Abstract
Background and Objectives
To improve the likelihood of achieving a margin-free resection, neoadjuvant induction chemotherapy with GTX (gemcitabine, docetaxel, and capecitabine) followed by 5-FU-IMRT was administered to patients with borderline resectable pancreatic cancer. The utility of CT, EUS, PET, and CA 19-9 during diagnostic workup and assessment of response was also examined.
Methods
Seventeen patients with borderline resectable pancreatic cancer received a median of 3 cycles of neoadjuvant GTX induction chemotherapy followed by 5-FU-IMRT with dose painting. CA 19-9, CT mass size, and PET SUV were examined before and after neoadjuvant treatment.
Results
Diagnostic EUS and CT scans displayed similar mean mass sizes and extent of vascular involvement. Eight of the 17 patients achieved an R0 resection. Median CA 19-9 levels, CT mass size, and PET SUV all significantly decreased after neoadjuvant therapy. The median progression-free survival and overall survival were 10.48 months and 15.64 months respectively. Six patients are still alive.
Conclusions
Neoadjuvant GTX induction chemotherapy followed by 5-FU-IMRT shows promise in improving the likelihood of resectability with negative margins in borderline resectable pancreatic cancer. CT and EUS play complimentary roles during diagnostic workup. CT scans, CA 19-9, and PET scans are useful in judging response to neoadjuvant therapy.
Keywords: chemotherapy, borderline resectable, pancreatic cancer, GTX, neoadjuvant
Introduction
Long-term survival after a diagnosis of pancreatic cancer is best achieved in patients who are able to undergo a pancreaticoduodenectomy (Whipple procedure) with negative margins and who are diagnosed prior to the development of distant metastases. Unfortunately, only 20% of pancreatic cancer patients have resectable disease at presentation [1]. The median overall survival of patients who undergo complete resection with negative margins ranges between 12 and 26 months [2].
Approximately 40% of patients with pancreatic cancer present with locally advanced disease that is not amenable to surgical resection [3]. One-third of these patients will be marginally or “borderline resectable.” Numerous retrospective studies have demonstrated an association of positive margins with higher local failure rates and poorer overall and disease-free survivals for patients with borderline resectable pancreatic cancer [4–6]. Attempting a resection upfront for this disease is associated with a higher probability of necessitating vascular reconstruction and obtaining positive margins. The application of multimodality neoadjuvant therapies can have the greatest impact on borderline resectable pancreatic cancer by increasing the likelihood of obtaining negative margins at the time of resection and yielding survival comparable to that achieved by upfront resectable disease.
To our knowledge, a completed study reporting clinical outcomes for one particular neoadjuvant regimen for borderline resectable pancreatic cancer has never been published. Numerous clinical trials have taken place with different multiagent neoadjuvant chemotherapeutic regimens with radiation for locally advanced pancreatic cancer, including 5-FU, gemcitabine, cisplatin, mitomycin, paclitaxel, and streptozotocin [7–11]. Percent resectability has ranged between 9% and 32% among the trials. Evaluating the literature in this setting is complicated by combining borderline resectable patients with those patients who actually have locally advanced unresectable disease.
It is hypothesized that a period of induction chemotherapy might allow for the selection of those locally advanced patients who might truly benefit from subsequent chemoradiation. In a retrospective analysis conducted by Krishnan et al [12], median overall survival was superior in the patients who received induction chemotherapy with a gemcitabine based regimen compared to the chemoradiation alone group (11.9 vs. 8.5 mo). Fogelman et al first reported the use of GTX consisting of gemcitabine, docetaxel, and capecitabine as induction chemotherapy followed by gemcitabine-based chemoradiation [13]. The abstract described a series of 14 patients with locally advanced pancreatic cancer who received the GTX induction regimen. Eight patients (57%) were downstaged to resectability with negative margins. GTX was developed based on the demonstration of preclinical in vitro synergy of the combination. In the metastatic setting, GTX chemotherapy has shown a response rate that approaches 30–40% with a median survival of 11.2 months [14].
In the past, the determination of whether pancreatic cancer was resectable, borderline resectable, or unresectable was made at surgical exploration. The development of modern imaging techniques with improved resolution has allowed for the preoperative staging of patients. Computed Tomography (CT), Endoscopic Ultrasound (EUS), and Positron Emission Tomography (PET) scans are available imaging options that can be used during the diagnostic workup and management of pancreatic cancer. These modalities can offer valuable information during the management of borderline pancreatic cancer, however a comparison of their utility has not been formally performed.
