Abstract
The NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome is a caspase-1-containing cytosolic protein complex that is essential for processing and secretion of IL-1β. The U1-small nuclear ribonucleoprotein (U1-snRNP) which includes U1-small nuclear RNA (U1-snRNA) is a highly conserved intranuclear molecular complex involved in splicing premRNA. Antibodies against this self nuclear molecule are characteristically found in autoimmune diseases like systemic lupus erythematosus (SLE), suggesting a potential role of U1-snRNP in autoimmunity. Although endogenous DNA and microbial nucleic acids are known to activate the inflammasomes, it is unknown whether endogenous RNA-containing U1-snRNP could activate this molecular complex. Here we show that U1-snRNP activates the NLRP3 inflammasome in CD14+ human monocytes dependently of anti-U1-snRNP antibodies, leading to IL-1β production. Reactive oxygen species (ROS) and K+ efflux were responsible for this activation. Knocking down the NLRP3 or inhibiting caspase-1 or TLR 7/8 pathway decreased IL-1β production from monocytes treated with U1-snRNP in the presence of anti-U1-snRNP antibodies. Our findings indicate that endogenous RNA-containing U1-snRNP could be a signal that activates the NLRP3 inflammasome in autoimmune diseases like SLE where anti-U1-snRNP antibodies are present.
Introduction
The innate immune cells detect molecular signals from the invading microorganisms or host cells in danger through specialized receptors, leading to the development of inflammation and cytokine production (1). The cytosolic protein complex inflammasome activates caspase-1 which is involved in processing and secretion of the pro-inflammatory cytokine IL-1β (2). The production of IL-1β requires the activation of two pathways (3): 1) the pattern recognition receptors such as TLRs with increased pro-IL-1β expression through NF-κB activation; and 2) inflammasomes that convert pro-IL-1β to IL-1β. An array of inflammasome activators has been identified, linking it to various pathologic conditions (2). These activators include self-derived uric acid, cholesterol crystals and DNA as well as ones originating from environments and microorganisms like alum, asbestos, silica and viral nucleic acids (4–8). The NLRP3 inflammasome that comprises NLRP3, the adaptor protein ASC and procaspase-1 is the best characterized one (9). Up on detecting triggering molecules, NLRP3 recruits ASC and procaspase-1, which subsequently cleaves pro-IL-1β to IL-1β. Recent studies support the important role for the NLRP3 inflammasome in host defense against pathogens as well as in the pathogenesis of inflammatory diseases such as gout and atherosclerosis (2).
The U1-snRNP is an intranuclear molecular complex involved in splicing pre-mRNA (10). This complex which contains U1-snRNA, Sm and U1-specific proteins is highly conserved from human to insects and the most abundant snRNP in the cell (10, 11). Antibodies to U1-snRNP are found in patients with SLE and mixed connective tissue disease (MCTD), the autoimmune diseases characterized by the systemic inflammation affecting multiple organs including the skin and dysregulated immune responses to self nuclear antigens (10). Of interest, the translocation of U1-snRNP from the nucleus to the cell membrane was found in apoptotic keratinocytes induced by UV light, a known trigger for SLE, suggesting a potential role for this molecule in initiating and/or propagating autoimmunity (12, 13). Indeed, plasmacytoid dendritic cells (pDC) and B cells, which are involved in the lupus pathogenesis, were activated by the RNA sequences within the U1-snRNP or immune complexes containing U1-snRNP through TLR7 and 8 triggering (14–16).
Although endogenous DNA and microbial nucleic acids are known to activate the inflammasomes (2, 6, 17–20), it is unknown whether and how endogenous RNA-containing U1-snRNP could activate this molecular complex. Here we investigated this critical question, focusing on human monocytes, the primary cellular source of IL-1β (21). The results of our study show that U1-snRNP can activate the NLRP3 inflammasome in human CD14+ monocytes dependently of anti-U1-snRNP antibodies, leading to IL-1β production. This phenomenon is driven by the generation of reactive oxygen species (ROS) and K+ efflux as well as dependent on the TLR7 and 8. Our findings indicate that endogenous U1-snRNP could be a signal that activates the NLRP3 inflammasome in autoimmune diseases like SLE where anti-U1-snRNP antibodies are present.
Materials and Methods
Human monocytes and sera
Human peripheral blood was obtained from the N.Y. Blood Center or healthy adult donors with informed consent. Fresh monocytes were purified from peripheral blood using a negative cell purification kit (Stem Cell Technologies, Vancouver, Canada). ANA-positive sera with or without anti-U1-snRNP Abs were obtained from the L2 Diagnostic Laboratory and patients with SLE. Anti-U1-snRNP Abs were measured by ELISA (DiaSorin, Stillwater, MN). Healthy control sera were obtained from peripheral blood of healthy donors. This work was approved by the institutional review committee of Yale University.
Cell stimulation
Purified monocytes (5 × 104) were resuspended in 100 µl of RPMI 1640 media supplemented with 10% FCS, penicillin and streptomycin. Bovine U1-snRNP was purchased from AroTec Diagnostics Limited (New Zealand). Monocytes were stimulated with or without bovine U1-snRNP (5 µg/ml) in the presence or absence of serum (final concentration of 5 %) or total IgG with or without Abs to U1-snRNP. Total IgG purification from sera was done using a NAb spin kit for Ab purification (Thermo scientific, Rockford, IL) according to the manufacturer’s protocol. Some monocytes were treated with zymosan (10 µg/ml). In some experiments, monocytes were additionally pre-treated with ant-CD32 (FcγRII) Abs (2.5 µg/ml, R&D systems, Minneapolis, MN), NF-κB inhibitors (Bay11-7082 (5 µM) and Cenostrol (5 µM), Invivogen, San Diego, CA), caspase-1 inhibitor (5 µM, Enzo Life Sciences International Inc, PA), DPI (50 µM, Sigma-Aldrich) or cathepsin B inhibitor CA-074-Me (20 µM, EMD Chemicals, Gibbstown, NJ). Inhibitory nucleic acid sequence for TLR7 (ODN) was purchased from Invivogen. Inhibitory nucleic acid sequences for TLR8/9 (ORNs) were synthesized at Bioneer, Alameda, CA as previously described (15). Each inhibitory nucleic acid sequence (5 µM final concentration) was pre-incubated for 15 min with DOTAP at room temperature and then added to monocytes.
