Abstract
Objective
Massive transfusion protocols have emphasized the importance of ratio-based transfusion of plasma and platelets relative to packed red blood cells (PRBCs), however the risks attributable to crystalloid resuscitation in patients requiring massive transfusion remain largely unexplored. We hypothesized that an increased crystalloid:PRBC (C:PRBC) ratio would be associated with increased morbidity and poor outcome following massive transfusion.
Methods
Data were obtained from a multi-center prospective cohort study evaluating outcomes in blunt injured adults with hemorrhagic shock. Patients requiring massive transfusion (≥10u PRBCs in first 24hrs) were analyzed. The C:PRBC ratio was computed by the ratio of crystalloid infused in liters (L) to the units of PRBCs transfused in the first 24hrs post-injury. Logistic regression modeling was used to characterize the independent risks associated with the 24hr C:PRBC ratio, after controlling for important confounders and other blood component transfusion requirements.
Results
Logistic regression revealed that the 24hr C:PRBC ratio was significantly associated with a greater independent risk of MOF, acute respiratory distress syndrome (ARDS) and abdominal compartment syndrome (ACS). No association with mortality or nosocomial infection was found. A dose response analysis revealed patients with a C:PRBC ratio >1.5:1, had over a 70% higher independent risk of MOF, and over a 2-fold higher risk of ARDS and ACS.
Conclusion
In patients requiring massive transfusion, crystalloid resuscitation in a ratio greater than 1.5:1 per unit of PRBCs transfused was independently associated with a higher risk of MOF, ARDS, and ACS. These results suggest overly aggressive crystalloid resuscitation should be minimized in these severely injured patients. Further research is required to determine if incorporation of the C:PRBC ratio into massive transfusion protocols improves outcome.
Introduction
An expanding body of recent literature has focused on the role of hemostatic resuscitation as part of massive transfusion to avoid the coagulopathy and associated morbidity seen after large volume blood transfusion.1–5 Massive transfusion (MT) protocols have emphasized the importance of ratio-based transfusion of hemostatic blood components relative to packed red blood cells (PRBCs), and multiple recent studies have sought to identify the ideal ratios of fresh frozen plasma (FFP), platelets, and cryoprecipitate that should be used as part of the massive transfusion resuscitation strategy.6–11 In addition to these resuscitation strategies, the use of crystalloid remains common practice in patients experiencing significant hemorrhage.12 However, despite the abundance of literature focusing on the outcomes of FFP and platelet use as part of massive transfusion protocols, the use of crystalloid in massive transfusion remains largely unexplored.
Seminal work by Shires, Moore, and colleagues established crystalloid as an initial approach to fluid resuscitation in hemorrhagic shock in the 1950s and 1960s.13,14 The use of balanced salt solution then became and now remains a critically important tool in the approach to the bleeding patient. However, large volume crystalloid resuscitation has been shown to be associated with cardiac, pulmonary and coagulopathic complications that occur secondary to the cellular and metabolic disturbances associated with crystalloid use following hemorrhage.15,16 Although there are significant risks associated with crystalloid use, it remains common, especially in the early resuscitation of the hemorrhaging trauma patient. Despite its near ubiquitous use, the role of crystalloid in massive transfusion protocols and the outcomes of massive transfusion patients receiving crystalloid infusion have not been thoroughly characterized. We hypothesized that an increased crystalloid:PRBC (C:PRBC) ratio would be associated with increased morbidity and poor outcome following massive transfusion.
Methods
Data were derived from the ongoing multi-center prospective cohort study known as the Inflammation and the Host Response to Injury Large Scale Collaborative Program, (www.gluegrant.org) supported by the National Institute of General Medical Sciences (NIGMS), which is designed to characterize the genomic and proteomic response in injured patients at risk for multiple organ failure following traumatic injury and hemorrhagic shock.17 Standard operating procedures were developed and implemented across all institutional centers to minimize variation in post-injury care, including: early goal directed resuscitation, strict glycemic control, venous thrombo-embolism prophylaxis, appropriate low tidal volume ventilation, ventilator associated pneumonia management, and restrictive transfusion guidelines.17–22 Patients admitted to one of seven institutions, over a 6 year period (11/03-10/08), were included in the analysis. Inclusion criteria for the overall cohort study included: blunt mechanism of injury, presence of pre-hospital or emergency department systolic hypotension (< 90 mmHg) or an elevated base deficit (≥ 6 meq/L), blood transfusion requirement within the first 12hrs, and any body region exclusive of the brain with an abbreviated injury score (AIS) ≥ 2, allowing exclusion of patients with isolated traumatic brain injury. Patients < 16 or > 90 years of age and those with cervical spinal cord injury were also excluded from enrolment. Clinical data were entered and stored in TrialDb, a web-based data collection platform, by trained research nurses.23 Integrity of the data was maintained through ongoing curation and external data review by an independent chart abstractor.
