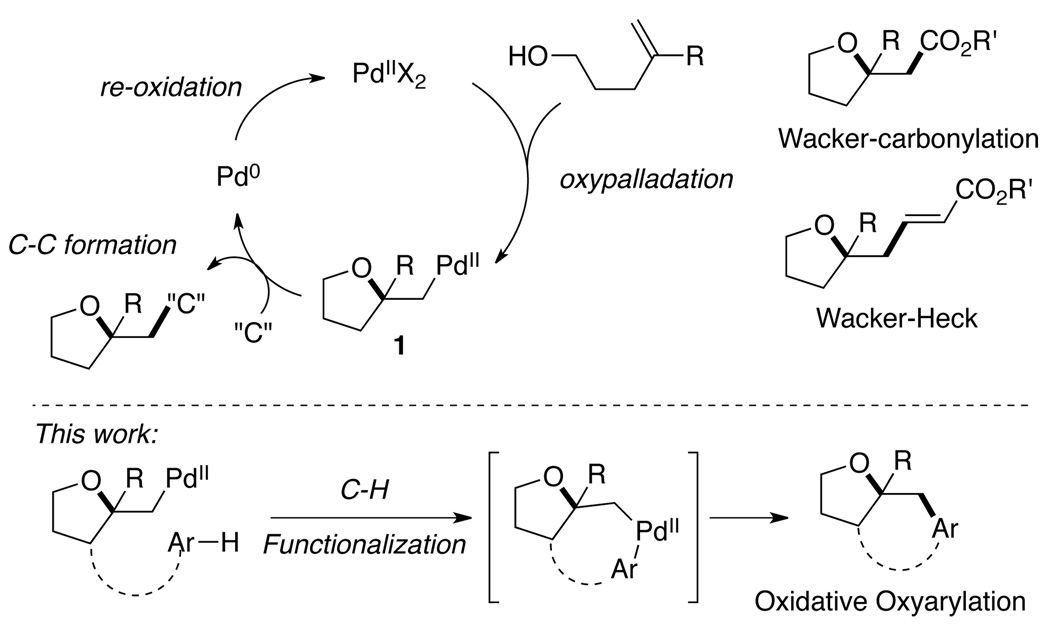
Originating from the classic Wacker process,1 the palladium(II)-catalyzed oxidative difunctionalization of alkenes has emerged as an attractive strategy for the rapid generation of molecular complexity due to its ability to form multiple carbon–carbon/carbon–heteroatom bonds and stereogenic centers in a single step.2,3,4 This versatility, combined with the broad functional group compatibility and air- and moisture-tolerance, renders the Pd(II)-catalyzed oxidative difunctionalization a powerful tool for synthetic chemists. One of the most synthetically relevant transformations of this class is the Pd(II)-catalyzed oxidative carboetherification of hydroxyalkenes, which leads to a variety of interesting oxygenated heterocycles.5 Pioneered by Semmelhack,6 the oxidative carboetherification in the presence of CO or electronically biased olefins has been developed with wide application to complex molecule synthesis.7 Mechanistically such reactions are believed to proceed via a β-alkoxy-alkylpalladium(II) intermediate 1 generated from nucleophilic oxypalladation (Scheme 1). Compared to the more often reported carbon–heteroatom bond forming processes, the scope of oxidative carbon–carbon bond formation from 1 is still limited.8 It is both synthetically and mechanistically interesting to expand the scope of this type of transformation in order to access more structurally diverse molecules.
Scheme 1.
Scope of the Pd(II)/Pd(0) catalyzed oxidative carboetherification.
Aiming to develop a viable method for the catalytic oxidative oxypalladation/arylation of unactivated hydroxyalkenes, we were inspired by the recent development in the field of Pd(II)-catalyzed directed arene C–H activation/C–C bond formation.9 Noting that 1 could undergo cyclopalladation with a proximate arene ring under similar condition (Scheme 1), we envisioned the possibility of merging the two Pd(II)-catalyzed transformations, namely the oxypalladation and C–H activation/C–C bond formation.
The success of this strategy lies in the identification of a catalytic system efficient for both the oxypalladation and C–H functionalization steps, as well as the ability to avoid oxidation of the alcohol functional group.10 Herein we report a simple and mild Pd(II)-catalyzed method for the efficient construction of the tetrahydro-2H-indeno-[2,1-b]furan framework from acyclic hydroxyalkenes bearing unactivated arenes.