Depletion of anti-U1-snRNP Abs from autoantibody-positive serum
Anti--U1-snRNP Ab-positive serum diluted with RPMI 1640 media (5% final concentration) was incubated overnight at 4 °C in a sterile ELISA plate coated with 20 µg/ml of U1-snRNP. Serum was collected and analyzed for anti-U1-snRNP Abs by ELISA to determine levels of depletion.
Measuring the ROS synthesis
Monocytes were stimulated for 1 to 4 hours with or without U1-snRNP in the presence or absence of healthy serum, anti-U1-snRNP Ab-positive serum (5% final concentration) or IgG purified from anti-U1-snRNP Ab-positive serum. ROS detection reagent carboxy-H2DCFDA (C400) (Invitrogen) was added at the end of stimulation. Cells were analyzed on a flow cytometer to determine the intracellular levels of ROS.
Measuring NF-κB activation
To determine the NF-κB activation, monocytes were stimulated for 1 to 4 hours with or without U1-snRNP in the presence or absence of healthy serum, anti-U1-snRNP Ab-positive serum (5% final concentration) or IgG purified from the Ab-positive serum. Stimulated cells were fixed, permeabilized and stained with anti-phophorylated NF-κB p65 Abs (pS529) (BD bioscience, San Diego, CA). Cells were analyzed on a flow cytometer
Measuring caspase-1 activation
Monocytes were stimulated for 3 to 10 hours with or without healthy serum, anti-U1-snRNP Ab-positive serum (5% final concentration) or IgG purified from anti-U1-snRNP Ab-positive serum in the presence or absence of U1-snRNP. The caspase-1 activation was measured by flow cytometry using FAM FLICA Caspase-1 assay kit (Immunochemistry Technologies Inc, Bloomington, MN) according to the manufacturer’s instruction.
Knocking down the NLRP3 gene
psiRNA-hNLRP3 plasmid and scrambled plasmid were purchased from Invivogen. Purified human Monocytes were transfected with scrambed siRNA or NLRP3-specific siRNA using the Amaxa transfection system (Lonza, Walkersville, MD). Transfected cells were rested for 6 hours followed by incubation for 18 hours with or without U1-snRNP and anti-U1-snRNP Ab-positive serum. Knock down of the NLRP3 gene was confirmed by qPCR with a primer set: forward primer 5’CCACAAGATCGTGAGAAAACCC3’ and reverse primer 5’CGGTCCTATGTGCTCGTCA3’
ELISA and qPCR
IL-1β in culture supernatants was measured by ELISA (ebioscience, San Diego, CA). Intracellular pro-IL-1β protein was measured using a human pro-IL-1β ELISA kit (R&D systems). Pro-IL1B gene was determined by qPCR with a primer set: forward primer 5’ CACGATGCACCTGTACGATCA3’ and reverse primer 5’GTTGCTCCATATCCTGTCCCT3’. Total RNA was extracted from cells using RNeasy Plus Midi kit (QIAGEN) and cDNA was synthesized. Each real-time PCR reaction was performed in a mix of 10-µl reaction mixture containing 50 ng of cDNA, 2× Brilliant SYBR green master mix (Stratagene), and 3 µM of each primer. The reaction mixture was denatured for 10 min at 94°C and incubated for 40 cycles (denaturing for 15 s at 95°C and annealing and extending for 1 min at 60°C) using Mx3005P QPCR system (Stratagene). ACTINB was used as an endogenous reference. The comparative CT method (ΔΔCT) was used for quantification of gene expression.