For the current secondary data analysis, only patients who required massive transfusion, defined as ≥ 10 units of packed red blood cells in the initial 24hrs post injury, and those who survived beyond 24hrs post-injury were included in the analysis. Exclusion of early mortality in the study cohort allowed the analysis to focus on the risks of morbidity attributable to the C:PRBC ratio beyond the 24hr transfusion and resuscitation period. The primary outcomes of interest were in-hospital mortality (beyond 24hrs), nosocomial infection (NI), multiple organ failure (MOF), acute respiratory distress syndrome (ARDS), and abdominal compartment syndrome (ACS).
While patients were admitted to the ICU, multiple organ dysfunction scores for renal, hepatic, cardiovascular, metabolic, hematologic, respiratory, and neurological systems were determined daily.24–26 All nosocomial infectious complications were monitored for and recorded (infection type, culture specimen source, and bacteriology). The diagnosis of MOF required a maximum Marshall Multiple Organ Dysfunction score > 5, while diagnosis of NI required specific clinical criteria along with positive culture evidence. Diagnosis of a ventilator associated pneumonia required a quantitative culture threshold of ≥ 104 CFU/ml for broncho-alveolar lavage specimens.19 Diagnosis of catheter-related blood stream infections required positive peripheral cultures with the identical organism obtained from either a positive semi-quantitative culture (>15 CFU/segment), or positive quantitative culture (>103 CFU/segment) from a catheter segment specimen. Urinary tract infections required > 105 organisms/ml of urine. ARDS was defined as a Pao2/Fio2 ratio less than 200, bilateral infiltrates on chest x-ray, a measured pulmonary artery occlusion pressure < 18mmHg or the absence of clinical evidence for elevated left atrial pressure.27 ACS diagnosis required opening of the abdominal cavity for intra-abdominal pressures > 25cm H2O with at least one of the following: oliguria (<30cc/hr), diminished cardiac output (<2.5L/min/m2), elevated static airway pressures (>45cm H2O) or a Pao2/Fio2 ratio less than 200.28
Resuscitation and transfusion requirements for crystalloid (per liter) and PRBCs (per unit) in the initial 24 hours following injury were used to determine a C:PRBC ratio for each massive transfusion patient (n= 452). To assess the uniformity of the study cohort, the C:PRBC ratio was divided at its median (50th percentile) and demographics and injury characteristics of patients in the LOW C:PRBC (n=227) and HIGH C:PRBC (n=225) groups were compared in a univariate fashion. To better assess differences in overall resuscitation requirements across the entire cohort, the C:PRBC variable was then split by quartile cut-points (25th%, 50th%, 75th%) into four groups and transfusion and resuscitation requirements were compared using analysis of variance (ANOVA). Multivariate logistic regression modeling was then utilized to determine the independent risks associated with the C:PRBC ratio for the primary outcomes of interest (in-house mortality, NI, MOF, ARDS and ACS) after controlling for important confounders. The C:PRBC quartile cut-points (25th%, 50th%, 75th%) were then again used for the regression analysis. These quartile groups corresponded to 4 different ratio groups. (C:PRBC ratio <0.5:1, n=114; C:PRBC ratio ≥ 0.5:1 and < 1:1, n=113; C:PRBC ratio ≥ 1:1 and < 1.5:1, n=111; and a C:PRBC ratio ≥ 1.5:1, n=114) The three highest C:PRBC ratio groups were placed into the logistic regression model (analyzed relative to the lowest C:PRBC ratio <0.5:1) to determine if any dose response relationship existed.
As changes in resuscitation practice have occurred over time we additionally performed a stratified regression analysis after the study cohort was divided into EARLY (years 2003–2005) time period and LATE ( years 2006–2008) time period groups to determine if changes in resuscitative practice had an effect on the independent risks associated with the C:PRBC ratio. Finally, to determine whether the outcome risks associated with the C:PRBC ratio interacted with or were affected by the proportion of fresh frozen plasma to PRBC (FFP:PRBC ratio) each massively transfused patient received, we also performed a stratified regression analysis across LOW FFP:PRBC ratio and HIGH FFP:PRBC ratio patients (median split, 50th%, corresponding to FFP:PRBC ratio ≤ 1:2 vs. > 1:2, respectively).