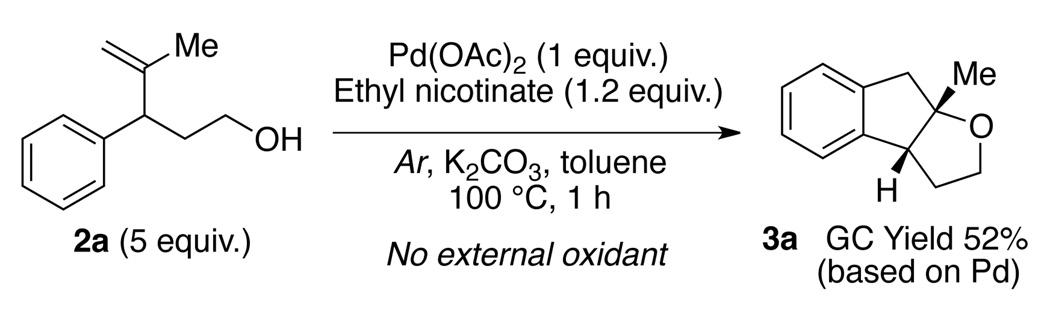
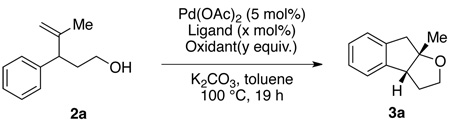
We began our study by subjecting 4-methyl-3-phenylpent-4-en-1-ol (2a) to a catalytic amount of palladium acetate and a variety of different stoichiometric oxidants. Several common combinations for Pd(II)-catalyzed oxidative C–H activations such as Pd(II)/Cu(II), Pd(II)/Ag(I) and Pd(II)/PIDA were shown to be ineffective for this transformation (Table 1, entries 1–3).8,11 Simple Pd(OAc)2/pyridine/O2 (1 atm) system,12 however, was able to efficiently catalyze the desired transformation, affording 3a in moderate yield (entry 5). The use of bidentate pyridine-based ligands were found to significantly retard the reaction (entries 6, 7).13 Tuning the substituents on the pyridine ligand (entries 8–10) led to the identification of ethyl nicotinate14 as the optimal ligand. The ligand loading could also be lowered to 6 mol% without loss of yield (entry 12).
Table 1.
Representative optimization of the reaction conditions.a
 | |||
|---|---|---|---|
| Entry | Ligand (x mol%) | Oxidant (y equiv.) | Yield %b |
| 1 | None | CuCl2 (2) | <5 |
| 2 | None | AgOAc (2) | 18 |
| 3 | None | PhI(OAc)2 (2) | <5 |
| 4 | None | O2 (1 atm) | 22 |
| 5 | Pyridine(20) | O2 (1 atm) | 66 |
| 6 | 2,2’-bipyridyl(10) | O2 (1 atm) | 13 |
| 7 | 1,10-phen(10) | O2 (1 atm) | 5 |
| 8 | 3-cyanopyridine(20) | O2 (1 atm) | 71 |
| 9 | 4-DMAP(20) | O2 (1 atm) | 82 |
| 10 | Ethyl nicotinate(20) | O2 (1 atm) | 88 |
| 11c | Ethyl nicotinate(20) | O2 (1 atm) | <5 |
| 12 | Ethyl nicotinate(6) | O2 (1 atm) | 89 |
Reaction conditions: Pd(OAc)2 (5 mol%), Ligand (x mol%), Oxidant (y equiv.), K2CO3 (0.5 equiv.), 2a (0.1 mmol), toluene (1 mL), 100 °C, 19 h.
GC Yield using dodecane as an internal standard.
Without Pd(OAc)2.