Given the lack of completed prospective studies specifically involving borderline resectable pancreatic cancer, we have performed a retrospective analysis of our institution’s experience with neoadjuvant GTX induction chemotherapy followed by 5-FU-IMRT for this disease. An ECOG prospective trial (E1200) for borderline resectable disease was recently published describing tolerability and resectability after a particular neoadjuvant regimen, however the trial was not completed as it closed early due to poor accrual [15]. The utility of our particular regimen for borderline resectable pancreatic cancer has not been formally published elsewhere. The main objective was to determine if GTX induction chemotherapy followed by 5-FU-IMRT optimizes the likelihood of achieving a margin-free resection. Comparisons were made between the findings of CT, EUS, PET, and CA 19-9 to offer insight into their utility during diagnostic workup and treatment. To our knowledge, this particular analysis has not formally been performed specifically for borderline resectable pancreatic cancer.
Materials and Methods
Patient Population
Clinical data on 18 borderline resectable pancreatic cancer patients between February 2006 and February 2009 treated at the Moffitt Cancer Center and Research Institute were retrieved from a database maintained by the GI Pancreatic Tumor Program. None of the 18 patients were exposed to previous chemotherapy or radiation to the pancreas. Approximately 250 new cases of pancreatic cancer are seen per year at Moffitt. Approximately 30 patients per year were likely borderline resectable during the timeframe of the retrospective study. The number of patients that now receive neoadjuvant therapy at our institution has significantly increased based on the results of this study. Prior to initiating treatment, all patients had leukocyte counts >3000/uL, ANC ≥1000/uL, Creatinine clearance ≥ 60 mL/min/1.73m2, T Bilirubin ≤ULN, and AST and ALT ≤ 2.5 × ULN. Performance status was recorded using the Eastern Cooperative Oncology Group (ECOG) scale [16].
H. Lee Moffitt Cancer Center Diagnostic Algorithm for Borderline Resectable Pancreatic Cancer
A baseline evaluation of all patients consisted of a detailed history and physical examination, complete blood count, blood chemistries, and CA 19-9 level. At Moffitt Cancer Center, we attempt to obtain an initial EUS, a thin cut pancreatic protocol CT, and PET scan for all patients with possible borderline resectable disease. PET scanning as a modality for the diagnosis, staging, and monitoring of treatment of pancreatic cancer and other cancers is currently being assessed nationally in a Medicare outcomes study. Our center is a participant in the National Oncologic PET Registry, which provides us the opportunity to evaluate this imaging modality in pancreatic cancer. Data from our institution reported by Farma et al [17] has shown that in patients with potentially resectable pancreatic cancer, PET/CT imaging can change management in 11% of patients. After the initial assessment, patients were reviewed by our GI Tumor Board and an agreement for the diagnosis of borderline resectable pancreatic cancer was made for each patient.
For this retrospective study, mass sizes and vessel involvement displayed by the diagnostic CT and EUS studies were retrieved. The diagnosis of borderline resectable pancreatic cancer was mainly made by CT or EUS. Three of the 18 patients were found to be borderline resectable during an initial surgical resection attempt based on a CT or EUS diagnosis of resectable pancreatic cancer. A successful tissue diagnosis was made from a biopsy using EUS in 14 patients and by using CT guidance in 4 patients. If a patient had an EUS or CT that displayed evidence of locally advanced pancreatic cancer that was not consistent with borderline resectable disease, he or she was not included in the database.
The H. Lee Moffitt Cancer Center utilizes specific criteria for the classification of borderline resectable pancreatic cancer, which encompasses definitions used elsewhere [18,19]. Borderline resectable patients were those who had circumferential tumor abutment with the superior mesenteric vein (SMV), portal vein (PV), or superior mesenteric artery (SMA) ≤180°. Short segment (approximately 1.5 cm) encasement of the PV or SMV that was amenable to partial vein resection and reconstruction was also classified as borderline. Patients who had encasement of the gastroduodenal artery up to the origin of the hepatic artery were also considered borderline resectable. Involvement of both the PV/SMV and SMA that would require resection and reconstruction of both arterial and venous systems was classified as unresectable. Patients who had encasement of the superior mesenteric artery (SMA), celiac artery, aorta, or inferior vena cava (IVC) were classified as unresectable.
