Abstract
In response to potential bioterrorism with smallpox, members of the Japanese Self-Defense Forces were vaccinated with vaccinia virus (VACV) strain LC16m8, an attenuated smallpox vaccine derived from VACV strain Lister. The serological response induced by LC16m8 to four virion-surface proteins and the intracellular mature virus (IMV) and extracellular enveloped virus (EEV) was investigated. LC16m8 induced antibody response against the IMV protein A27 and the EEV protein A56. LC16m8 also induced IMV-neutralizing antibodies, but unlike the VACV strain Lister, did not induce either EEV-neutralizing antibody or antibody to EEV protein B5, except after revaccination. Given that B5 is the only target for EEV-neutralizing antibody and that neutralization of both IMV and EEV give optimal protection against orthopoxvirus challenge, these data suggest that immunity induced by LC16m8 might be less potent than that deriving from strain Lister. This potential disadvantage should be balanced against the advantage of the greater safety of LC16m8.
Smallpox was eradicated in 1979 by widespread vaccination with vaccinia virus (VACV) and thereafter smallpox vaccination was discontinued (Fenner et al., 1988). However, due to the potential threat of bioterrorism, limited smallpox vaccination programmes have been restarted, and the World Health Organization (WHO) and several nation states are replenishing their smallpox vaccine stockpiles.
The WHO reference smallpox vaccine was the strain Lister, but several other VACV strains were also used including New York City Board of Health (NYCBH/Dryvax), EM-63 and Tian Tan (Fenner et al., 1988). Although these strains protected against smallpox, they could also cause adverse reactions and eczema, immunodeficiency and pregnancy were recognized as contraindications for smallpox vaccination (Lane et al., 1969). The concern about vaccine safety led to the development of attenuated vaccines by empirical passage, such as LC16m8 (Hashizume et al., 1985) or modified vaccinia virus Ankara (MVA) (Stickl & Hochstein-Mintzel, 1971), or by genetic engineering, such as NYVAC (Tartaglia et al., 1992). However, these strains were not used in countries where smallpox was endemic and, consequently, evidence that they protect against smallpox is lacking. Nonetheless, in animal models, they can protect against disease caused by other orthopoxviruses (OPVs), such as monkeypox virus (Earl et al., 2004; Saijo et al., 2006). Here, we have examined the immunogenicity of LC16m8 by evaluating the neutralizing antibody response to both intracellular and extracellular virions and four individual proteins on the surface of these virions. These results were compared with the immunogenicity of the VACV strain Lister.
LC16m8 is a small plaque variant of the VACV strain Lister (Elstree) obtained by repeated passage of Lister in primary rabbit kidney cells at low temperature (Hashizume et al., 1985). LC16m8 is attenuated in animal models and in man (Hashizume et al., 1985; Saito et al., 2009) and was used to vaccinate over 50 000 children in Japan in the 1970s and members of the Japanese Self-Defense Forces between 2002 and 2005 (Kenner et al., 2006; Saito et al., 2009). The small plaque phenotype of LC16m8 is due to a mutation of the B5R gene, resulting in the truncation of the ORF after codon 91 (Takahashi-Nishimaki et al., 1991) and expression of a truncated B5 protein (Meseda et al., 2009). This mutation and additional alterations elsewhere in the genome contributed to the attenuated phenotype of LC16m8 (Takahashi-Nishimaki et al., 1991). Spontaneous mutations in the LC16m8 B5R gene that restore the plaque size to normal and increase virulence can occur, but this could be prevented by deletion of the entire gene (Kidokoro et al., 2005). Recently, it was demonstrated that whereas T-cells are needed to prevent development of progressive vaccinia in macaques immunized with ACAM200 (a plaque purified derivative of Dryvax), LC16m8 was unable to spread and cause disease even in the absence of T-cells, demonstrating its greater safety (Gordon et al., 2011).