Confounders for the multivariate models were chosen to adjust for differences in injury characteristics, shock severity, operative and ICU interventions, transfusion and resuscitation requirements and presence of comorbidities. Model confounders in addition to the C:PRBC variable for the final regression model included: patient age, gender, Injury Severity Score (ISS), presenting Glasgow Coma Score (GCS), initial 24-hour per unit transfusion and resuscitation (per liter) requirements (blood, FFP, platelet, and colloid), initial base deficit, the requirement of early operative intervention (exploratory laparotomy or thoracotomy/sternotomy), early hyperglycemia (glucose >200mg/dl in first 24hrs), and the presence of pre-existing comorbidities (prior MI, hypertension, CHF, COPD, liver disease, renal disease, alcoholism).
All data were summarized as mean ± SD, median [IQR, inter-quartile range], or percentage (%). Student-t or Mann-Whitney statistical tests were used to compare continuous variables, while Chi-Square or Fischer’s Exact test were used for categorical variables. The Institutional Review Board of each participating center approved the cohort study, while the Institutional Review Board at the University of Pittsburgh Medical Center approved this current secondary data analysis.
Results
Of the 1,710 blunt injured patients enrolled during the study period, 452 patients required ≥ 10u PRBCs in the first 24hrs post-injury and survived beyond this time period and constituted the study population. Median crystalloid transfusion for the cohort over the initial 24 hours was over 17 liters while median blood transfusion was just under 16 units (Table 1). The overall inhospital mortality for the study population was 22.6%, while the rates of MOF, NI, ARDS and ACS were 63.5%, 56.2%, 36.3% and 15.1% respectively. This cohort of massively transfused patients had a mean age of 41±17 years and, as expected, were significantly injured with a median ISS of 34 [IQR 25,43].
Table 1.
Overall massive transfusion cohort resuscitation and transfusion requirements
| Resuscitation Component | Median [IQR] |
|---|---|
| Crystalloid (Liter) | 17.2 [12,24] |
| Blood (unit) | 16.0 [11,24] |
| FFP (unit) | 8.4 [4,13] |
| Platelet (unit) | 1.6 [0.6,2.8] |
| Colloid (Liter) | 0.0 [0.0,0.5] |
Those patients with a HIGH C:PRBC ratio as compared to those with a LOW C:PRBC ratio were similar across patient demographics, injury characteristic and severity, and shock parameters (Table 2). Importantly, there were no differences in early central venous pressure (CVP) measurements or the requirement of vasopressors or stress dose steroids across the entire study cohort. Patients in the LOW C:PRBC group were, however, more likely to receive colloid resuscitation and require early operative intervention while those in the HIGH C:PRBC more commonly had pre-existing renal disease. When the C:PRBC was divided into quartiles, resuscitation and transfusion comparison revealed that as the C:PRBC ratio increased, the volume of crystalloid resuscitation received also increased (Table 3). Importantly, as the C:PRBC ratio increased, the volume of blood component transfusion received significantly decreased. Those patients who were in the highest C:PRBC ratio group received significantly less blood, FFP, platelets and colloid (hextend®/Hespan® (hetastarch), or albumin), relative to the lower C:PRBC ratio groups.
Table 2.
Baseline demographics and characteristics for patients with a HIGH C:PRBC ratio versus a LOW C:PRBC ratio.
| HIGH C:PRBC (n=225) | LOW C:PRBC (n=227) | p- value | |
|---|---|---|---|
| Age (years) | 43.6±19 | 41.7±17 | 0.261 |
| Gender (%male) | 72.5% | 67.3% | 0.225 |
| Mechanism of Injury (%) | |||
| MVC | 70.7% | 68.3% | 0.847 |
| Ped Struck | 14.9% | 15.4% | |
| Fall | 7.8% | 9.0% | |
| Other | 6.6% | 7.3% | |
| ED GCS | 7.8±5 | 7.7±5 | 0.936 |
| ISS | 34 [24,43] | 34[27,43] | 0.398 |
| Initial Base Deficit (meq/L) | 9.72±5 | 10.6±6 | 0.132 |
| 6 Hour Lowest pH | 7.16±0.1 | 7.15±0.1 | 0.320 |
| 6 Hour Lowest Central Venous Pressure (CVP) | 11.4±7 | 11.1±6 | 0.692 |
| Presenting INR | 1.67±1 | 1.69±1 | 0.875 |
| Received Colloid Resuscitation (%) | 22.5% | 43.1% | <0.001* |
| 24 Hour Vasopressor Requirement | 33.3% | 36.3% | 0.512 |
| 24hr Stress Dose Steroids | 11.7% | 12.4% | 0.826 |
| Early (24hr) Operative Intervention (exlap/thoracotomy/sternotomy) | 57.2% | 68.6% | 0.013* |
| History of MI | 3.6% | 2.2% | 0.380 |
| History of CHF | 0.9% | 1.8% | 0.424 |
| History of Hypertension | 16.2% | 11.9% | 0.194 |
| History of Diabetes | 10.4% | 6.2% | 0.109 |
| History of COPD | 3.2% | 3.1% | 0.973 |
| History of Liver Disease | 5.4% | 6.2% | 0.721 |
| History of Renal Disease | 1.8% | 0.0% | 0.043* |
| History of Alcohol Abuse | 14.4% | 11.5% | 0.359 |
MVC=motor vehicle collision, ISS=injury severity score, ED=emergency department, GCS = Glasgow coma scale, ICU = intensive care unit, MI=myocardial infarction, CHF=congestive heart failure, COPD, chronic obstructive pulmonary disease.