Neoadjuvant Treatment
All of the borderline resectable pancreatic cancer patients received induction chemotherapy with GTX. Gemcitabine (Eli Lilly) was given at 750mg/m2 on Days 4 and 11. Docetaxel (Sanofi Aventis) was administered at 30mg/m2 on Days 4 and 11. Capecitabine (Genentech) was given at 750mg/m2 BID on Days 1–14. This cycle was repeated every 21 days, as used in the original metastatic study involving GTX [14].
One week after completing induction GTX chemotherapy, the patients began concurrent chemoradiation with 5-FU (continuous infusion, 225mg/m2) and Intensity-Modulated Radiation Therapy (IMRT). With IMRT, radiation oncologists are able to deliver the same or higher doses to the target while sparing more normal tissue. IMRT was administered with dose painting in the majority of cases, with the intent to deliver a higher dose each fraction to gross disease and a lower dose each fraction to the microscopic disease at highest risk.
All cases in this series were planned by the same GI radiation oncologist. 4D CT simulation was performed with the patient in the supine position using immobilization with the patient’s arms over their head. An initial scan using IV and oral contrast was performed in free breathing unless there was a contraindication such as a high creatinine or a contrast allergy. Neither abdominal compression nor respiratory gating techniques were utilized since the patient was receiving a 5 week course of therapy. Two target volumes were drawn. The first was the GITV, the gross internal target volume, taking into account the primary disease in all phases of respiration. A second was a subjective CITV, the clinical internal target volume, reflecting the microscopic sites of highest risk. No attempt was made to cover all potential sites of nodal risk. Since image guidance was not utilized for the treatment, a margin of 1cm was placed around the GITV and the CITV for the creation of the respective PTV 50 and PTV 45. The intent of treatment was thus to irradiate the gross disease target at 200cGy per fraction for 25 fractions while delivering a lower dose of 180cGy per fraction to the microscopic clinical volume felt to be at highest risk.
After induction chemotherapy and chemoradiation, the patients were restaged. CT and PET scans were performed approximately 4 weeks after completing chemoradiation. A CA 19-9 level was drawn at this time as well. The changes in CA 19-9, CT mass size, and PET SUV were calculated using the values obtained prior to and after neoadjuvant therapy.
Surgical Assessment
After completing neoadjuvant treatment and reviewing the data, all potentially resectable patients were discussed by our GI Tumor Board. A consensus was made on the potential for resectability based on standard NCCN radiologic guidelines, including a lack of distant metastases, tumor thrombus, or vascular abutment. For those patients who underwent surgery and had a resection, the operating surgeon and pathologist determined if an R0 or R1 resection was achieved. The operation was defined as an R0 resection if there was no microscopic tumor found at the margin and as an R1 resection if a margin was microscopically positive. Pancreaticoduodenectomy was performed in the standard fashion. Segmental resection of the SMA, PV, or SMV/PV confluence was performed when the operating surgeon could not separate the pancreatic head from these vessels without leaving tumor on the vessel.
Follow-up and Statistical Analyses
The patients were seen in clinic approximately 1 month after surgery and then every 3–4 months thereafter. The visits included a complete physical examination, complete blood count, and blood chemistries. Ordering CA 19-9 levels and CT scans were done in the surveillance time period, but not in fixed intervals. Overall survival was calculated from the initiation date of neoadjuvant therapy until the date of death or last contact. Progression free survival was calculated from the initiation date of neoadjuvant therapy until the last date at which the patient was known to be free of disease. Overall survival and progression-free survival curves were created by the Kaplan Meier method [20]. Median follow-up time for the patients who are still alive was calculated from the initiation of neoadjuvant therapy until the last follow-up visit.
Agreement between CT and EUS mass size was assessed by the Concordance Correlation Coefficient. Agreement of vascular involvement between CT and EUS was assessed by a kappa index. Correlation between CT mass size and CA 19-9/PET SUV was analyzed by calculating Pearson correlation coefficients. Statistical differences between pre and post-neoadjuvant CA 19-9 levels, PET SUV, and CT mass sizes were assessed using the Wilcoxon signed-rank test. The SPSS software version 15.0 was used for all statistical analyses.
