [DOI] [PubMed] [Google Scholar]
- 36.Osawa S, Funamoto S, Nobuhara M, Wada-Kakuda S, Shimojo M, Yagishita S, Ihara Y. Phosphoinositides suppress gamma-secretase in both the detergent-soluble and -insoluble states. The Journal of biological chemistry. 2008;283:19283–19292. doi: 10.1074/jbc.M705954200. [DOI] [PubMed] [Google Scholar]
- 37.Nesic I, Guix FX, Vennekens K, Michaki V, Van Veldhoven PP, Feiguin F, De Strooper B, Dotti CG, Wahle T. Alterations in phosphatidylethanolamine levels affect the generation of Abeta. Aging Cell. 2012;11:63–72. doi: 10.1111/j.1474-9726.2011.00760.x. [DOI] [PubMed] [Google Scholar]
- 38.Fong TM, McNamee MG. Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry. 1986;25:830–840. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- 39.Cevc G. How membrane chain-melting phase-transition temperature is affected by the lipid chain asymmetry and degree of unsaturation: an effective chain-length model. Biochemistry. 1991;30:7186–7193. doi: 10.1021/bi00243a021. [DOI] [PubMed] [Google Scholar]
- 40.Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, Ihara Y. Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci. 2005;25:436–445. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Contreras FX, Ernst AM, Haberkant P, Bjorkholm P, Lindahl E, Gonen B, Tischer C, Elofsson A, von Heijne G, Thiele C, Pepperkok R, Wieland F, Brugger B. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature. 2012;481:525–529. doi: 10.1038/nature10742. [DOI] [PubMed] [Google Scholar]
- 42.Koumanov KS, Tessier C, Momchilova AB, Rainteau D, Wolf C, Quinn PJ. Comparative lipid analysis and structure of detergent-resistant membrane raft fractions isolated from human and ruminant erythrocytes. Arch Biochem Biophys. 2005;434:150–158. doi: 10.1016/j.abb.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Fukumori A, Fluhrer R, Steiner H, Haass C. Three-amino acid spacing of presenilin endoproteolysis suggests a general stepwise cleavage of gamma-secretase-mediated intramembrane proteolysis. J Neurosci. 2010;30:7853–7862. doi: 10.1523/JNEUROSCI.1443-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furukawa K, Ohmi Y, Ohkawa Y, Tokuda N, Kondo Y, Tajima O. Regulatory mechanisms of nervous systems with glycosphingolipids. Neurochem Res. 2011;36:1578–1586. doi: 10.1007/s11064-011-0494-2. [DOI] [PubMed] [Google Scholar]
- 45.Bernardo A, Harrison FE, McCord M, Zhao J, Bruchey A, Davies SS, Jackson Roberts L, 2nd, Mathews PM, Matsuoka Y, Ariga T, Yu RK, Thompson R, McDonald MP. Elimination of GD3 synthase improves memory and reduces amyloid-beta plaque load in transgenic mice. Neurobiol Aging. 2009;30:1777–1791. doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 46.Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, Grinstein S, Parton RG. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. The Journal of cell biology. 2011;194:257–275. doi: 10.1083/jcb.201012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stokes CE, Hawthorne JN. Reduced phosphoinositide concentrations in anterior temporal cortex of Alzheimer-diseased brains. Journal of neurochemistry. 1987;48:1018–1021. doi: 10.1111/j.1471-4159.1987.tb05619.x. [DOI] [PubMed] [Google Scholar]
- 48.Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, Wenk MR, Shui G, Di Paolo G. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. The Journal of biological chemistry. 2011;287:2678–2688. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao JK, Wengenack TM, Curran GL, Poduslo JF. Reduced membrane lipids in the cortex of Alzheimer’s disease transgenic mice. Neurochem Res. 2009;34:102–108. doi: 10.1007/s11064-008-9673-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