Results
U1-snRNP induces IL-1β production from human monocytes in an anti-U1-snRNP antibody-dependent manner
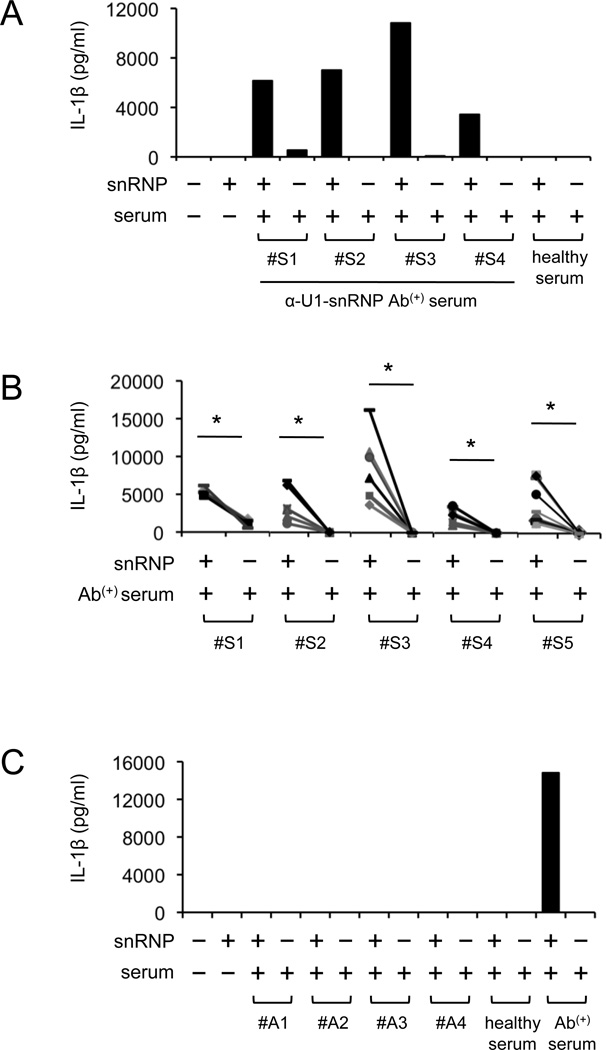
U1-snRNP induced IL-1β production from human monocytes in the presence of anti-U1-snRNP antibody-positive serum although U1-snRNP alone or a combination of U1-snRNP and healthy serum could not induce this cytokine production (Fig. 1A–B, Supplemental Fig. 1). Monocytes treated with only anti-U1-snRNP antibody-positive serum rarely produced IL-1β. In addition, IL-1β production was not detected from monocytes treated with U1-snRNP along with serum positive for antinuclear antibodies (ANA) but negative for U1-snRNP antibodies (Fig. 1C, Supplemental Fig. 1B).
FIGURE 1. U1-snRNP induces IL-1β production from human monocytes in the presence of U1-snRNP antibody-positive serum.
(A–C) IL-1β ELISA at 18 hours from cell culture supernatants of human monocytes incubated in the following conditions. (A–B) Monocytes from a single (A) or multiple donors (B, symbols indicate individual donors) were incubated with or without U1-snRNP (snRNP, 5 µg/ml) in the presence or absence of healthy serum or anti-U1-snRNP antibody-positive (Ab(+)) serum (5% final concentration) from multiple donors (#S1–#S5). (C) ANA-positive sera without anti-U1-snRNP antibodies (donors, #A1–#A4) were added to monocytes from a single donor in the presence or absence of U1-snRNP. Representative data from 2 independent experiments (A and C). *P < 0.05.
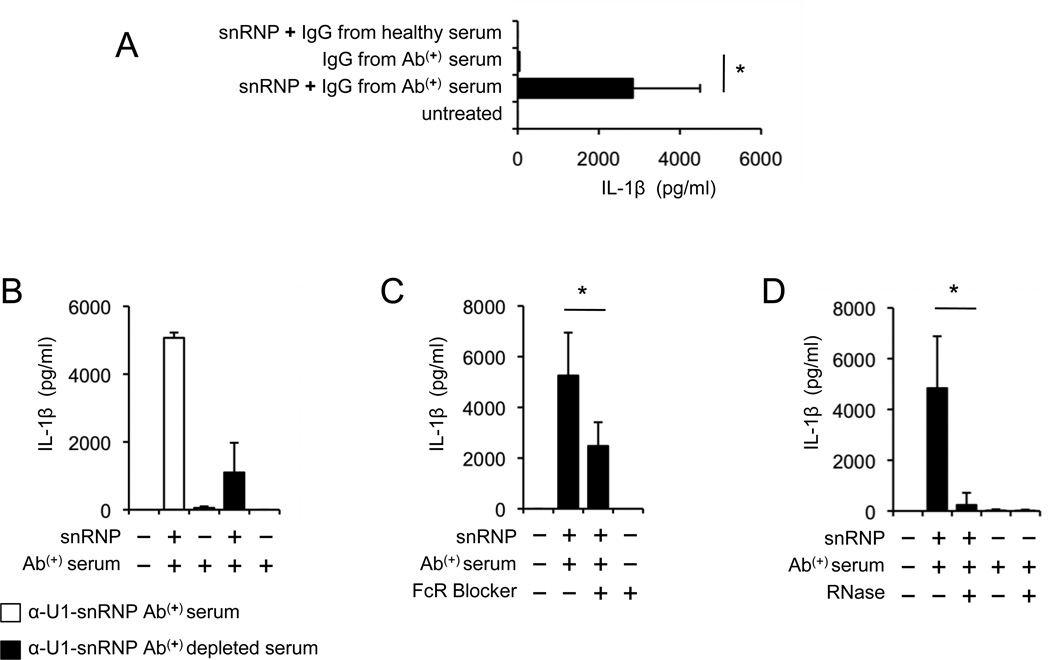
To further delineate the specific role of U-1snRNP antibodies in producing IL-1β, monocytes were incubated with IgG purified from anti-U1-snRNP antibody-positive serum in the presence or absence of U1-snRNP. Indeed, U1-snRNP induced IL-1β production from monocytes together with IgG purified from anti-U1-snRNP antibody-positive serum (Fig. 2A). Although we could only partially deplete anti-U1-snRNP antibodies in antibody-positive serum (data not shown), such depletion still decreased IL-1β production from monocytes treated with U1-snRNP (Fig. 2B). A previous study reported the involvement of the IgG receptor CD32 (FcγRII) in stimulating pDCs by DNA-containing lupus immune complexes (22). In fact, blocking the CD32 on monocytes with anti-CD32 antibodies reduced IL-1β production in response to a combination of U1-snRNP and anti-U1-snRNP antibody-positive serum (Fig. 2C). These findings further support the necessity of U1-snRNP antibodies in producing IL-1β from monocytes in the presence of U1-snRNP. The highly conserved molecule U1-snRNP contains U1-snRNA that can trigger TLR7 and 8 (15). Incubating monocytes with RNase-pretreated U1-snRNP and anti-U1-snRNP-positive serum almost abrogated IL-1β production (Fig. 2D), suggesting the role of snRNA in inducing IL-1β production. Overall, our findings indicate that U1-snRNP can induce IL-1β production from human monocytes in an anti-U1-snRNP antibody-dependent manner.