There are two infectious forms of VACV, the intracellular mature virus (IMV) and the extracellular enveloped virus (EEV), which have different numbers of membranes and distinct surface antigens (Roberts & Smith, 2008). IMV has a single membrane, whereas EEV has a second membrane and promotes spread within an infected host. Despite studies showing that antibodies against EEV are important for protection against disease (Boulter & Appleyard, 1973; Law et al., 2005), immune responses against EEV have been less intensively studied than those against IMV. There are multiple targets for neutralizing antibodies on the IMV surface, including A27 and H3 (Davies et al., 2005; Pütz et al., 2006), but B5 is the only target of EEV-neutralizing antibodies (Bell et al., 2004; Pütz et al., 2006), and is conserved in all strains of variola virus that have been sequenced (Aguado et al., 1992; Massung et al., 1994; Shchelkunov et al., 1994, 1995; Esposito et al., 2006). B5 is also important for virus spread from cell to cell and for virulence (Engelstad et al., 1992; Isaacs et al., 1992; Engelstad & Smith, 1993; Wolffe et al., 1993). The production of only a truncated B5 protein by LC16m8 is therefore relevant to the efficacy of this virus as a vaccine for smallpox, although, in animal models, LC16m8 induces neutralizing antibodies against both IMV and EEV and can protect from a lethal orthopoxvirus challenge (Empig et al., 2006; Meseda et al., 2009). A recent study of the immunogenicity of LC16m8 in man, investigated the seroconversion rate of vaccinees and IMV-neutralizing antibody titres (Saito et al., 2009), but the immunity to individual antigens, including those specific to EEV, and the ability to neutralize EEV remain unknown.
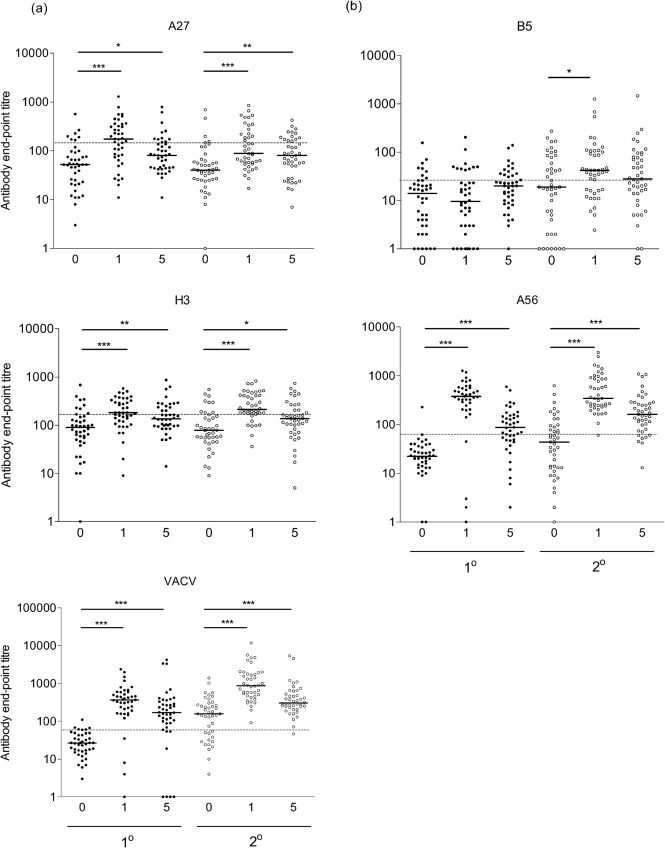
Here, the antibody responses to four VACV antigens were measured by ELISA and the IMV- and EEV-neutralizing titres were determined by plaque reduction assay, as described previously (Pütz et al., 2005, 2006). The antigens selected were the IMV-surface proteins (A27 and H3) and the EEV-surface proteins (B5 and A56), which were produced and purified from bacterial (A27 and H3) or mammalian (B5 and A56) expression systems (Pütz et al., 2006; Midgley et al., 2008). The total anti-VACV antibody titre was also measured by ELISA against the VACV strain Western Reserve (WR)-infected cell lysates (Pütz et al., 2006; Midgley et al., 2008). Serum samples (pre-vaccination and 1 and 5 months after vaccination) were obtained from 42 primary vaccinees and 43 persons vaccinated previously, most likely with the VACV strain Lister (revaccinees), as described previously (Saito et al., 2009). The pre-vaccination sera from primary vaccinees were used to calculate cut-off titres defining seropositivity, defined as three times the geometric mean titre (GMT) of the pre-vaccinated sera. The cut-off titre for each antigen was defined as the maximum dilution of serum that gave a positive-antibody response; these were: B5, 1 : 28; A56, 1 : 63; A27, 1 : 145; H3, 1 : 254; VACV, 1 : 82 and are shown by the dashed line in Fig. 1. The vast majority of pre-vaccination sera were below the cut-offs, with the following specificities: B5, 81 %; A56, 98 %; A27, 83 %; H3, 83 %; VACV, 90 %. Any values below this cut-off were deemed seronegative and given an arbitrary value of one-half of that titre to allow calculations of GMT and to determine effective seroconversion or boosting.