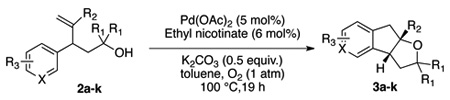
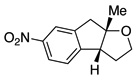
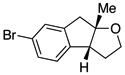
A variety of α-aryl-γ-hydroxyalkenes could be cyclized to the corresponding tetrahydro-2H-indeno-[2,1-b]furan derivatives using this Pd(II)-catalyzed intramolecular tandem oxypalladation/C–H activation protocol. Illustrative examples of the reaction scope are shown in Table 2. Both alkyl and aryl substituents on the carbon-carbon double bond were tolerated (3b, 3c), as well as tertiary alcohol nucleophile (3d). In addition, a wide range of electron-rich, -neutral and -deficient arenes were found to participate in the C–H activation process (3e–h, 3j). An aryl bromide-containing substrate afforded the desired product (3i) in modest yield, although additional quantities of copper(II) chloride proved to be necessary,15 this observed orthogonal reactivity relative to the Pd(0)-catalyzed cross-coupling chemistry is useful for the further elaboration of the arene ring. meta-Substituted arene (entry 10) cyclized to give a 3:1 mixture of regioisomers favoring the cleavage of the less hindered C–H bond (3j, 3j’). Finally it was found that a pyridyl group could also participate in the C–H activation process with functionalization solely at the 4-position (3k).
Table 2.
Pd(II)-catalyzed oxidative oxyarylation of hydroxyalkenes.a
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Yield%b | |
| 1 |  |
 |
3a | 81 |
| 2 | 3b | 58 | ||
| 3 | 3c | 74 | ||
| 4 | 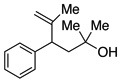 |
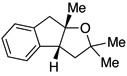 |
3d | 74 |
| 5 | 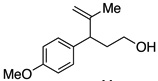 |
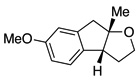 |
3e | 80 |
| 6 | 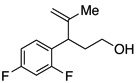 |
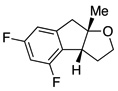 |
3f | 66 |
| 7 | 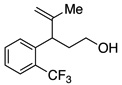 |
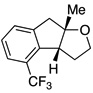 |
3g | 87 |
| 8 | 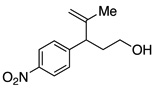 |
 |
3h | 69 |
| 9 | 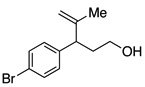 |
 |
3i | 48c |
| 10 | 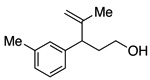 |
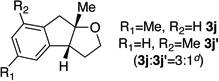 |
67 | |
| 11 | 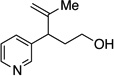 |
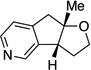 |
3k | 73 |
Reaction conditions: Pd(OAc)2 (5 mol%), ethyl nicotinate (6 mol%), O2 (1 atm), K2CO3 (0.5 equiv.), 2 (0.5 mmol), toluene (5 mL), 100 °C, 19 h.
Isolated yields, average of two runs.
With 5 mol% CuCl2 as co-oxidant and 30 mol% ethyl nicotinate.
Ratio determined by 1H NMR.
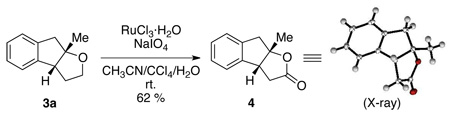 |
(1) |
Lactone 4 can be accessed by one step oxidation of the oxyarylation adduct 3a (eq. 1). The relative stereochemistry of 4 was confirmed by X-ray diffraction. Lactone 4 is also structurally related to a class of sulindac-derived biologically active molecules which have been used for “precancerous treatment.”16
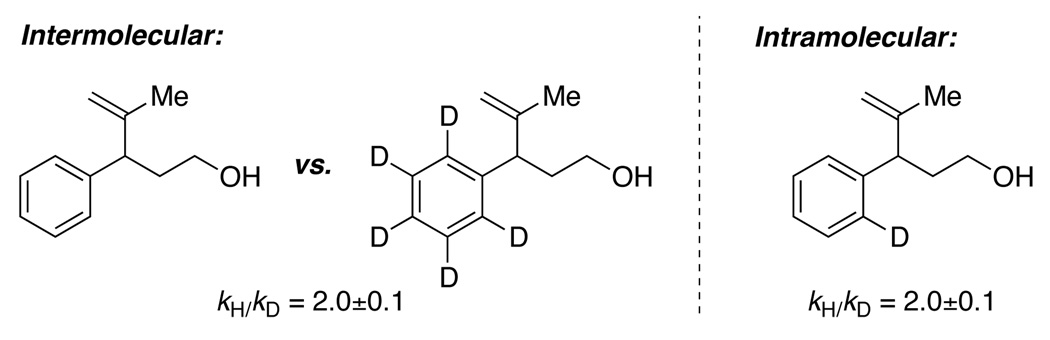
To gain an insight into the reaction mechanism, deuterium labeling experiments were performed. The observed kinetic isotope effect for both intermolecular and intramolecular cases were found to be approximately 2.17 This observation is consistent with a reaction mechanism in which irreversible C–H bond cleavage is rate-limiting. Next, treating 2a with palladium acetate under an argon atmosphere in the presence of ethyl nicotinate also afforded cyclization product 3a (Scheme 2). The formation of 3a in the absence of external re-oxidant is consistent with the Pd(II)/Pd(0) catalytic cycle depicted in Scheme 1.