Table 3.
Transfusion and resuscitation requirements compared across C:PRBC quartile groups for the massively transfused cohort. (*significant by Kruskall-Wallace test)
| 1–25th% C:PRBC (n=114) | 26–50th% C:PRBC (n=113) | 51–75th% C:PRBC (n=111) | 76–100th% C:PRBC (n=114 ) | p-value | |
|---|---|---|---|---|---|
| Crystalloid Resuscitation (liter) | 11.0 [8,17] | 15.6 [11,22] | 16.3 [14,21] | 24.6[21,31] | <0.001* |
| Blood Transfusion (unit) | 28.0 [16,38] | 19.2 [14,26] | 13.3 [11,18] | 12.8 [11,15] | <0.001* |
| FFP Transfusion (unit) | 9.6 [5,16] | 9.6 [4,16] | 6.5 [3,11] | 7.5 [4,11] | <0.001* |
| Platelet Transfusion (unit) | 2.0[1,4] | 1.9 [0.7,3] | 1.5 [0.6,2] | 0.8 [0,2] | <0.001* |
| Colloid Resuscitation (liter) | 0.0 [0,2] | 0.0 [0,1] | 0.0 [0,0.5] | 0.0 [0,0] | <0.001* |
Colloid= Hextend®/Hespan® (Hetastarch), or Albumin
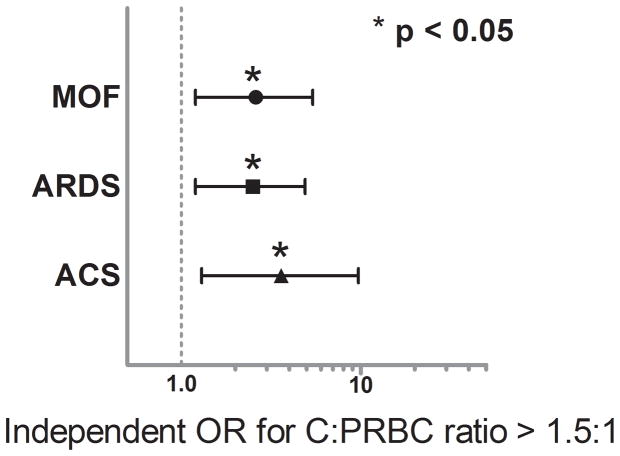
Multivariate logistic regression revealed no significant association for the C:PRBC ratio with in-hospital mortality or the development of NI. (Mortality OR = 0.9, 95%CI 0.58–1.45, p = 0.716 and NI OR = 1.3, 95%CI 0.68–2.5, p = 0.408), however, after controlling for differences in age, gender, GCS, injury and shock severity, transfusion and resuscitation requirements, operative interventions and comorbidities, the C:PRBC ratio was significantly associated with an independent higher risk of MOF (OR = 1.7, 95% CI 1.2–2.6, p = 0.008), ARDS (OR = 2.2, 95% CI 1.5–3.1, p < 0.001) and ACS (OR = 2.3, 95% CI 1.4–3.8, p = 0.001). When a dose response relationship was evaluated using the C:PRBC quartile cut-points, regression analysis revealed that a C:PRBC ratio > than 1.5:1 was associated with over a 2-fold higher independent risk of MOF (OR 2.6, 95%CI 1.2–5.4, p = 0.011) and ARDS (OR 2.5, 95%CI 1.2–4.9, p = 0.010) and over a 3-fold higher independent risk of ACS (OR 3.6, 95%CI 1.3–9.7, p = 0.009) (Figure 1).
Figure 1.
Independent odds ratios (ORs) associated with Infusion of crystalloid in a ratio >1.5:1 relative to PRBCs and the development of MOF, ARDS, and ACS.