FIGURE 2. The production of IL-1β from human monocytes in response to U1-snRNP requires anti-U1-snRNP antibodies.
(A–D) IL-1β ELISA at 18 hours from cell culture supernatants of human monocytes incubated in the following conditions. (A) Monocytes were incubated with or without U1-snRNP in the presence or absence of total IgG purified from anti-U1-snRNP antibody-positive (Ab(+)) serum or healthy serum. (B) Monocytes were incubated with or without U1-snRNP in the presence or absence of serum depleted or un-depleted of anti-U1-snRNP antibodies (Bars and error bars indicate mean and SEM, n = 2). (C) Monocytes were treated with anti-CD32 antibodies (FcR blocker) followed by incubation with U1-snRNP and anti-U1-snRNP antibody-positive (Ab(+)) serum. (D) Monocytes were incubated with anti-U1-snRNP antibody-positive (Ab(+)) serum or U1-snRNP and anti-U1-snRNP antibody-positive serum in the presence or absence of RNase. Bars and error bars indicate mean and SEM, respectively (n = 5–7 donors for A, C and D). *P < 0.05.
U1-snRNP induces IL-1β production from human monocytes by activating NF-κB through triggering TLR7 and 8 in the presence of anti-U1-snRNP antibody-positive serum
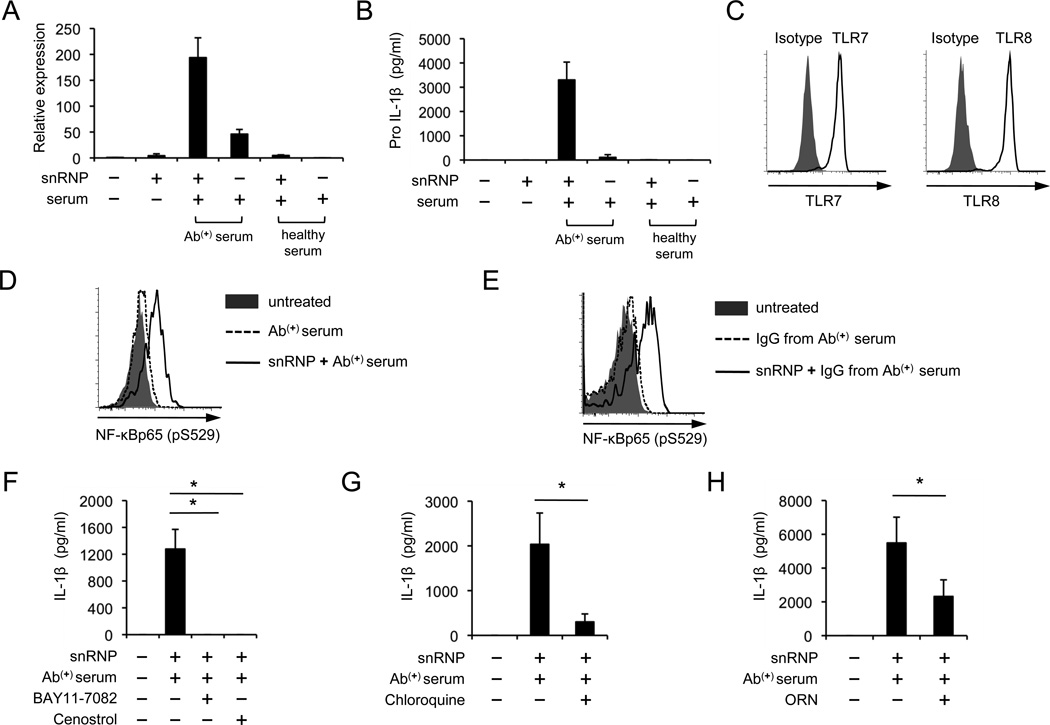
The production of IL-1β requires the activation of two pathways (3): 1) the pattern recognition receptors like TLRs with increased pro-IL-1β expression through NF-κB activation; and 2) inflammasomes that convert pro-IL-1β to IL-1β. Thus, we determined whether U1-snRNP could increase pro-IL-1β gene and protein synthesis in monocytes in the presence of anti-U1-snRNP antibodies. Indeed, pro-IL1β gene and protein were highly expressed in monocytes treated with U1-snRNP along with anti-U1-snRNP antibody-positive serum (Fig. 3A–B). Viral RNAs can trigger TLR7 and 8, leading to NF-κB activation that induces pro-IL-1β synthesis (20). Also, endogenous RNA could stimulate pDCs and B cells by triggering TLR7 and 8 (14, 16, 23, 24). Thus, we explored whether TLR7 and 8 with NF-κB activation are involved in producing IL-1β from monocytes in response to a combination of U1-snRNP and its autoantibodies. Human monocytes that expressed these TLRs had activation of NF-κB upon stimulation with U1-snRNP in the presence of anti-U1-snRNP antibody-positive serum or IgG purified from the autoantibody-positive serum (Fig. 3C–E). However, U1-snRNP alone or in combination with healthy serum hardly induced the activation of NF-κB in monocytes (Supplemental Fig. 2). Furthermore, IL-1β production from these cells was blocked by NF-κB inhibitors (Fig. 3F). TLR7 and 8 recognize their target molecules in the endosome (1). The endosomal inhibitor chloroquine and inhibitory nucleic acid sequences for TLR7 and 8 (14) reduced both pro-IL-1β and IL-1β production by moncytes treated with U1-snRNP and anti-U1-snRNP antibody-positive serum (Fig. 3G–H; Supplemental Fig. 3A–B). Incubating monocytes with RNase-pretreated U1-snRNP and anti-U1-snRNP-positive serum also reduced pro-IL-1β production (Supplemental Fig. 3C). These findings indicate that NF-κB activation by U1-snRNP through TLR7 and 8 triggering in the endosome is essential for IL-1β production from monocytes in the presence of anti-U1-snRNP antibodies.