Scheme 2.
Oxyarylation in the absence of external oxidant.
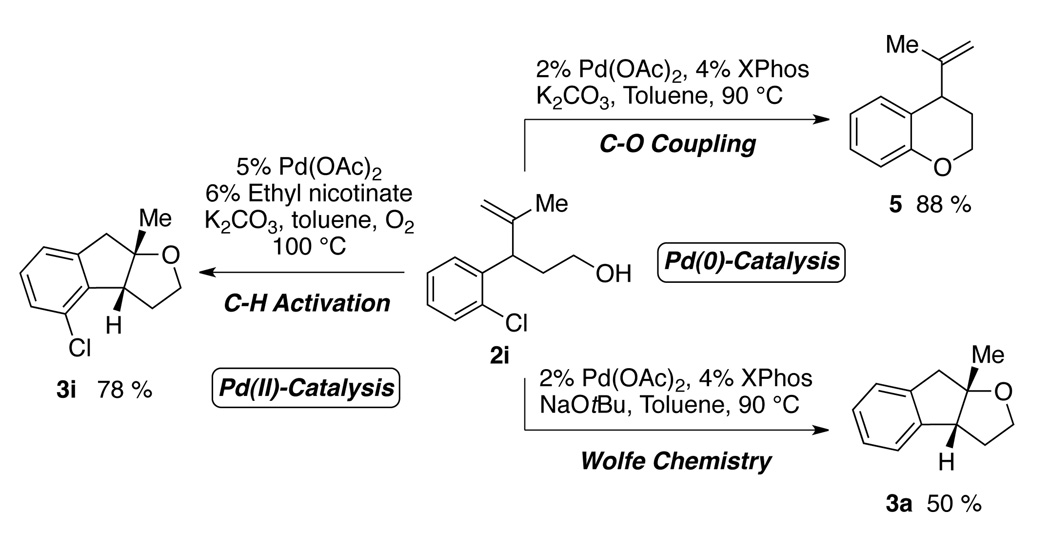
One of the most interesting aspects of the Pd(II)-catalyzed oxidative process is its orthogonal reactivity compared with Pd(0)-catalyzed transformations (Scheme 3). Treating hydroxyalkene 2i with a catalytic amount of palladium acetate and XPhos in the presence of K2CO3 afforded chroman derivative 5 by a classic Pd(0)-catalyzed C–O coupling.18 The alkene functional group remained intact. However, switching the base to NaOtBu resulted in the formation of cyclization product 3a via Pd(0)-catalyzed carboetherification of the alkene originally developed by Wolfe and co-workers.5 On the other hand, subjecting 2i to the standard conditions described in this work led to exclusive formation of 3i via the desired Pd(II)-catalyzed oxypalladation/C–H functionalization pathway. Dechlorinated material was not detected. This demonstrates the potential of diversified modification of a single compound containing multiple functional groups using different palladium catalyst systems.
Scheme 3.
Divergent Pd(II)-catalysis and Pd(0)-catalysis.
In conclusion, we have developed an efficient protocol for the intramolecular oxidative oxyarylation of hydroxyalkenes using a Pd(II)-catalyzed tandem oxypalladation/C–H functionalization strategy. This methodology allows rapid access to tetrahydro-2H-indeno-[2,1-b]furan framework from simple acyclic hydroxyalkene bearing unactivated arenes or heteroarenes. Further mechanistic studies and application of this strategy to the synthesis of other heterocyclic systems are currently under investigation.