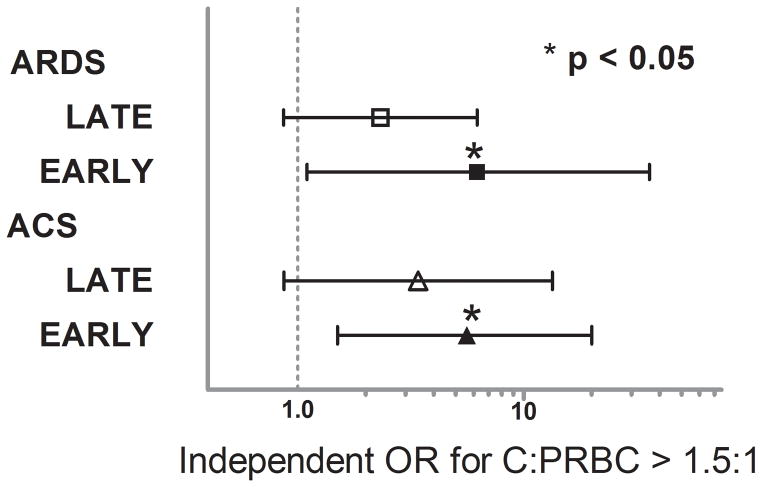
When median crystalloid resuscitation was compared across EARLY (years 2003–2005) and LATE (years 2006–2008) patient accrual periods, a significant decrease in crystalloid administration was found (EARLY 19 [14,27] vs. LATE 15 [10,21], p< 0.001). A concurrent increase in injury severity (median ISS) in the LATE period was found as crystalloid use decreased (EARLY 29 [22,41] vs. LATE 34 [24,41], p=0.006). To further characterize and incorporate any effect these crystalloid changes would have on the outcome risks associated with the C:PRBC ratio, a stratified analysis was performed with the regression models for the two time periods. The strength of the association between the C:PRBC and MOF was not altered when stratified by EARLY and LATE time periods, however, the independent risks associated ARDS and ACS were remained significant only in the EARLY time period, when crystalloid use was highest, and demonstrated a much higher, exaggerated, increased risk. The stratified regression analysis revealed that in the EARLY time period, a C:PRBC ratio > 1.5:1 was independently associated with the over a 5-fold higher independent risk of ARDS (OR 5.6, 95%CI 1.5–20, p = 0.009) and over a 6-fold higher independent risk of ACS (OR 6.2, 95%CI 1.1–36, p = 0.044) (Figure 2) despite a lower injury severity for this time period.
Figure 2.
Independent odds ratios (ORs) associated with Infusion of crystalloid in a ratio >1.5:1 relative to PRBCs and the development of ARDS and ACS stratified by EARLY and LATE time periods.
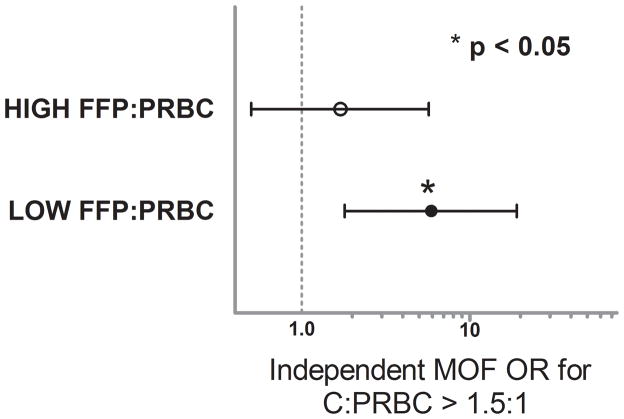
Changes in massive transfusion practice have occurred based upon evidence for a beneficial effect of aggressively treating the early coagulopathy which complicates severe injury with ratio based blood component transfusion, specifically, elevated FFP:PRBC transfusion ratios.7,10,29,30 To determine if the proportion of FFP:PRBC given in a massively transfused patient has an effect on the risks associated with the C:PRBC, we first tested for interaction between FFP:PRBC and C:PRBC ratios in our regression models. The interaction variable was not significant for ARDS and ACS (p=0.609 and p=0.893, respectively) indicating that the independent risks associated with a C:PRBC ratio > 1.5:1 for the development of ARDS and ACS were not altered by a patient’s FFP:PRBC ratio which they received in the first 24hrs post-injury. Interestingly, the interaction variable in the MOF model was highly significant (p= 0.002), indicating that the independent risks associated with a C:PRBC ratio >1.5:1 for the development of MOF was affected by the proportion of FFP:PRBC a patient received. To characterize this interaction we again performed a stratified regression analysis after dividing the cohort into HIGH FFP:PRBC ratio (> 1:2 FFP:PRBC) and LOW FFP:PRBC ratio (≤ 1:2 FFP:PRBC) groups (median cut-point, 50th%). This revealed that the independent risk of MOF associated with a C:PRBC ratio > 1.5:1 was strongest, significant and most pertinent to patients who received a LOW FFP:PRBC ratio (OR 5.9, 95%CI 1.8–19, p =0.003) while there was no significant association between a C:PRBC ratio > 1.5:1 and the development of MOF in patients with a HIGH FFP:PRBC ratio (OR 1.7, 95%CI 0.5–5.7, p = 0.308) (Figure 3). Due to the importance of any interaction between the risks attributable to a elevated C:PRBC ratio and the FFP:PRBC ratio a patient received, and these stratified results, we went back and included the FFP:PRBC ratio into the primary logistic regression models for MOF, ARDS and ACS. Despite the results of the stratified analysis, the inclusion of the FFP:PRBC to each regression model had no effect on the magnitude, direction or significance of the risks attributable to a C:PRBC ratio > 1.5:1.