Fig. 1.
Antibody responses in humans after LC16m8 vaccination. Antibody end-point titres against (a) IMV and (b) EEV proteins were detected pre-vaccination (0) and 1 and 5 months post-vaccination for primary vaccinees (filled circles) and revaccinees (open circles) by ELISA as described in Pütz et al. (2006). IgG end-point titres were defined as the reciprocal serum dilution giving twice the average optical density obtained from BSA. A control serum from an individual vaccinated multiple times was used to normalize end-point titres between plates and assays (titres: B5, 1 : 809; A56, 1 : 1213; A27, 1 : 563; H3, 1 : 394; VACV, 1 : 5785). Median values of whole population (black bars), cut-off titres for seropositivity (dashed line) and significant differences between groups, as determined by Mann–Whitney test (*P<0.05, **P<0.005, ***P<0.0001) are shown.
Following vaccination the greatest increase in antibody titre was for antibodies against A56, and VACV-infected cells in which there was a statistically significant increase in mean antibody titre from the pre-vaccination serum to the 1 and 5 months post-vaccination sera in both primary vaccinees and revaccinees (Fig. 1). There were also significant increases in mean titre for antibodies against A27 and H3, although increases were lower than for A56- and VACV-infected cells. In contrast, no antibody response was detected against B5 in primary vaccinees (P = 0.1). However, there was a boosting of B5 responses in revaccinees from pre-vaccination to 1 month post-vaccination (P = 0.02). GMTs for each antigen also increased following vaccination of primary vaccinees, with the exception of B5 where no increase was seen (Table 1). An increase in GMTs was seen for all antigens in revaccinees, including B5.
Table 1. IgG GMTs.
GMTs and 95 % confidence intervals are given for each VACV antigen and the total VACV antigen in infected cells in ELISA before and after vaccination for primary vaccinees and revaccinees. The fold increase in GMT from pre-vaccination to 1 month post-vaccination is also given.
| Antigen | Primary vaccinees | Revaccinees | ||||||
| Pre | 1 month | 5 months | Fold increase | Pre | 1 month | 5 months | Fold increase | |
| B5 | 9 (1–17) | 9 (0–20) | 18 (9–26) | 1.0 | 16 (0–34) | 43 (0–109) | 30 (0–97) | 2.7 |
| A56 | 21 (11–31) | 288 (189–386) | 75 (38–111) | 13.7 | 36 (1–72) | 436 (248–624) | 166 (89–242) | 12.1 |
| A27 | 48 (18–78) | 150 (76–223) | 86 (41–132) | 3.1 | 42 (6–79) | 115 (57–173) | 76 (48–104) | 2.7 |
| H3 | 75 (36–114) | 173 (130–215) | 145 (95–195) | 2.3 | 82 (45–119) | 240 (182–297) | 127 (78–176) | 2.9 |
| VACV | 24 (18–31) | 267 (120–414) | 130 (0–407) | 11.1 | 122 (40–204) | 942 (309–1575) | 356 (47–665) | 7.7 |
Rates of seroconversion in primary vaccinees and boosting in revaccinees, defined as a fourfold increase in end-point titre from the pre- to post-vaccination sera, varied for each antigen. In primary vaccinees, the IMV antigens A27 and H3 and total VACV had seroconversion rates of 19.0, 2.4 and 76.2 %, respectively. For the EEV antigens, only 2.4 % of primary vaccinees seroconverted to B5, compared with 85.7 % for A56. For revaccinees, the antibody response against IMV antigens A27 and H3 and total VACV were boosted in 16.7, 9.5 and 69.0 % of vaccinees, respectively. For EEV antigens B5 and A56, an effective booster response was seen in 28.6 and 76.2 % of revaccinees, respectively. The observation that B5 responses are boosted in revaccinees, despite little or no antibody response in primary vaccines, is interesting and is likely attributable to the production of a short fragment of the B5 protein up to aa 91 (Meseda et al., 2009).