Results
Patient Characteristics
As displayed in Table I, the median age at diagnosis was 67 with a range from 45–82 for the 18 patients involved in this retrospective analysis. All patients began with an ECOG Performance Status ≤ 1 prior to starting therapy. Twelve of the patients were male and 6 were female. Seventy-two percent of the masses (n=13) involved the pancreatic head, 11.1% (n=2) were located in the body, and 16.7% (n=3) involved the body/tail. A pretreatment CT was performed in all 18 patients, while 14 of the patients underwent an EUS as well. Mean mass size by CT was 3.4 cm (n=17) and 3.3 cm by EUS (n=14). The median number of administered cycles of GTX was 3 in our retrospective analysis. Thirteen patients received 3 cycles, one was given 4 cycles, and four received 2 cycles. Median time from the date of diagnosis to start of chemotherapy was 18 days. The median IMRT dose was 5000cGy. Thirteen patients received 5000cGy, three were given 4500cGy, and one received 5200cGy. One patient did not receive IMRT because of disease progression while receiving induction chemotherapy, thus only 17 patients finished the complete neoadjuvant regimen.
Table I.
Characteristics of the 18 Borderline Resectable Pancreatic Cancer Patients
| Total Patients | 18 | (100%) |
| Age (y) | ||
| Median | 67 | |
| Range | 45–82 | |
| Gender, n (%) | ||
| Male | 12 | (66.6%) |
| Female | 6 | (33.3%) |
| Location of Pancreatic Mass, n (%) | ||
| Head | 13 | (72.2%) |
| Body | 2 | (11.1%) |
| Body/Tail | 3 | (16.7%) |
| Performance Status, n (%) | ||
| 0 | 11 | (61.1%) |
| 1 | 7 | (38.9%) |
| Mean Mass Size (cm) * | ||
| by CT (n=17) | 3.4 | |
| by EUS (n=14) | 3.3 | |
mean mass size by CT did not differ when compared to EUS for those patients who had both studies (p=0.52, n=13); Concordance correlation coefficient of r=0.85
Sites of vascular abutment that defined the diagnosis of borderline resectable disease are illustrated in Table II. Abutment could be assessed in 15 of the 18 patients by CT and in 10 of the 14 patients who had an EUS. The majority of the borderline resectable cases consisted of portal vein (PV) and superior mesenteric vein (SMV) abutment. Utilizing CT, 22% of the cases displayed abutment with the PV, 28% with the SMV, and 17% with the PV/SMV confluence. EUS suggested that 29% of the cases had abutment with the PV, 21% with the SMV, and 14% with the PV/SMV confluence. Three patients were not found to have vessel abutment by the initial CT and EUS, but abutment was seen when taken for immediate resection. Surgery was aborted in these 3 cases and they were then diagnosed as having borderline resectable disease.
Table II.
Tumor Vascular Abutment by CT vs. EUS
| Vessel | By CT
|
By EUS
|
||
|---|---|---|---|---|
| n | % | n | % | |
| PV | 4 | 22.2 | 4 | 28.6 |
| SMA | 2 | 11 | 1 | 7.1 |
| SMA and SMV | 1 | 5.6 | - | - |
| SMV | 5 | 27.8 | 3 | 21.4 |
| SMV/PV confluence | 3 | 16.7 | 2 | 14.3 |
| none | 3 | 16.7 | 4 | 28.6 |
| Total | 18 | 100 | 14 | 100 |
Three patients were not found to have vessel abutment by initial CT or EUS, but abutment was seen when taken for immediate resection; PV = portal vein; SMA = superior mesenteric artery; SMV = superior mesenteric vein
Pretreatment CA19-9 levels and PET SUV are displayed in Table III. The median CA 19-9 level (n=18) prior to treatment was 483. The median pretreatment max SUV for the 15 patients who had a PET scan was 5.7.
Table III.