Figure 3.
Independent odds ratios (ORs) associated with Infusion of crystalloid in a ratio >1.5:1 relative to PRBCs and the development of MOF stratified HIGH and LOW FFP:PRBC ratios.
Discussion
Massive transfusion is commonly required when resuscitating the trauma patient with significant hemorrhage, and recent data have suggested that optimizing ratios of FFP and platelets (hemostatic resuscitation) as part of massive transfusion protocols may improve outcomes.1,9,11 Using the same dataset as the present analysis, we have found that early use of a high FFP:PRBC ratio results in an early survival benefit.1 Despite the evolving interests into these hemostatic resuscitation agents, no research to date has specifically investigated the role of one of the most common resuscitation tools, crystalloid, in massive transfusion. Crystalloid use has been independently associated with an increase in multiple morbidities following trauma, largely due to perturbations in coagulation and an increased incidence of abdominal compartment syndrome and post-operative pulmonary edema.31–33 However, unlike FFP and platelets, no specific guidelines for the use of crystalloid as part of massive transfusion protocols exist, and the independent risk of crystalloid use in these patients has never been investigated.
The results of the current analysis suggest that high ratios of crystalloid use relative to PRBCs (>1.5:1 crystalloid to PRBC) are associated with a dramatic increase in the risk of multiple organ failure, abdominal compartment syndrome, and ARDS. In this critically ill patient population, the use of high volumes of crystalloid was common as part of massive transfusion, with a median of 17.2 L of crystalloid used, with over 50% of the patients receiving greater than a 1:1 (liter/unit) ratio of crystalloid to PRBCs. Interestingly, the odds ratios for the development of each morbidity increased as the ratio of crystalloid to PRBCs increased in the dose response regression analysis, suggesting that higher volume crystalloid use leads to worse outcomes. These findings held true in a logistic regression model which controlled for the use of blood, FFP and platelets and colloid, thus identifying an independent risk for the crystalloid:PRBC ratio in the development of major morbidity following massive transfusion. An important additional observation regarding the dose response analysis is that the patients in the highest quartile (>1.5:1) actually received not only more crystalloid but also significantly less blood transfusion products. Multiple studies have linked blood product transfusion to major morbidities such as ARDS, however, in this cohort, the patients who had the highest risk for the development of severe morbidity actually received the least total blood and blood component transfusion. These findings suggest that patients who receive aggressive crystalloid resuscitation despite lower overall blood component transfusion have significantly higher independent risks of poor outcome. Interestingly, early central venous pressure, shock parameters (pH, base deficit) and the requirement for early vasopressors were similar across the entire study cohort, suggesting that end points for resuscitation across different C:PRBC ratios were similar.
Because of the widespread trend towards increased use of hemostatic resuscitation, we sought to address whether crystalloid use changed over time. After identifying the mid-point of patient accrual in the database, we found that the latter half of patients enrolled in the ‘Glue Grant’ received significantly less crystalloid than those at early time points, which fits the trend towards increased hemostatic resuscitation and minimizing crystalloid.12 The patients in this later group also had a higher overall injury severity as measured by ISS. When the ratio of crystalloid:PRBC was stratified over time, there was no statistically significant increase in MOF, ACS, or ARDS during the lower volume crystalloid period (LATE time period) despite the fact that the patients had an overall higher ISS. Meanwhile, the odds ratios for the development of ACS and ARDS were dramatically increased in the EARLY time period. The risks of morbidity associated with higher ratios of crystalloid were most pertinent to and robust in the time period when crystalloid use was highest, further suggesting a causal relationship.
Although our initial regression analyses controlled for the total amount of FFP received, the focus of recent literature in massive transfusion has been on the ratio of FFP to PRBCs. Thus, we tested for interaction between FFP:PRBC and C:PRBC ratios in our subsequent regression models. Because of the significant interaction between the C:PRBC and FFP:PRBC ratios for MOF, we subdivided the cohort into HIGH FFP:PRBC and LOW FFP:PRBC ratios (FFP:PRBC >1:2 vs. ≤ 1:2) values relative to the median and found that the cohort of patients receiving HIGH C:PRBC as well as receiving LOW FFP:PRBC had a dramatic increase in the odds ratio for the development of MOF. There was no increase in those patients who received HIGH FFP:PRBC. These results suggests that high volume crystalloid and low volume FFP resuscitation may create a worst case scenario and identify a patient cohort and resuscitation strategy at particularly elevated risk for MOF. It may be that in the absence of aggressive treatment of the early coagulopathy post-injury, the risk of crystalloids promoting an excessive inflammatory response are accentuated. Similarly, obviating FFP resuscitation with crystalloid resuscitation may overwhelm the already primed innate immune response and results in higher organ failure and poor outcome.
