Abstract
Most endocrine hormones are produced in tissues and organs with permeable microvessels that may provide an excess of hormones to be transported by the blood circulation to the distal target organ. Here, we investigate whether leptin, an endocrine hormone, induces the formation of vascular fenestrations and permeability, and we characterize its angiogenic property in the presence of other angiogenic factors. We provide evidence that leptin-induced new blood vessels are fenestrated. Under physiological conditions, capillary fenestrations are found in the leptin-producing adipose tissue in lean mice. In contrast, no vascular fenestrations were detected in the adipose tissue of leptin-deficient ob/ob mice. Thus, leptin plays a critical role in the maintenance and regulation of vascular fenestrations in the adipose tissue. Leptin induces a rapid vascular permeability response when administrated intradermally. Further, leptin synergistically stimulates angiogenesis with fibroblast growth factor (FGF)-2 and vascular endothelial growth factor (VEGF), the two most potent and commonly expressed angiogenic factors. These findings demonstrate that leptin has another new function—the increase of vascular permeability.
Keywords: leptin, obesity, vascular fenestrations, neovascularization
Obesity is a global public health problem that is associated with diabetes mellitus, coronary heart disease, tumors, and sleep breathing problems. A key gene product that regulates body weight is leptin. Leptin is a circulating hormone that regulates adipose tissue mass through hypothalamic effects on satiety and energy expenditure. Recently, leptin was found to stimulate angiogenesis in animal models (1, 2). Whereas adipose tissue is considered to be the key site for leptin production, this hormone is also produced in actively angiogenic tissues such as the placenta and fetal tissues such as heart, bone, and hair follicles, which suggests that it may stimulate neovascularization in these tissues (3). Thus, leptin may act both as an endocrine hormone and as a paracrine growth factor.
Most endocrine hormones are produced in tissues and organs with fenestrated endothelium (4) that may provide an excess of hormones to be transported by blood circulation to the distal target organ. This function seems to be particularly important for the adipose tissue because a global control of body weight, food intake, energy expenditure, and thermogenesis requires release of both endocrine hormones and small molecules into the circulatory system. To accomplish these functions, vascular fenestrations should be regulated in an organ or tissue in such a way that fenestrations can be switched on and off. Vascular fenestrations are an important structural basis for vascular permeability (4, 5)
Several soluble growth factors are found to regulate vascular fenestrations and permeability. Examples are vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) and VEGF-C (6, 7). Although these factors participate in the control of vascular leakage, they have several other angiogenesis-related functions in vivo (6). However, their roles in the maintenance and regulation of vascular fenestrations and in permeability in the adipose tissue are not yet fully understood. In most leptin-producing tissues, including the placenta and the adipose tissue, other angiogenic factors are also coexpressed (6). Thus, it is important to investigate the angiogenic features of leptin in the presence of several other angiogenic factors. Here, we investigate whether leptin induces the formation of vascular fenestrations and permeability, and we study its angiogenic property in the presence of other angiogenic factors.
Materials and Methods
Reagents and Animals.
Recombinant human leptin and VEGF165 were obtained from R & D Systems. Recombinant human FGF-2 was obtained from Scios Nova (Mountain View, CA). C57BL/6J ob/ob mice were obtained from M & B A/S, Ry, Denmark. Male 5- to 6-week-old C57BL/6J and female BALB/c mice were acclimated and caged in groups of six or fewer. Animals were anesthetized in a methoxyflurane chamber before all procedures and killed with a lethal dose of CO2. All animal studies were reviewed and approved by the animal care and use committee of the Stockholm Animal Board.
Endothelial Cell Proliferation Assay.
A 72-h bovine capillary endothelial (BCE) cell proliferation assay was performed as described (8). BCE cells were maintained in DMEM containing 10% heat-inactivated bovine calf serum (BCS) and 3 ng/ml recombinant human FGF-2. Cells growing in gelatinized 6-well plates were dispersed in 0.05% trypsin solution and resuspended with DMEM containing 5% BCS. Approximately 10,000 cells in 0.5 ml were added to each gelatinized well of 24-well plates and incubated at 37°C for 1 h. Leptin and FGF-2 samples in triplicates were added to cells to a final volume of medium of 1 ml per well. After a 72-h incubation, cells were trypsinized, resuspended in Isoton II solution (Beckman Coulter AB, Sweden), and counted with a Coulter Counter.
Mouse Corneal Angiogenesis Assay.
The mouse corneal assay was performed according to procedures described (9). Corneal micropockets were created with a modified von Graefe cataract knife in both eyes of male 5- to 6-week-old C57BL/6J mice. A micropellet (0.35 × 0.35 mm) of sucrose and aluminum sulfate coated with Hydron polymer type NCC (Interferon Sciences, New Brunswick, NJ) containing 160 ng of leptin, 160 ng of VEGF, or 80 ng of FGF-2 was implanted into each pocket. The pellet was positioned 0.6–0.8 mm from the corneal limbus. After implantation, erythromycin ophthalmic ointment was applied to each eye. The eyes were examined by a slit-lamp biomicroscope on day 5 or day 6 after pellet implantation. Vessel length and clock-hours of circumferential neovascularization were measured.
Immunohistochemistry.