Biochemical and Imaging Characteristics before and after Neoadjuvant Therapy
| Pre-GTX/IMRT | Post-GTX/IMRT | p-value | ||
|---|---|---|---|---|
| Median CA 19-9 (units/mL) | 483 | 50 | <0.01 | * |
| Range | 0–9590 | 0–5450 | ||
| Median PET SUV max | 5.7 | 1.6 | <0.001 | # |
| Range | 3.4–13.9 | 0–4 | ||
| Median Pancreatic Mass Size by CT (cm) | 3.35 | 2.70 | 0.002 | |
| Range | 2.0–5.0 | 0–5.0 | ||
8/16 patients had a > 85% decrease in CA 19-9 levels
7/14 patients had a 100% decrease in PET SUV max
Response to Neoadjuvant Therapy
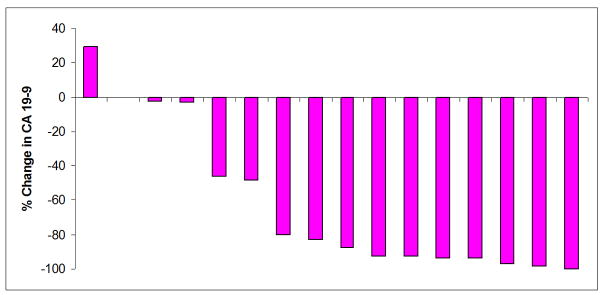
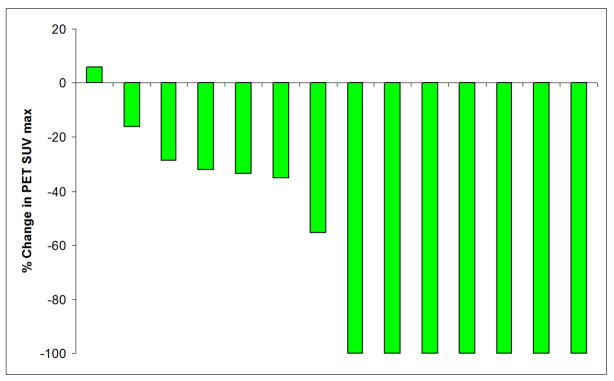
CA 19-9, CT mass size, and PET SUV significantly decreased after neoadjuvant therapy (Table III). Sixteen patients had both pre and post-therapy CA 19-9 levels checked. Median CA 19-9 decreased from 483 to 50 (p<0.01), representing a median 83% decrease. Eight out of the 16 patients had a >85% decrease in CA 19-9 levels. Fourteen patients had both pre and post-therapy PET scans. Median PET max SUV decreased from 5.7 to 1.6 (p<0.001). Seven of the 14 patients (50%) had a 100% decrease in PET SUV. Graphical representations of the significant change in CA 19-9 levels and PET SUV after neoadjuvant therapy are illustrated in Figure 1 and 2 for each patient who had pre and post CA 19-9 levels and PET scans. Median CT mass size decreased from 3.35 to 2.70cm after neoadjuvant therapy (p=0.002).
Figure 1.
Percent change in CA 19-9 level after neoadjuvant therapy for each of the 16 patients who had pre and post CA 19-9 levels drawn. Eight patients had >85% decrease in their CA 19-9 levels.
Figure 2.
Percent change in PET SUV after neoadjuvant therapy for each of the 14 patients who had pre and post PET scans performed. Seven patients had a complete loss of PET avidity.
Table IV displays the surgical outcomes after neoadjuvant therapy. Seventeen out of the 18 patients from the database finished neoadjuvant therapy. One patient progressed while receiving induction chemotherapy, so neoadjuvant therapy was not completed. Following a discussion at our GI Tumor Board, 14 out of the 17 patients (82%) who completed neoadjuvant therapy met radiologic evidence for resectability based on NCCN guidelines and were subsequently brought to surgery. Of these 14 patients who went to surgery, pancreatic mass resections were performed in 9 cases (64%). Two of the cases involved reconstruction of the PV/SMV. Pancreatic disease could not be resected in 5 patients at exploration due to unexpected encasement of the SMA/SMV in 2 cases, encasement of the SMA/SMV/PV in 1 case, mesenteric disease and SMV encasement in 1 case, and peritoneal metastases in another case that were not found on the post-neoadjuvant scans. From the 9 patients who were able to undergo resections, an R0 resection was achieved in 8 of these cases (89%). Overall, 8 out of the 17 patients (47%) who finished our neoadjuvant protocol achieved an R0 resection. By ITT analysis, 8 of 18 borderline resectable patients underwent a successful Whipple procedure with negative margins. The common bile duct, proximal gastric, pancreatic, retroperitoneal, and duodenal margins were examined for each resection. Of the 8 R0 resection specimens, the pathologist noted that 7 displayed minimally residual disease that was consistent with significant treatment effect. The other specimen did not reveal any carcinoma and was completely fibrotic. The median time from the initiation of neoadjuvant therapy to the date of surgery was 5.82 months.
Table IV.