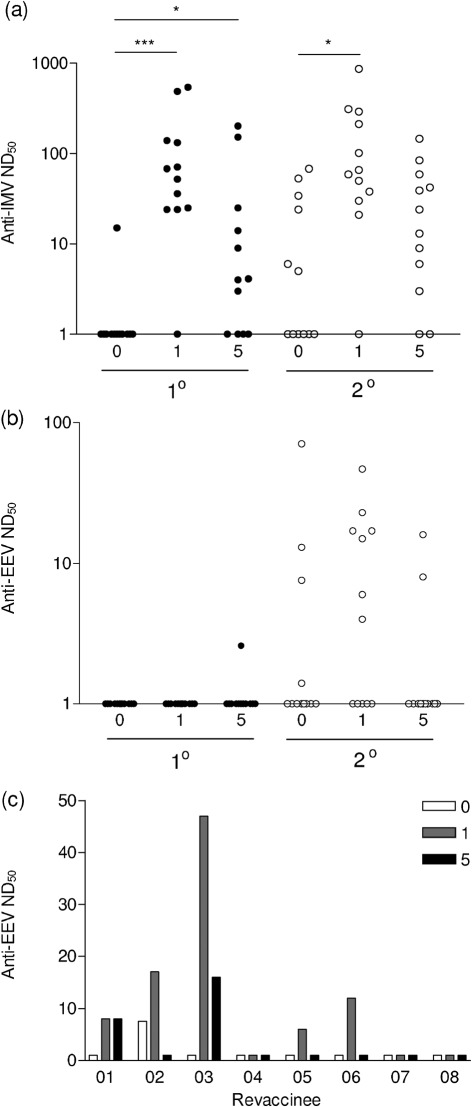
Some sera were also tested for their ability to neutralize IMV and EEV from the VACV strain WR by plaque-reduction neutralization assay (Pütz et al., 2006). A statistically significant increase in neutralizing antibody titres against IMV was seen from the pre-vaccination sera to the 1 month post-vaccination sera for both primary vaccinees and revaccinees (P = 0.0002 and P = 0.0043, respectively; Fig. 2). There was also a significant increase in IMV-neutralizing antibody titres from the pre-vaccination sera to the 5 months post-vaccination sera for primary vaccinees (P = 0.018). However, there was not a significant increase for revaccinees for this time point (P = 0.13). Neutralizing antibody titres correlated well with end-point titres against IMV antigens. In contrast, sera from primary vaccinees did not neutralize EEV, except for one sample that showed a very weak response (Fig. 2b). This correlated with the weak or no anti-B5 responses and was in contrast to the high titres of anti-A56 antibodies. It was also consistent with the prior observation that B5 is the only target for EEV-neutralizing antibody (Bell et al., 2004; Pütz et al., 2006). Several sera from revaccinees neutralized EEV before and after vaccination with LC16m8 (Fig. 2b). In individuals for whom pre-, 1 and 5 months post-vaccination sera were tested, four of eight showed effective boosting of EEV-neutralizing antibodies following LC16m8 vaccination (Fig. 2c), perhaps due to the presence of a truncated B5 protein. It is worth noting that non-neutralizing antibodies against EEV, such as those against A56, may activate the complement system (Benhnia et al., 2009).
Fig. 2.
Neutralizing antibody responses were detected by plaque-reduction neutralization against (a) IMV and (b) EEV for pre-vaccination (0) and 1 and 5 months post-vaccination sera from primary vaccinees (filled circles) and revaccinees (open circles) as described in Pütz et al. (2006). (c) Shows the response against EEV from eight individual vaccinees. IMV from the VACV strain WR was purified from sucrose density gradients, whereas EEV was harvested from cell supernatant following 24 h post-infection and then incubated with anti-IMV antibody (raised against L1 and A27). ND50 values were defined as the reciprocal of the dilution of serum giving a 50 % reduction in plaque number. Significant differences between groups were determined by Mann–Whitney test and shown (*P<0.05, ***P<0.0001).