Experimental Section
An oven-dried 50 mL Schlenk tube equipped with a Teflon-coated magnetic stir bar was charged with palladium acetate (5.6 mg, 0.05 equiv.), potassium carbonate (34.5 mg, 0.5 equiv.). The tube was then briefly evacuated and backfilled with oxygen (This sequence was repeated a total of four times). Ethyl nicotinate (4.5 mg, 0.06 equiv.) and hydroxyalkene (0.50 mmol, 1.0 equiv.) were added to the tube followed by anhydrous toluene (5.0 mL) via syringe. The sealed tube was placed in a pre-heated 100 °C oil bath. After stirring at the same temperature for 19 h the mixture was allowed to cool to room temperature. Ether (5 mL), methanol (0.25 mL), and sodium borohydride (9.5 mg, 0.5 equiv.) were then added and the resulting mixture was stirred for a further 5 min at room temperature. The mixture was then filtered through a short plug of silica gel and concentrated in vacuo. The residue was purified by silica gel flash column chromatography (EtOAc/hexane) to afford the cyclization product.
Supplementary Material
Figure 1.
Observed kinetic isotope effect.
Footnotes
We thank the National Institutes of Health (GM46059) for financial support of this project. The Varian 400 MHz NMR spectrometer used in this work was supported by grants from the National Institutes of Health (1S10RR13886-01). We thank the National Science Foundation for departmental X-ray diffraction instrumentation (CHE-0946721).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Smidt J, Hafner W, Jira R, Sedlmeier J, Sieber R, Rüttinger R, Kojer H. Angew. Chem. 1959;71:176. [Google Scholar]; b) Smidt J, Hafner W, Jira R, Sieber R, Sedlmeier J, Sabel A. Angew. Chem. 1962;74:93. Angew. Chem. Int. Ed.1962, 1, 80. [Google Scholar]
- 2.For selected reviews, see: Zeni G, Larock RC. Chem. Rev. 2004;104:2285. doi: 10.1021/cr020085h. Jensen KH, Sigman MS. Org. Biomol. Chem. 2008;6:4083. doi: 10.1039/b813246a. McDonald RI, Liu G, Stahl SS. Chem. Rev. 2011;111:2981. doi: 10.1021/cr100371y. Wolfe JP. Eur. J. Org. Chem. 2007:571.
- 3.For recent examples of Pd(II)-catalyzed oxidative dihetero-functionalization of unactived alkenes: Alexanian EJ, Lee C, Sorensen EJ. J. Am. Chem. Soc. 2005;127:7690. doi: 10.1021/ja051406k. Liu G, Stahl SS. J. Am. Chem. Soc. 2006;128:7179. doi: 10.1021/ja061706h. Desai LV, Sanford MS. Angew. Chem. 2007;119:5853. doi: 10.1002/anie.200701454. Angew. Chem. Int. Ed.2007, 46, 5737; Li Y, Song D, Dong VM. J. Am. Chem. Soc. 2008;130:2962. doi: 10.1021/ja711029u. Wang A, Jiang H, Chen H. J. Am. Chem. Soc. 2009;131:3846. doi: 10.1021/ja900213d. Streuff J, Hövelmann CH, Nieger M, Muñiz K. J. Am. Chem. Soc. 2005;127:14586. doi: 10.1021/ja055190y. Muñiz K. J. Am. Chem. Soc. 2007;129:14542. doi: 10.1021/ja075655f. Iglesias Á, Pérez EG, Muñiz K. Angew. Chem. 2010;122:8286. doi: 10.1002/anie.201003653. Angew. Chem. Int. Ed.2010, 49, 8109; Zhang Y, Sigman MS. J. Am. Chem. Soc. 2007;129:3076. doi: 10.1021/ja070263u. Jensen KH, Pathak TP, Zhang Y, Sigman MS. J. Am. Chem. Soc. 2009;131:17074. doi: 10.1021/ja909030c. Jensen KH, Webb JD, Sigman MS. J. Am. Chem. Soc. 2010;132:17471. doi: 10.1021/ja108106h. Sibbald PA, Rosewall CF, Swartz RD, Michael FE. J. Am. Chem. Soc. 2009;131:15945. doi: 10.1021/ja906915w. Sibbald PA, Michael FE. Org. Lett. 2009;11:1147. doi: 10.1021/ol9000087.