Surgical Outcomes for Patients who Finished Neoadjuvant Therapy
| Total Patients * | 17 | |
| Surgery | 14 | |
| Percent of Total | 82% | |
| Resected | 9 | |
| Percent Resected from those who had Surgery | 64% | |
| R0 Resections | 8 | |
| Percent R0 from Total Resected | 89% | |
| Percent R0 Resections from those who finished Therapy | 47% | |
One out of the 18 patients progressed while receiving induction chemotherapy and did not finish neoadjuvant therapy
Survival analysis is not complete as 6 out of the 18 patients are still alive. Three of these 6 patients are without evidence of disease. Of the initial 18 patients, one patient progressed to develop liver metastases during induction chemotherapy and could not finish neoadjuvant therapy. Nine other patients progressed after neoadjuvant therapy. However, only 3 of these patients progressed after resection. Progression involved development of liver metastases (n=1), peritoneal metastases (n=1), lung metastases (n=2), malignant ascites/growth of tumor (n=1), increased lymphadenopathy/growth of tumor (n=1), vascular encasement (n=1), and significantly worsening CA 19-9 levels/symptoms (n=2). Kaplan-Meier curves for progression-free survival and overall survival are illustrated in Figure 3. Median progression-free survival was 10.48 months (95% CI: 6.01–14.55) and median overall survival was 15.64 months (95% CI: 14.49–23.92); however, 6 patients are still alive. Median follow-up time for the 6 patients who are still alive was 13.27 months.
Figure 3.
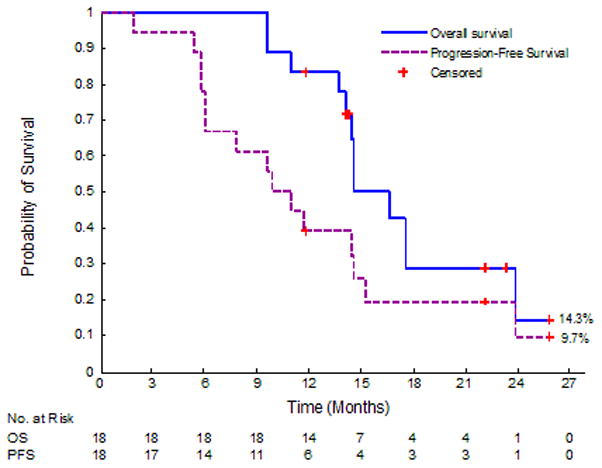
Kaplan-Meier curves for progression-free survival and overall survival for the 18 borderline resectable pancreatic cancer patients. Median OS was 15.64 mo and the median PFS was 10.48 mo. Six patients are still alive (censored).
Toxicities and Complications
The toxicities related to neoadjuvant therapy did not prevent any of the patients from completing treatment or cause any subsequent surgical morbidity. Significant temporary toxicities related to treatment consisted of hand-foot syndrome (n=7), mucositis (n=8), abdominal pain (n=1), diarrhea (n=3), febrile neutropenia (n=1), and thrombocytopenia (n=3). Post-operative complications included a pulmonary embolism (n=1), wound infection (n=1), seizures (n=1), and acute renal failure (n=1). There were no deaths from neoadjuvant therapy or surgery. All patients who were admitted for surgery were discharged home.
Correlation between Diagnostic Modalities
The mean mass size determined by CT did not differ when compared to that measured by EUS. Primary tumor size as measured by EUS was significantly correlated with the size measured by CT scan. The Concordance correlation coefficient was 0.85 for patients who had both CT and EUS (n=13, 95% CI: 0.596–0.950), consistent with statistical agreement. As shown in Table V, CT and EUS agreed upon the extent of vascular involvement in 12 out of the 14 patients (86%) who had both pretreatment CT and EUS assessments (p=0.02 Chi-square test). Statistical reproducibility was demonstrated by a Kappa index of 0.59 (95% CI: 0.1–1.0).
Table V.
Vessel Abutment Agreement by CT and EUS for Patients who had Both Studies
| Vessel Abutment by CT | |||
|---|---|---|---|
| Yes | No | Total | |
| Vessel Abutment by EUS | |||
| Yes | 10 | 0 | 10 |
| No | 2 | 2 | 4 |
| Total | 12 | 2 | 14 |
When investigating for a possible association between CA 19-9 and CT mass size, the Pearson correlation coefficient (r) was found to be −0.084. Therefore, CA19-9 was not correlated with tumor size as measured by CT scan (p = 0.75). Less than 1% of the variability in CA19-9 could be explained by the variability in size by CT scan as indicated by r2 = 0.0071.
When evaluating for a possible association between PET SUV and CT mass size, the Pearson correlation coefficient (r) was found to be 0.38. Thus, SUV max was not significantly correlated with tumor size as measured by CT scan (p = 0.18). Only 15% of the variability in SUV max could be explained by the variability in size by CT scan as indicated by r2 = 0.1475.