FIGURE 3. U1-snRNP induces IL-1β production from human monocytes by activating NF-κB through triggering TLR7 and 8 in the presence of anti-U1-snRNP antibody-positive serum.
(A–B) Pro-IL-1β qPCR (A) and ELISA (B) of human monocytes incubated for 6 (qPCR) or 10 (ELISA) hours with or without U1-snRNP (snRNP, 5 µg/ml) in the presence or absence of healthy or anti-U1-snRNP antibody-positive serum (5% final concentration) (Bars and error bars indicate mean and SEM, respectively, n = 2 and 3 donors for A and B). (C) Flow cytometric analysis of TLR7 and 8 expression by CD14+ human monocytes. (D–E) Flow cytometric analysis of NF-κB activation in monocytes treated for 3 hours with anti-U1-snRNP antibody-positive (Ab(+)) serum (D) or IgG purified from anti-U1-snRNP antibody-positive (Ab(+)) serum (E) in the presence or absence of U1-snRNP. (F–H) IL-1β ELISA of culture supernatants from monocytes incubated for 18 hours with U1-snRNP and anti-U1-snRNP antibody-positive (Ab(+)) serum in the presence or absence of the NF-κB inhibitors (F, Bay11-7082 and Cenostrol), chloroquine (G) or inhibitory nucleic acid sequences for TLR7/8 (H, ORN). Representative data from 2–3 independent experiments (C–E). Bars and error bars indicate mean and SEM, respectively (n = 4–8 donors for F–H). *P < 0.05.
The production of IL-1β from human monocytes in response to U1-snRNP and anti-U1-snRNP antibody-positive serum is dependent on caspase-1 activation, ROS synthesis, K+ efflux and NLRP3
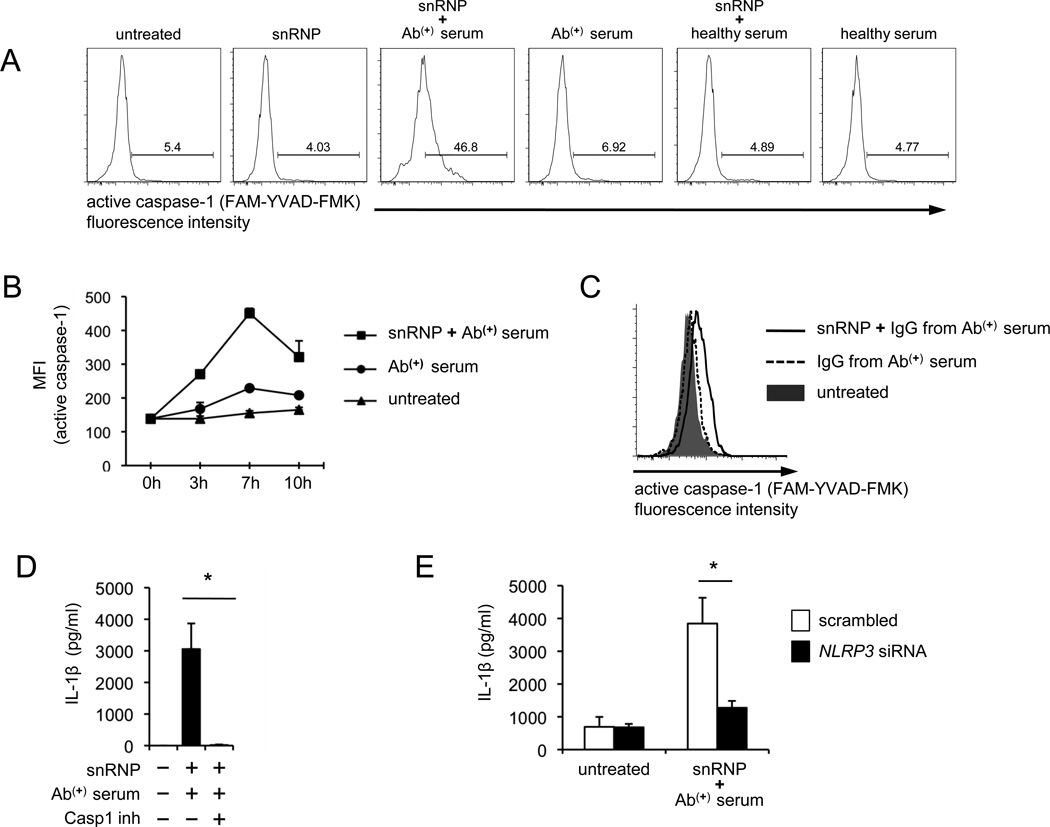
Active caspase-1 cleaves pro-IL-1β into the mature form IL-1β for secretion (3). Thus, we next determined whether U1-snRNP could activate caspase-1 in monocytes. Active caspase-1 was detected at high levels in monocytes treated with U1-snRNP in the presence of anti-U1-snRNP-positive serum (Fig. 4A–B). However, U1-snRNP alone or in combination with healthy serum could not induce the activation of caspase-1. A similar finding was found when monocytes were stimulated with IgG purified from the autoantibody-positive serum (Fig. 4C). The caspase-1 inhibitor suppressed IL-1β production from monocytes treated with a combination of U1-snRNP and anti-U1-snRNP antibody-positive serum (Fig. 4D), indicating the essential role of caspase-1 activation. To determine whether this caspase-1 activation was dependent on the NLRP3 inflammasome, we knocked down NLRP3 gene expression in human monocytes and measured IL-1β production from these cells in response to U1-snRNP and anti-U1-snRNP antibody-positive serum. Indeed, reducing NLRP3 gene expression decreased IL-1β production (Fig. 4E).