Our study does have several potential limitations. First, this analysis is a secondary analysis of a prospective cohort study looking at the genomic and proteomic response following severe injury and hemorrhagic shock. As with any secondary analysis, data were not recorded to answer our specific hypothesis stated for this study. Important variables including daily weights, overall fluid in and out, and the timing of crystalloid volumes which were given in the initial 24 hours were limitations of the dataset. Similarly, these patients were blunt injured patients who presented in hemorrhagic shock and ultimately received MT. The results and conclusions may not apply to other MT cohorts. Importantly, these results are not specific for those patients who do not require > 10 units of PRBCs in the initial 24 hours post-injury. Our ability to predict those patients who ultimately require MT is somewhat limited a priori, however, an expanding pool of literature suggests that patients who ultimately require massive transfusion can be determined or predicted early in the initial resuscitation.34,35 Potential unknown or unmeasured confounding variables may be responsible for the associations described and the conclusions formulated. Factor VIIa use was not originally a data point recorded for the overall cohort analysis. This is an important variable to consider as part of massive transfusion resuscitation, however, it was not prospectively recorded throughout the enrolment of patient in the Glue Grant. One important observed difference in the univariate comparison between patients who received HIGH versus LOW C:PRBC ratios (median split, 50th%) was that patients in the LOW C:PRBC ratio cohort were more likely to undergo early surgical intervention. Despite controlling for early operative interventions in the regression modeling, it may be that the transfusion and resuscitation characteristics that occur in the OR may be quite different than in the ICU following severe injury. Despite otherwise similar injury characteristics across the entire cohort and adjustment for differences which were apparent using multivariate regression analysis, patients who received higher crystalloid ratios soon after injury may be an inherently different population relative to those who received low crystalloid ratios, as they were not randomized.
Finally, a frequent concern in similar analyses assessing outcomes after resuscitation in such a critically ill cohort is the “survivor bias.” This potential bias is thought to result from that fact that patients who receive more crystalloid may be those patients who survive the longest. We attempted to minimize this potential by excluding early mortality. Although exclusion of mortality allowed us to focus on the risks of morbidity associated with the C:PRBC ratio, we recognize that this can confound the analysis by eliminating a significant percentage of patients. For this reason, we separately analyzed the data for all outcomes previously mentioned and with no exclusion criteria in the secondary analysis. Inclusion of early mortality had no significant impact on the reported results, with the risk of development of MOF, ARDS, and ACS remaining significantly elevated in the most elevated C:PRBC group (C:PRBC > 1.5:1). Despite these attempts, the C:PRBC ratio may have varied in specific patients over the first 24hr period and represents a significant limitation of the analysis.
In summary, this study raises significant concern regarding the morbidity associated with high volume crystalloid use relative to packed red blood cells in massive transfusion and suggests that overly aggressive crystalloid resuscitation should be minimized in these severely injured patients. These findings were most robust and pertinent to patients in a period when overall crystalloid use was highest and in those who received a low FFP:PRBC ratio (≤ 1:2). Further, higher level research is required to verify these findings and determine if incorporation of the C:PRBC ratio into massive transfusion protocols improves outcome and reduces this attributable morbidity.
Acknowledgments
Funding/Support: NIH NIGMS U54 GM062119-1 and NIH NIGMS K23GM093032-1.
Footnotes
This paper was presented as an oral presentation at the annual meeting of the Eastern Association for the Surgery of Trauma in Naples, Florida, Feb 28-Mar 6, 2010.