Discussion
The current retrospective analysis displays promising outcomes for a particular neoadjuvant regimen specifically in borderline resectable pancreatic cancer. Completed prospective studies specifically involving only borderline resectable disease do not exist. It is known that long-term survival after a diagnosis of pancreatic cancer is best achieved in patients who are diagnosed prior to the development of metastases and who are able to undergo a Whipple procedure with negative margins. Multimodality neoadjuvant therapies can have the greatest impact on borderline resectable pancreatic cancer patients by increasing the likelihood of obtaining an R0 resection, as shown in a meta-analysis specifically examining neoadjuvant therapy for pancreatic cancer [21]. Induction chemotherapy followed by chemoradiation has led to efficacious results in locally advanced unresectable pancreatic cancer [12,13]. The fact that most successfully resected patients display recurrence provides evidence that micrometastases are likely to present at an early stage of the disease. Induction chemotherapy not only offers the advantage of eradicating micrometastatic disease, but it can also theoretically decrease tumor size allowing better response to subsequent chemoradiation and serve as a screening test for response to the chosen chemotherapeutic agents.
Our analysis demonstrates that neoadjuvant GTX induction chemotherapy followed by 5-FU IMRT has significant activity in borderline resectable pancreatic cancer while satisfying the goal of achieving clinical outcomes comparable to upfront resectable disease. Median CA 19-9 levels, CT mass size, and PET SUV all significantly decreased after neoadjuvant therapy. The data illustrate that all three modalities, CA 19-9, CT scans, and PET scans allow the opportunity to judge response to neoadjuvant therapy. Overall, 8 out of the 17 patients who finished our neoadjuvant regimen achieved a successful Whipple procedure with negative margins. These surgical outcomes compare favorably with historical data [7–11,13,22–24] that utilized various neoadjuvant regimens to yield resections for locally advanced disease with negative margins. In addition, only 2 patients needed vascular reconstruction after neoadjuvant therapy. Based on historical data, the percentage of patients necessitating vascular reconstruction would be expected to be much higher if taken for resection upfront. Our analysis yielded a progression-free survival of 10.48 months and a median overall survival of 15.64 months. Six out of the 18 patients are still alive. It is evident that neoadjuvant GTX induction chemotherapy followed by chemoradiation can achieve the goal of an R0 resection in borderline resectable pancreatic cancer and can lead to survival that is similar to what has been found for resectable pancreatic cancer at diagnosis [2]. This neoadjuvant regimen also involves minimal toxicity with a convenient dosing schedule.
The retrospective analysis also facilitated an assessment of the utility of CA 19-9, CT scans, and PET scans in the diagnosis and management of borderline resectable disease that has not formally been published in the past. The mean mass size and extent of vascular abutment determined by CT did not differ when compared to EUS, which suggests that CT and EUS can play complimentary roles during diagnostic workup. In terms of these diagnostic tools, the study was important as it did not illustrate statistical associations between CT mass size and PET SUV and between CA 19-9 levels and CT mass size. Although known to be quite helpful in the majority of the cases, CT and PET scans failed to identify occult disease in 5 patients after neoadjuvant therapy. These 5 patients were brought to the operating room, but their disease could not be resected due to gross visual evidence of occult disease.
Major strengths and weaknesses are evident when examining the current study. The retrospective analysis mostly consisted of patients that were treated by a dedicated multi-disciplinary team at a single institution. A multidisciplinary approach allows uniformity in obtaining tissue diagnoses, accurate radiologic interpretation, adherence to the definition of resectability, consistent treatment approaches, and standardized follow-up assessments. The study is only made up of borderline resectable pancreatic cancer that allows the opportunity to closely examine the various diagnostic modalities and treatment effects on this particular disease. Neoadjuvant therapy was completed in 17 out of 18 patients, which allows accurate assessment for the ability to obtain an R0 resection with the particular regimen. The study also allowed an opportunity to examine the utility of CT, PET, and CA 19-9 during diagnosis and during the assessment of response. The main weakness of the analysis was that it was retrospective, although the patients were treated in a uniform fashion. The small sample size involved in the imaging and CA 19-9 comparisons, as well as in assessing surgical response to neoadjuvant therapy, also represents a limitation. However, studies involving large sample sizes do not exist for the borderline resectable pancreatic cancer population.