FIGURE 4. The production of IL-1β from human monocytes in response to U1-snRNP and anti-U1-snRNP antibody-positive serum requires caspase-1 activation and NLRP3 inflammasome.
(A–C) Flow cytometric analysis of active caspase-1 in human monocytes stimulated for 7 (A) or indicated hours (B) with or without U1-snRNP (5 µg/ml) in the presence or absence of anti-U1-snRNP (snRNP) antibody-positive (Ab(+)) serum (5% final concentration), healthy serum (A–B) or total IgG purified from anti-U1-snRNP antibody-positive (Ab(+)) serum (C). (D) IL-1β ELISA of culture supernatants from monocytes incubated for 18 hours with U1-snRNP and anti-U1-snRNP antibody-positive (Ab(+)) serum in the presence or absence of caspase-1 inhibitor. (E) IL-1β ELISA of culture supernatants from monocytes transfected with scrambled or NLRP3-specific siRNA followed by the incubation for 18 hours with U1-snRNP and anti-U1-snRNP antibody-positive (Ab(+)) serum. Representative data from 3 independent experiments (A, C). Numbers in histograms indicate the frequency of cells stained positive. Symbols and error bars indicate mean + SD (n = 2) (B). Bars and error bars indicate mean and SEM, respectively (n = 4–7 donors for D–E). *P < 0.05.
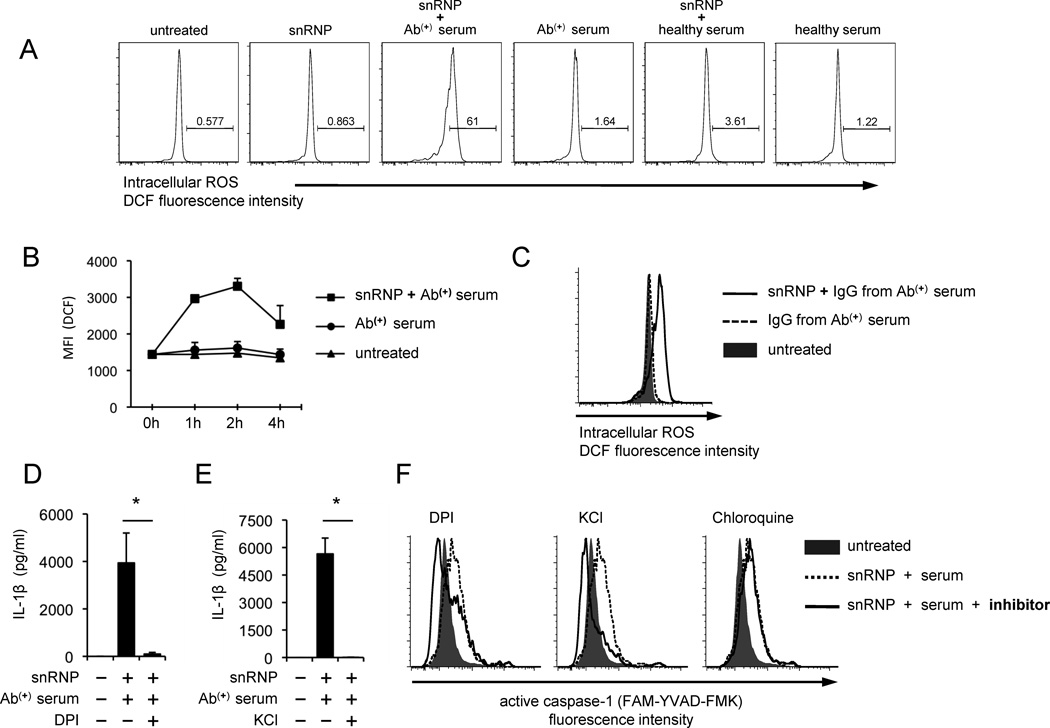
ROS is an essential mediator for the activation of the NLRP3 inflammasome by multiple molecules such as asbestos, silica and cholesterol crystals (9). ROS could induce the separation of thioredoxin interacting protein from thioredoxin, resulting in the NLRP3 inflammasome activation through binding thioredoxin interacting protein to NLRP3 (25). Thus, we measured ROS in monocytes treated with U1-snRNP in the presence of serum or purified IgG containing antibodies to this molecule. The increased generation of ROS was found in such treated monocytes (Fig. 5A–C). However, monocytes treated with U1-snRNP alone or a combination of U1-snRNP and healthy serum could not increase ROS synthesis. Furthermore, blocking the generation of ROS with the NADPH oxidase inhibitor diphenylene iodonium (DPI) decreased IL-1β production (Fig. 5D). In addition to ROS, K+ efflux has been suggested as an activator for the NLRP3 inflammasome (9). Indeed, monocytes stimulated with U1-snRNP and anti-U1-snRNP antibody-positive serum in the presence of exogenous KCl had decreased IL-1β production (Fig. 5E). DPI and exogenous KCl also decreased caspase-1 activation in monocytes treated with U1-snRNP and anti-U1-snRNP antibody-positive serum although the endolysomal inhibitor chroloquine could not suppressed caspase-1 activation (Fig. 5F). The latter finding suggests that the activation of TLR7 and 8 by U1-snRNP is primarily involved in the generation of pro-IL-1β rather than caspase-1 activation. This point is further supported by the suppression of pro-IL-1β production in monocytes treated with chloroquine or inhibitory nucleic acid sequences for TLR7 and 8 (Supplemental Fig. 3A–B). Of interest, the levels of active caspase-1 in monocytes treated with DPI or KCl were lower that those in untreated monocytes. This is likely secondary to the presence of constitutively activated caspase-1 at low levels in unstimulated human monocytes as previously reported (21). Overall, our findings indicate the role for ROS and K+ efflux in activating the NLRP3 inflammasome and inducing IL-β production from human monocytes in response to U1-snRNP in the presence of anti-U1-snRNP antibodies.