- 4.For recent examples of Pd(II)-catalyzed oxidative carboamination and carboamidation of unactived alkenes: Hegedus LS, Allen GF, Olsen DJ. J. Am. Chem. Soc. 1980;102:3583. Yip K-T, Yang M, Law K-L, Zhu N-Y, Yang D. J. Am. Chem. Soc. 2006;128:3130. doi: 10.1021/ja060291x. He W, Yip K-T, Zhu N-Y, Yang D. Org. Lett. 2009;11:5626. doi: 10.1021/ol902348t. Yip K-T, Yang D. Chem. Asian J. 2011;6:2166. doi: 10.1002/asia.201100242. Yip K-T, Yang D. Org. Lett. 2011;13:2134. doi: 10.1021/ol2006083. Shinohara T, Arai MA, Wakita K, Arai T, Sasai H. Tetrahedron Lett. 2003;44:711. Tsujihara T, Shinohara T, Takenaka K, Takizawa S, Onitsuka K, Hatanaka M, Sasai H. J. Org. Chem. 2009;74:9274. doi: 10.1021/jo901778a. Cernak TA, Lambert TH. J. Am. Chem. Soc. 2009;131:3124. doi: 10.1021/ja809897f. Rosewall CF, Sibbald PA, Liskin DV, Michael FE. J. Am. Chem. Soc. 2009;131:9488. doi: 10.1021/ja9031659. Nicolai S, Piemontesi C, Waser J. Angew. Chem. 2011;123:4776. doi: 10.1002/anie.201100718. Angew. Chem. Int. Ed.2011, 50, 4680.
- 5.For elegant studies by Wolfe and co-workers on the Pd(0)-catalyzed carboamination and carboetherification using arylhalides as electrophiles: Wolfe JP, Rossi MA. J. Am. Chem. Soc. 2004;126:1620. doi: 10.1021/ja0394838. Lira R, Wolfe JP. J. Am. Chem. Soc. 2004;126:13906. doi: 10.1021/ja0460920. Nakhla JD, Kampf JW, Wolfe JP. J. Am. Chem. Soc. 2006;128:2893. doi: 10.1021/ja057489m. Leathen ML, Rosen BR, Wolfe JP. J. Org. Chem. 2009;74:5107. doi: 10.1021/jo9007223. Rosen BR, Ney JE, Wolfe JP. J. Org. Chem. 2010;75:2756. doi: 10.1021/jo100344k.
- 6.a) Semmelhack MF, Bodurow C. J. Am. Chem. Soc. 1984;106:1496. [Google Scholar]; b) Semmelhack MF, Zhang N. J. Org. Chem. 1989;54:4483. [Google Scholar]; c) Semmelhack MF, Epa WR. Tetrahedron Lett. 1993;34:7205. [Google Scholar]
- 7.a) For an example of the Wacker-Heck reaction in natural product synthesis: Tietze LF, Sommer KM, Zinngrebe J, Stecker F. Angew. Chem. 2005;117:262. doi: 10.1002/anie.200461629. Angew. Chem. Int. Ed.2005, 44, 257; b) for a representative application of the Wacker-carbonylation: Xiao Q, Ren W-W, Chen Z-X, Sun T-W, Li Y, Ye Q-D, Gong J-X, Meng F-K, You L, Liu Y-F, Zhao M-Z, Xu L-M, Shan Z-H, Shi Y, Tang Y-F, Chen J-H, Yang Z. Angew. Chem. 2011;123:7511. doi: 10.1002/anie.201103088. Angew. Chem. Int. Ed.2011, 50, 7373.
- 8.a) For a Pd(II)/Pd(IV)-mediated oxyalkynylation using hypervalent iodine: Nicolai S, Erard S, González DF, Waser J. Org. Lett. 2010;12:384. doi: 10.1021/ol9027286. b) For a Pd(II)-catalyzed oxyindolation via quinone methide intermediate: Pathak TP, Gligorich KM, Welm BE, Sigman MS. J. Am. Chem. Soc. 2010;132:7870. doi: 10.1021/ja103472a. c) During the preparation of this manuscript, a Pd(II)/Pd(IV)-mediated oxyarylation using PhI(OAc)2 was reported: Matsuura BS, Condie AG, Buff RC, Karahalis GJ, Stephenson CRJ. Org. Lett. 2011 doi: 10.1021/ol202881q. ASAP.