In summary, we report an effective neoadjuvant regimen for borderline resectable pancreatic cancer that can lead to increasing the likelihood of R0 resections. This analysis provides the first published data displaying the response of borderline resectable disease to GTX induction chemotherapy followed by 5-FU IMRT. GTX chemotherapy displays activity in borderline resectable disease based on our results. The neoadjuvant regimen proved to be well tolerated and did not pose difficulties in administration or scheduling. The results obtained by utilizing this regimen are favorable to historical data and will need to be explored further. Our group hopes to perform a prospective study to accomplish this objective. We will also explore novel radiotherapy approaches, such as incorporating Stereotactic Body Radiotherapy (SBRT).
Acknowledgments
Dr Springett receives grant support from the Robert Wood Johnson Foundation, Amos Faculty Development Program, and NIH R01 CA131400-2.
References
- 1.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Mehta VK, Fisher G, Ford JA, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2001;5(1):27–35. doi: 10.1016/s1091-255x(01)80010-x. [DOI] [PubMed] [Google Scholar]
- 3.Warshaw AL, Gu Z-Y, Whittenberg J, et al. Preoperative staging and assessment of resectability of pancreatic cancer. Arch Surg. 1990;125:230–233. doi: 10.1001/archsurg.1990.01410140108018. [DOI] [PubMed] [Google Scholar]
- 4.Millikan KW, Deziel DJ, Silverstein JC, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg. 1999;65:618–623. [PubMed] [Google Scholar]
- 5.Pingpank JF, Hoffman JP, Ross EA, et al. Effect of preoperative chemoradiotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. J Gastrointest Surg. 2001;5:121–130. doi: 10.1016/s1091-255x(01)80023-8. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman JP, Weese JL, Solin LJ, et al. A pilot study of preoperative chemoradiation for patients with localized adenocarcinoma of the pancreas. Am J Surg. 1995;169(1):71–78. doi: 10.1016/s0002-9610(99)80112-3. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998;16(1):317–323. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 9.Snady H, Bruckner H, Cooperman A, et al. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma: an outcomes trial. Cancer. 2000;89(2):314–327. doi: 10.1002/1097-0142(20000715)89:2<314::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Wilkowski R, Thoma M, Schauer R, et al. Effect of chemoradiotherapy with gemcitabine and cisplatin on locoregional control in patients with primary inoperable pancreatic cancer. World J Surg. 2004;28(10):1011–1018. doi: 10.1007/s00268-004-7338-z. [DOI] [PubMed] [Google Scholar]
- 11.Safran H, Dipetrillo T, Iannitti D, et al. Gemcitabine, paclitaxel, and radiation for locally advanced pancreatic cancer: a phase I trial. Int J Radiat Oncol Biol Phys. 2002;54(1):137–141. doi: 10.1016/s0360-3016(02)02902-4. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110(1):47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 13.Fogelman DR, Schreibman S, Sherman W, et al. Neoadjuvant GTX and radiation for unresectable pancreatic cancer: a prospective phase II trial. Proceedings from the 4th Annual Gastrointestinal Cancers Symposium; January 19–21, 2007; Orlando, FL. Abstract 143. [Google Scholar]
- 14.Fine RL, Fogelman DR, Schreibman SM, et al. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: a retrospective analysis. Cancer Chemother Pharmacol. 2008;61(1):167–175. doi: 10.1007/s00280-007-0473-0. [DOI] [PubMed] [Google Scholar]
- 15.Landry J, Catalano PJ, Staley C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Onc. 2010;101(7):587–592. doi: 10.1002/jso.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 17.Farma JM, Santillan AA, Melis M, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15(9):2465–71. doi: 10.1245/s10434-008-9992-0. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed June 1,2010];National Comprehensive Cancer Network (NCCN) Practice Guidelines for Pancreatic Cancer. Available at: http://www.nccn.org.
- 19.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035–46. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. American Statistical Association. 1958;53:457–481. [Google Scholar]
- 21.Gillen S, Schuster T, Meyer zum Buschenfelde C, et al. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jessup JM, Steele G, Mayer RJ, et al. Neoadjuvant therapy for unresectable pancreatic adenocarcinoma. Arch Surg. 1993;128(5):559–64. doi: 10.1001/archsurg.1993.01420170093014. [DOI] [PubMed] [Google Scholar]
- 23.Crane CH, Abbruzzese JL, Evans DB, et al. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys. 2002;52(5):1293–1302. doi: 10.1016/s0360-3016(01)02740-7. [DOI] [PubMed] [Google Scholar]
- 24.Crane CH, Ellis LM, Abbruzzese JL, et al. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol. 2006;24(7):1145–1151. doi: 10.1200/JCO.2005.03.6780. [DOI] [PubMed] [Google Scholar]