FIGURE 5. The production of IL-1β and activation of caspase-1 by human monocytes in response to U1-snRNP and anti-U1-snRNP antibody-positive serum requires ROS synthesis and K+ efflux.
(A–C) Flow cytometric analysis of reactive oxygen species (ROS) in monocytes stimulated for 3 (A) or indicated hours (B) with or without U1-snRNP (5 µg/ml) in the presence or absence of anti-U1-snRNP (snRNP) antibody-positive (Ab(+)) serum (5% final concentration), healthy serum (A–B) or total IgG purified from anti-U1-snRNP antibody-positive (Ab(+)) serum (C). (D–E) IL-1β ELISA of culture supernatants from monocytes incubated for 18 hours with U1-snRNP and anti-U1-snRNP antibody-positive (Ab(+)) serum in the presence or absence of the ROS inhibitor DPI (D) or KCl (E). (F) Flow cytometric analysis of active caspase-1 in monocytes treated for 7 hours with U1-snRNP and anti-U1-snRNP antibody-positive (Ab(+)) serum in the presence or absence of DPI, KCl or chloroquine. Representative data from 3–4 independent experiments (A, C, F). Symbols and error bars indicate mean + SD (n = 2) (B). Bars and error bars indicate mean and SEM, respectively (n = 4–7 donors for D–E). *P < 0.05.
Discussion
The NLRP3 inflammasome is a caspase-1-activating cytosolic protein complex that is essential for processing and secretion of IL-1β. U1-snRNP which contains U1-snRNA is a highly conserved intranuclear molecular complex. Although endogenous DNA and microbial nucleic acids are known to activate the inflammasomes (2, 6, 17–20), it is unknown whether endogenous RNA-containing U1-snRNP could activate this molecular complex. Here we show that U1-snRNP activates the NLRP3 inflammasome in CD14+ human monocytes dependently of anti-U1-snRNP antibodies, leading to IL-1β production. This phenomenon is driven by the generation of ROS and K+ efflux as well as dependent on NLRP3, caspase-1, TLR7 and 8 pathways. Our findings indicate that endogenous RNA-containing U1-snRNP could be a signal that activates the NLRP3 inflammasome in autoimmune diseases like SLE where anti-U1-snRNP antibodies are present.
The activators of the NLRP3 inflammasome are heterogeneous, ranging from self-derived ones to molecules from the environments and pathogens (2). It has been a subject of intense research of how molecules with distinct structures and chemical properties can activate the NLRP3 inflammasome. Several models have been suggested including the ones mediated by ROS synthesis and K+ efflux (9). The diverse types of NLRP3 inflammasome activators such as uric acid, asbestos, silica and extracellular ATP could induce ROS synthesis and/or K+ efflux, leading to the caspase-1 activation and IL-1β secretion (reviewed in (9)). We found that U1-snRNP induced ROS production in monocytes in the presence of anti-U1-snRNP antibodies. Furthermore, the NADPH oxidase inhibitor DPI blocked caspase-1 activation and IL-1β production by such treated monocytes, indicating the involvement of NADPH oxidase in inducing this ROS. In fact, NADPH oxidase was implicated in generating ROS by the monocytic THP1 cells in response to asbestos and monosodium urate crystals (7). In addition to ROS, K+ efflux is likely linked to activating the NLRP3 inflammsome in monocytes in response to U1-snRNP and anti-U1-snRNP antibodies in that adding KCl to tissue culture media blocked caspase-1 activation and IL-1β production by these monocytes. A role of cathepsin B in activating NLRP3 inflammasome has been reported (5, 26, 27). In fact, inhibiting cathepsin B also reduced caspase-1 activation and IL-1β production by monocytes in the presence of U1-snRNP and anti-U1-snRNP antibody-positive serum (Supplemental Fig. 4), suggesting the potential role of this molecule in U1-snRNP-mediated activation of NLRP3 inflammasome.
Autoantibodies to U1-snRNP are found in patients with autoimmune diseases like SLE. The immune complex containing this molecule could activate pDC via the triggering of TLR7 and 8, leading to increased IFN-α production in lupus patients (14, 15). Increased IL-1β gene or protein expression is found in the peripheral blood mononuclear cells (PBMCs) and skin lesions of lupus patients as well as in the kidneys of lupus-prone mice (28–32). Furthermore, IL-1β-deficient mice were resistant to induction of lupus (33), and the recombinant IL-1 receptor antagonist anakinra improved arthritis in lupus patients (34). Although these findings suggest a pathogenic role of IL-1β in lupus and the NLRP3 inflammasome is essential for IL-1β production (21), little is known about the mechanism for increased IL-1β production in lupus. Indeed, the results of our studies provide a possible mechanistic explanation for increased IL-1β production in lupus.