A comparison of antibody titres in pre-vaccination sera between primary vaccinees and revaccinees showed residual antibody from prior immunization. Differences in the median end-point titres were seen for A56 (P = 0.029), VACV (P<0.0001) and B5, although the latter was not statistically significant (P = 0.085). Pre-existing antibodies were not detected for A27 (P = 0.40) or H3 (P = 0.86) although these sera did neutralize IMV and EEV, as seen by high titres of neutralizing antibodies in pre-vaccination sera from some revaccinees (Fig. 2). The smallpox eradication campaign in Japan ceased in 1976, showing that these immune responses are still active at least 35 years after vaccination.
Antibody responses to LC16m8 were qualitatively and quantitatively different from those seen for Lister, the VACV strain used most widely in the smallpox eradication campaign. In primary Lister vaccinees, the fold-increases in GMT against antigens B5, A27, H3 and VACV were 13.7, 10.0, 1.8 and 17.1, respectively (Pütz et al., 2006); all but H3 were higher than the responses to LC16m8 (Table 1). LC16m8 also induced lower responses than those following primary Dryvax inoculation [fold increases of B5, 18.8; A27, 17.2; H3, 5.7; VACV, 18.1 (Midgley et al., 2008)]. However, LC16m8 performed favourably compared to the attenuated vaccine NYVAC (B5, 2.2; A27, 1.0; H3, 1.8; VACV, 1.9), apart from in its anti-B5, and therefore anti-EEV, responses (Midgley et al., 2008). In comparison, the human antibody responses to MVA are lower than those to Dryvax in primary vaccinees, but similar in revaccinees (Davies et al., 2007; Parrino et al., 2007). Overall, LC16m8 induces quantitatively lower antibody responses in primary vaccinees than Lister or Dryvax, but stronger responses than NYVAC. LC16m8 also induces qualitatively different responses to each of these vaccines, as shown by the absence of B5- or EEV-neutralizing antibodies in primary vaccinees. It is important to note that LC16m8 has an excellent safety record with less complications and contraindications than either Lister or Dryvax.
In summary, an analysis of the serological responses induced by VACV LC16m8 in primary vaccinees showed that IMV-neutralizing antibodies were induced and there was a good response to the A27 IMV-surface proteins and total VACV antigen from infected cells. In primary vaccinees, LC16m8 failed to induce EEV-neutralizing antibody and consistent with this antibodies to EEV protein B5 were not produced; however, a boosting response against B5 protein was observed in revaccinees. Since B5 is the only EEV antigen that is the target for EEV-neutralizing antibody, and since it is conserved in all strains of variola virus, these data suggest that immunity induced by LC16m8 might be less potent than that deriving from the VACV strain Lister. Nevertheless, several studies showed that, similar to Lister, LC16m8 can protect animals against disease caused by some orthopoxviruses (Empig et al., 2006; Meseda et al., 2009). In addition, it should be noted that the exact correlates of protection against smallpox remain uncertain, and that there is evidence for involvement of both antibodies and cellular immunity in protection against orthopoxviruses, for review see Moss (2011). The potential disadvantage of reduced immunogenicity of LC16m8 should be considered together with the advantage of increased safety of this vaccine.
Acknowledgements
The authors thank Huw Davies, University of California, Irvine, for providing soluble H3 protein and Mike Pütz and Claire Midgley, Imperial College London, for helpful discussions. This work was supported by grants from the UK Department of Health and the Medical Research Council. G. L. S. is a Wellcome Trust Principal Research Fellow.