Bibliography
- 1.Sperry JL, Ochoa JB, Gunn SR, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008 Nov;65(5):986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 2.Alam HB, Bice LM, Butt MU, et al. Testing of blood products in a polytrauma model: results of a multi-institutional randomized preclinical trial. J Trauma. 2009 Oct;67(4):856–864. doi: 10.1097/TA.0b013e3181b5ae75. [DOI] [PubMed] [Google Scholar]
- 3.Duchesne JC, Islam TM, Stuke L, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma. 2009 Jul;67(1):33–37. doi: 10.1097/TA.0b013e31819adb8e. discussion 37–39. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB. Damage control resuscitation. J Trauma. 2007 Jun;62(6 Suppl):S36–37. doi: 10.1097/TA.0b013e3180654134. [DOI] [PubMed] [Google Scholar]
- 5.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003 Jul;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 6.Dente CJ, Shaz BH, Nicholas JM, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009 Jun;66(6):1616–1624. doi: 10.1097/TA.0b013e3181a59ad5. [DOI] [PubMed] [Google Scholar]
- 7.Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009 May;197(5):565–570. doi: 10.1016/j.amjsurg.2008.12.014. discussion 570. [DOI] [PubMed] [Google Scholar]
- 8.Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009 Jan;66(1):41–48. doi: 10.1097/TA.0b013e31819313bb. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Lustenberger T, Rhee P, et al. The impact of platelet transfusion in massively transfused trauma patients. J Am Coll Surg. Nov;211(5):573–579. doi: 10.1016/j.jamcollsurg.2010.06.392. [DOI] [PubMed] [Google Scholar]
- 10.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007 Oct;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 11.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008 Aug;65(2):261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 270–261. [DOI] [PubMed] [Google Scholar]
- 12.Duchesne JC, Kimonis K, Marr AB, et al. Damage control resuscitation in combination with damage control laparotomy: a survival advantage. J Trauma. Jul;69(1):46–52. doi: 10.1097/TA.0b013e3181df91fa. [DOI] [PubMed] [Google Scholar]
- 13.Carrico CJ, Canizaro PC, Shires GT. Fluid resuscitation following injury: rationale for the use of balanced salt solutions. Crit Care Med. 1976 Mar-Apr;4(2):46–54. [PubMed] [Google Scholar]
- 14.Moore FD, Shires G. Moderation. Ann Surg. 1967 Aug;166(2):300–301. doi: 10.1097/00000658-196708000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotton BA, Guy JS, Morris JA, Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006 Aug;26(2):115–121. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 16.Schmand JF, Ayala A, Morrison MH, Chaudry IH. Effects of hydroxyethyl starch after trauma-hemorrhagic shock: restoration of macrophage integrity and prevention of increased circulating interleukin-6 levels. Crit Care Med. 1995 May;23(5):806–814. doi: 10.1097/00003246-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Maier RV, Bankey P, McKinley B, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: Patient-Oriented Research Core--standard operating procedures for clinical care. Foreward. J Trauma. 2005 Sep;59(3):762–763. [PubMed] [Google Scholar]
- 18.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006 Jul;61(1):82–89. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 19.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core--standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006 May;60(5):1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 20.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: Patient-Oriented Research Core--standard operating procedures for clinical care. I. Guidelines for mechanical ventilation of the trauma patient. J Trauma. 2005 Sep;59(3):764–769. [PubMed] [Google Scholar]
- 21.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the host response to injury, a large-scale collaborative project: Patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma. 2006 Aug;61(2):436–439. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 22.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care: VI. Blood glucose control in the critically ill trauma patient. J Trauma. 2007 Sep;63(3):703–708. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 23.Brandt CA, Deshpande AM, Lu C, et al. TrialDB: A web-based Clinical Study Data Management System. AMIA Annu Symp Proc. 2003:794. [PMC free article] [PubMed] [Google Scholar]
- 24.Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986 Feb;121(2):196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 25.Marshall JC. Organ dysfunction as an outcome measure in clinical trials. Eur J Surg Suppl. 1999;(584):62–67. doi: 10.1080/11024159950188583. [DOI] [PubMed] [Google Scholar]
- 26.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995 Oct;23(10):1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 28.Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006 Nov;32(11):1722–1732. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007 Jan;62(1):112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 30.Duchesne JC, Holcomb JB. Damage control resuscitation: addressing trauma-induced coagulopathy. Br J Hosp Med (Lond) 2009 Jan;70(1):22–25. doi: 10.12968/hmed.2009.70.1.37690. [DOI] [PubMed] [Google Scholar]
- 31.Balogh Z, McKinley BA, Cocanour CS, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003 Jun;138(6):637–642. doi: 10.1001/archsurg.138.6.637. discussion 642–633. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell RA, Fabian TC, Croce MA, Davis KA. Secondary abdominal compartment syndrome: an underappreciated manifestation of severe hemorrhagic shock. J Trauma. 1999 Dec;47(6):995–999. doi: 10.1097/00005373-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Arieff AI. Fatal postoperative pulmonary edema: pathogenesis and literature review. Chest. 1999 May;115(5):1371–1377. doi: 10.1378/chest.115.5.1371. [DOI] [PubMed] [Google Scholar]
- 34.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? The Journal of trauma. 2009 Feb;66(2):346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 35.Cotton BA, Dossett LA, Haut ER, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. The Journal of trauma. 2010 Jul;69( Suppl 1):S33–39. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]