- 9.For selected reviews: Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem. 2009;121:5196. doi: 10.1002/anie.200806273. Angew. Chem. Int. Ed.2009, 48, 5094; Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem. Rev. 2007;107:5318. doi: 10.1021/cr068006f.
- 10.a) Gligorich KM, Sigman MS. Chem. Commun. 2009:3854. doi: 10.1039/b902868d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Stahl SS. Angew. Chem. 2004;116:3480. Angew. Chem. Int. Ed.2004, 43, 3400; [Google Scholar]; c) Muzart J. Tetrahedron. 2003;59:5789. [Google Scholar]; d) Schultz MJ, Sigman MS. Tetrahedron. 2006;62:8227. [Google Scholar]
- 11.a) Chen X, Li J-J, Hao X-S, Goodhue CE, Yu J-Q. J. Am Chem. Soc. 2006;128:78. doi: 10.1021/ja0570943. [DOI] [PubMed] [Google Scholar]; b) Lu Y, Leow D, Wang X, Engle KM, Yu J-Q. Chem. Sci. 2011;2:967. [Google Scholar]; c) Wang X, Lu Y, Dai H-D, Yu J-Q. J. Am. Chem. Soc. 2010;132:12203. doi: 10.1021/ja105366u. [DOI] [PubMed] [Google Scholar]
- 12.a) Nishimura T, Onoue T, Ohe K, Uemura S. Tetrahedron Lett. 1998;39:6011. [Google Scholar]; b) Nishimura T, Onoue T, Ohe K, Uemura S. J. Org. Chem. 1999;64:6750. doi: 10.1021/jo9906734. [DOI] [PubMed] [Google Scholar]; c) Trend RM, Ramtohul YK, Ferreira EM, Stoltz BM. Angew. Chem. 2003;115:2998. doi: 10.1002/anie.200351196. Angew. Chem. Int. Ed.2003, 42, 2892; [DOI] [PubMed] [Google Scholar]; d) Nishimura T, Kakiuchi N, Onoue T, Ohe K, Uemura S. J. Chem. Soc. Perkin Trans. 1. 2000:1915. [Google Scholar]
- 13.This adverse effect was possibly due to the lack of an available coordination site in the complex formed. In Table 1, entry 12 it is shown that a Pd:L (L=ethyl nicotinate) ratio of 1:1.2 also afforded high yield. Therefore monopyridine bound palladium species might account for the productive pathway. Fix SR, Brice JL, Stahl SS. Angew. Chem. 2002;114:172. doi: 10.1002/1521-3773(20020104)41:1<164::aid-anie164>3.0.co;2-b. Angew. Chem. Int. Ed.2002, 41, 164; Steinhoff BA, Stahl SS. Org. Lett. 2002;4:4179. doi: 10.1021/ol026988e.
- 14.Ferreira EM, Stoltz BM. J. Am. Chem. Soc. 2003;125:9578. doi: 10.1021/ja035054y. [DOI] [PubMed] [Google Scholar]
- 15.Only debrominated products were detected without coppeRII) chloride.
- 16.Gross P, Sperl G, Pamukcu R, Brendel K. 9603987 A1. PCT Int. Appl. WO. 1996 U.S. Patent US 5776962 A 1998.
- 17.Gómez-Gallego M, Sierra MA. Chem. Rev. 2011;111:4857. doi: 10.1021/cr100436k. [DOI] [PubMed] [Google Scholar]
- 18.a) Torraca KE, Huang X, Parrish CA, Buchwald SL. J. Am. Chem. Soc. 2001;123:10770. doi: 10.1021/ja016863p. [DOI] [PubMed] [Google Scholar]; b) Vorogushin AV, Huang X, Buchwald SL. J. Am. Chem. Soc. 2005;127:8146. doi: 10.1021/ja050471r. [DOI] [PubMed] [Google Scholar]; c) Maimone TJ, Buchwald SL. J. Am. Chem. Soc. 2010;132:9990. doi: 10.1021/ja1044874. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wu X, Fors BP, Buchwald SL. Angew. Chem. 2011;123:10117. doi: 10.1002/anie.201104361. Angew. Chem. Int. Ed.2011, 50, 9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.