References
- Aguado B., Selmes I. P., Smith G. L. (1992). Nucleotide sequence of 21.8 kbp of variola major virus strain Harvey and comparison with vaccinia virus. J Gen Virol 73, 2887–2902 10.1099/0022-1317-73-11-2887 [DOI] [PubMed] [Google Scholar]
- Bell E., Shamim M., Whitbeck J. C., Sfyroera G., Lambris J. D., Isaacs S. N. (2004). Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325, 425–431 10.1016/j.virol.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Benhnia M. R., McCausland M. M., Moyron J., Laudenslager J., Granger S., Rickert S., Koriazova L., Kubo R., Kato S., Crotty S. (2009). Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J Virol 83, 1201–1215 10.1128/JVI.01797-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter E. A., Appleyard G. (1973). Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol 16, 86–108 [PubMed] [Google Scholar]
- Davies D. H., McCausland M. M., Valdez C., Huynh D., Hernandez J. E., Mu Y., Hirst S., Villarreal L., Felgner P. L., Crotty S. (2005). Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol 79, 11724–11733 10.1128/JVI.79.18.11724-11733.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. H., Molina D. M., Wrammert J., Miller J., Hirst S., Mu Y., Pablo J., Unal B., Nakajima-Sasaki R., et al. & other authors (2007). Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics 7, 1678–1686 10.1002/pmic.200600926 [DOI] [PubMed] [Google Scholar]
- Earl P. L., Americo J. L., Wyatt L. S., Eller L. A., Whitbeck J. C., Cohen G. H., Eisenberg R. J., Hartmann C. J., Jackson D. L., et al. & other authors (2004). Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428, 182–185 10.1038/nature02331 [DOI] [PubMed] [Google Scholar]
- Empig C., Kenner J. R., Perret-Gentil M., Youree B. E., Bell E., Chen A., Gurwith M., Higgins K., Lock M., Rice A. (2006). Highly attenuated smallpox vaccine protects rabbits and mice against pathogenic orthopoxvirus challenge. Vaccine 24, 3686–3694 10.1016/j.vaccine.2005.03.029 [DOI] [PubMed] [Google Scholar]
- Engelstad M., Smith G. L. (1993). The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology 194, 627–637 10.1006/viro.1993.1302 [DOI] [PubMed] [Google Scholar]
- Engelstad M., Howard S. T., Smith G. L. (1992). A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology 188, 801–810 10.1016/0042-6822(92)90535-W [DOI] [PubMed] [Google Scholar]
- Esposito J. J., Sammons S. A., Frace A. M., Osborne J. D., Olsen-Rasmussen M., Zhang M., Govil D., Damon I. K., Kline R., et al. & other authors (2006). Genome sequence diversity and clues to the evolution of variola (smallpox) virus. Science 313, 807–812 10.1126/science.1125134 [DOI] [PubMed] [Google Scholar]
- Fenner F., Henderson D. A., Arita I., Jezek Z., Ladnyi I. D. (1988). Smallpox and its Eradication. Geneva: World Health Organisation [Google Scholar]
- Gordon S. N., Cecchinato V., Andresen V., Heraud J. M., Hryniewicz A., Parks R. W., Venzon D., Chung H. K., Karpova T., et al. & other authors (2011). Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis 203, 1043–1053 10.1093/infdis/jiq162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume S., Yoshizawa H., Morita M., Suzuki K. (1985). Properties of attenuated mutant of vaccinia virus, LC16m8, derived from Lister strain. In Vaccinia Viruses as Vectors for Vaccine Antigens, pp. 87–99 Edited by Quinnan G. V. New York: Elsevier Science Publishing Co [Google Scholar]
- Isaacs S. N., Wolffe E. J., Payne L. G., Moss B. (1992). Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J Virol 66, 7217–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenner J., Cameron F., Empig C., Jobes D. V., Gurwith M. (2006). LC16m8: an attenuated smallpox vaccine. Vaccine 24, 7009–7022 10.1016/j.vaccine.2006.03.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro M., Tashiro M., Shida H. (2005). Genetically stable and fully effective smallpox vaccine strain constructed from highly attenuated vaccinia LC16m8. Proc Natl Acad Sci U S A 102, 4152–4157 10.1073/pnas.0406671102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. M., Ruben F. L., Neff J. M., Millar J. D. (1969). Complications of smallpox vaccination, 1968 – National surveillance in the United States. N Engl J Med 281, 1201–1208 10.1056/NEJM196911272812201 [DOI] [PubMed] [Google Scholar]