The data from our study demonstrated that the highly conserved U1-snRNP activated the caspase-1 in human monocytes through triggering the NLRP3 inflammasome, leading to IL-1β production. This event that required U1-snRNP and anti-U1-snRNP antibodies raises a question of how U1-snRNP located in the nucleus can be recognized by anti-U1-snRNP antibodies. Of interest, keratinocytes damaged by the UV light, which is a well known precipitating factor for SLE, expressed this molecule on the cell surface in a form of apoptotic bodies, allowing the access of autoantibodies to the U1-snRNP (12, 13). Thus, U1-snRNP could become a danger signal that triggers the activation of the NLRP3 inflammasome in autoimmune diseases like SLE where anti-U1-snRNP antibodies are produced. Such activation with IL-1β production would induce and/or aggravate inflammation with tissue damage although this mechanism may not account for why breaching of self tolerance occurs at the beginning of autoimmunity.
Taken together, we show that U1-snRNP induces IL-1β production from human monocytes in the presence of serum or purified IgG containing U1-snRNP antibodies by activating the NLRP3 inflammasome. This process involves ROS synthesis and K+ efflux as well as the activation of TLR7, 8 and NF-κB. Our findings provide new insights into how the endogenous molecule U1-snRNP could serve as an NLRP3 inflammasome activator in the diseases with aberrant autoimmune responses to U1-snRNP, possibly contributing to the pathogenesis of such diseases.
Supplementary Material
Acknowledgements
We thank Ms. Laura Kramer and Yale Center for Clinical Investigation (UL1 RR024139) for assisting in the recruitment of human subjects. We are grateful to Dr. Mark Mamula for his critical review on the manuscript and Ms. Mary Lou Breitenstein for the identification of ANApositive sera. Insoo Kang is a participant of the World Class University Program of Republic of Korea.
The source of support
This work was supported in part by grants from the Department of Defense (W81XWH-10-1-0150) and the National Institute of Health (U19 AI082713, AG028069).
Abbreviations used in this article
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- U1-snRNP
U1-small nuclear ribonucleoprotein
- U1-snRNA
U1-small nuclear RNA
- SLE
systemic lupus erythematosus
- ROS
reactive oxygen species
- ANA
antinuclear antibodies
Footnotes
Disclosures
The authors declare no conflicts of interests.
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Davis BK, Wen H, Ting JP. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu Rev Immunol. 2010 doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 5.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 7.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 10.Kattah NH, Kattah MG, Utz PJ. The U1-snRNP complex: structural properties relating to autoimmune pathogenesis in rheumatic diseases. Immunol Rev. 2010;233:126–145. doi: 10.1111/j.0105-2896.2009.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark H, Luhrmann R. Cryo-electron microscopy of spliceosomal components. Annu Rev Biophys Biomol Struct. 2006;35:435–457. doi: 10.1146/annurev.biophys.35.040405.101953. [DOI] [PubMed] [Google Scholar]
- 12.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeFeber WP, Norris DA, Ryan SR, Huff JC, Lee LA, Kubo M, Boyce ST, Kotzin BL, Weston WL. Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest. 1984;74:1545–1551. doi: 10.1172/JCI111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, Simanski M, Glaser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R, Schauber J. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3:82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL- 1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savarese E, Chae OW, Trowitzsch S, Weber G, Kastner B, Akira s, Wagner H, Schmid RM, Bauer S, Krug A. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–3234. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- 24.Kelly KM, Zhuang H, Nacionales DC, Scumpia PO, Lyons R, Akaogi J, Lee P, Williams B, Yamamoto M, Akira S, Satoh M, Reeves WH. "Endogenous adjuvant" activity of the RNA components of lupus autoantigens Sm/RNP and Ro 60. Arthritis Rheum. 2006;54:1557–1567. doi: 10.1002/art.21819. [DOI] [PubMed] [Google Scholar]
- 25.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 26.Willingham SB, Bergstralh DT, O'Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell RA, Deshmukh M, Hoffman HM, Ting JP. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007;2:147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu J, Thomas LM, Watkins SC, Franchi L, Nunez G, Salter RD. Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J Leukoc Biol. 2009;86:1227–1238. doi: 10.1189/jlb.0309164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- 29.Popovic K, Ek M, Espinosa A, Padyukov L, Harris HE, Wahren-Herlenius M, Nyberg F. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005;52:3639–3645. doi: 10.1002/art.21398. [DOI] [PubMed] [Google Scholar]
- 30.Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141:3050–3054. [PubMed] [Google Scholar]
- 31.Boswell JM, Yui MA, Endres S, Burt DW, Kelley VE. Novel and enhanced IL-1 gene expression in autoimmune mice with lupus. J Immunol. 1988;141:118–124. [PubMed] [Google Scholar]
- 32.Brennan DC, Yui MA, Wuthrich RP, Kelley VE. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. J Immunol. 1989;143:3470–3475. [PubMed] [Google Scholar]
- 33.Voronov E, Dayan M, Zinger H, Gayvoronsky L, Lin JP, Iwakura Y, Apte RN, Mozes E. IL-1 beta-deficient mice are resistant to induction of experimental SLE. Eur Cytokine Netw. 2006;17:109–116. [PubMed] [Google Scholar]
- 34.Ostendorf B, Iking-Konert C, Kurz K, Jung G, Sander O, Schneider M. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann Rheum Dis. 2005;64:630–633. doi: 10.1136/ard.2004.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.