- Law M., Pütz M. M., Smith G. L. (2005). An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J Gen Virol 86, 991–1000 10.1099/vir.0.80660-0 [DOI] [PubMed] [Google Scholar]
- Massung R. F., Liu L. I., Qi J., Knight J. C., Yuran T. E., Kerlavage A. R., Parsons J. M., Venter J. C., Esposito J. J. (1994). Analysis of the complete genome of smallpox variola major virus strain Bangladesh-1975. Virology 201, 215–240 10.1006/viro.1994.1288 [DOI] [PubMed] [Google Scholar]
- Meseda C. A., Mayer A. E., Kumar A., Garcia A. D., Campbell J., Listrani P., Manischewitz J., King L. R., Golding H., et al. & other authors (2009). Comparative evaluation of the immune responses and protection engendered by LC16m8 and Dryvax smallpox vaccines in a mouse model. Clin Vaccine Immunol 16, 1261–1271 10.1128/CVI.00040-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley C. M., Putz M. M., Weber J. N., Smith G. L. (2008). Vaccinia virus strain NYVAC induces substantially lower and qualitatively different human antibody responses compared with strains Lister and Dryvax. J Gen Virol 89, 2992–2997 10.1099/vir.0.2008/004440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. (2011). Smallpox vaccines: targets of protective immunity. Immunol Rev 239, 8–26 10.1111/j.1600-065X.2010.00975.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrino J., McCurdy L. H., Larkin B. D., Gordon I. J., Rucker S. E., Enama M. E., Koup R. A., Roederer M., Bailer R. T., et al. & other authors (2007). Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naïve and vaccinia-immune individuals. Vaccine 25, 1513–1525 10.1016/j.vaccine.2006.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pütz M. M., Alberini I., Midgley C. M., Manini I., Montomoli E., Smith G. L. (2005). Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. J Gen Virol 86, 2955–2960 10.1099/vir.0.81265-0 [DOI] [PubMed] [Google Scholar]
- Pütz M. M., Midgley C. M., Law M., Smith G. L. (2006). Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nat Med 12, 1310–1315 10.1038/nm1457 [DOI] [PubMed] [Google Scholar]
- Roberts K. L., Smith G. L. (2008). Vaccinia virus morphogenesis and dissemination. Trends Microbiol 16, 472–479 10.1016/j.tim.2008.07.009 [DOI] [PubMed] [Google Scholar]
- Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H., Ogata M., Fukushi S., Mizutani T., et al. & other authors (2006). LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol 80, 5179–5188 10.1128/JVI.02642-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Fujii T., Kanatani Y., Saijo M., Morikawa S., Yokote H., Takeuchi T., Kuwabara N. (2009). Clinical and immunological response to attenuated tissue-cultured smallpox vaccine LC16m8. JAMA 301, 1025–1033 10.1001/jama.2009.289 [DOI] [PubMed] [Google Scholar]
- Shchelkunov S. N., Blinov V. M., Resenchuk S. M., Totmenin A. V., Olenina L. V., Chirikova G. B., Sandakhchiev L. S. (1994). Analysis of the nucleotide sequence of 53 kbp from the right terminus of the genome of variola major virus strain India-1967. Virus Res 34, 207–236 10.1016/0168-1702(94)90125-2 [DOI] [PubMed] [Google Scholar]
- Shchelkunov S. N., Massung R. F., Esposito J. J. (1995). Comparison of the genome DNA sequences of Bangladesh-1975 and India-1967 variola viruses. Virus Res 36, 107–118 10.1016/0168-1702(94)00113-Q [DOI] [PubMed] [Google Scholar]
- Stickl H., Hochstein-Mintzel V. (1971). [Intracutaneous smallpox vaccination with a weak pathogenic vaccinia virus (“MVA virus”)]. Munch Med Wochenschr 113, 1149–1153 (in German). [PubMed] [Google Scholar]
- Takahashi-Nishimaki F., Funahashi S., Miki K., Hashizume S., Sugimoto M. (1991). Regulation of plaque size and host range by a vaccinia virus gene related to complement system proteins. Virology 181, 158–164 10.1016/0042-6822(91)90480-Y [DOI] [PubMed] [Google Scholar]
- Tartaglia J., Perkus M. E., Taylor J., Norton E. K., Audonnet J. C., Cox W. I., Davis S. W., van der Hoeven J., Meignier B., Riviere M. (1992). NYVAC: a highly attenuated strain of vaccinia virus. Virology 188, 217–232 10.1016/0042-6822(92)90752-B [DOI] [PubMed] [Google Scholar]
- Wolffe E. J., Isaacs S. N., Moss B. (1993). Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J Virol 67, 4732–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]