The growth factor-implanted mouse eyes were enucleated at day 6 after implantation and immediately frozen on dry ice and stored at −80°C before use. Tissue sections of 14 μm were dissected with a cryostat and immersed in acetone for 10 min. Tissue slices were washed with PBS, blocked with 3% BSA in PBS for 20 min, and incubated for 1 h with a monoclonal rat antibody against mouse CD31 antigen (PharMingen). After washing with PBS, a secondary FITC-conjugated rabbit anti-rat IgG was incubated with the tissue sections for 1 h. The immunostained signals were examined under a fluorescence microscope. Corneal microvessels were counted in at least six sections at 20× magnification.
Electron Microscopy.
At day 6 after implantation of angiogenic factors, the animals were killed and the eyes were removed and immersed in a fixative containing 3% glutaraldehyde in 0.1 M sodium cacodylate–HCl buffer (pH 7.3) with 0.05 M sucrose. A few hours later, the parts of the corneas containing ingrowing blood vessels were dissected out, cut into small pieces, and put in fresh fixative. The subcutaneous adipose tissues of C57BL/6J wild-type mice and ob/ob mice were removed and immersed in glutaraldehyde fixative. After rinsing in buffer, the specimens were postfixed in 1.5% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.3) with 0.7% potassium ferrocyanate for 2 h at 4°C, dehydrated in ethanol (70%, 95%, and 100%), stained with 2% uranyl acetate in ethanol, and embedded in Spurr low-viscosity epoxy resin. Thin sections were cut with diamond knives on an LKB Ultrotome IV, picked up on carbon-coated Formvar films, stained with alkaline lead citrate, and examined in a Philips CM120 Twin electron microscope at 80 kV.
Permeability Assay.
Female 6-week-old BALB/c white mice were shaved. Four days later, the mice were anesthetized with a mixture of Hypnorm (Janssen Animal Health, Belgium) and Dormicum (Roche) (1:1) in an H2O solution, and 150 μl of 1% Evans blue dye solution was injected into the tail vein of each mouse. After 5 min, 50 ng of leptin or VEGF in a volume of 20 μl of PBS was injected at adjacent locations intradermally in the middorsum of the same animals. The extravasation of Evans blue dye was recorded with a digital camera for up to 4 h. As controls, 50 ng of BSA or FGF-2 was injected into the same positions in animals of a separate group. Five animals were used in each of the treated and control groups.
Statistics.
Statistical evaluation of the results was made by a two-tailed Student's t test with instat 1.1 and Microsoft excel 5 programs, and by a one-way ANOVA followed by a Newman–Keuls post hoc test. To relate the effects of individual and combined treatments, the effects of leptin and FGF-2 applied alone were summarized and compared with the effects of leptin and FGF-2 applied together by using the Mann–Whitney U test. The same method was used to compare the effects of leptin and VEGF used alone or together.
Results
Capillary Endothelial Cell Growth.
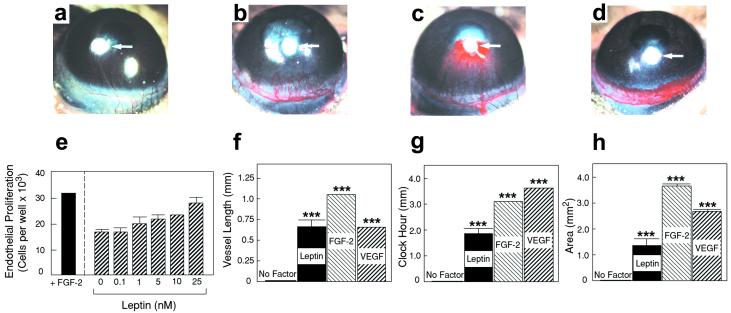
To study the effect of leptin on capillary endothelial cell growth, various concentrations of leptin were assayed on BCE cell proliferation and migration. Significant stimulation of BCE cell proliferation was detected at concentrations above 10 ng/ml. Human leptin stimulated BCE cell proliferation in a dose-dependent manner (Fig. 1e). These findings indicate that leptin stimulates capillary endothelial cell proliferation, which is a critical step for angiogenesis.
Figure 1.
Endothelial cell growth and corneal angiogenic responses induced by leptin, FGF-2, and VEGF. (e) BCE cells were incubated with various concentrations of leptin or 1 ng/ml FGF-2. After 72 h, cells were counted. Values represent means (±SEM) of triplicate of each sample. Statistically significant values were obtained at concentrations above 5 nM (P = 0.085 at 5 nM, P = 0.0014 at 10 nM, and P = 0.027 at 25 nM) (a) A cornea of the control group of mice implanted with sucrose aluminum sulfate and Hydron (without growth factors). Pellets containing sucrose aluminum sulfate, Hydron, and 160 ng of leptin (b), 80 ng of FGF-2 (c), or 160 ng of VEGF (d) were implanted into corneal micropockets of mice. Corneas were photographed with a stereomicroscope on day 5 after pellet implantation; positions of implanted pellets are indicated by white arrows. Maximal vessel length (f), clock-hours of circumferential neovascularization (g), and area of neovascularization (h) are presented as mean determinants (±SEM) of 9 or 10 corneas in each group. P values were calculated according to a standard two-tailed Student's t test. ***, P < 0.001.
Angiogenic Responses.
We chose the mouse corneal angiogenesis model to study the structure of microvessels induced by a single angiogenic agent because the cornea remains avascular under physiological conditions. The corneal angiogenic response induced by leptin (Fig. 1b) differed from those induced by FGF-2 (Fig. 1c) and VEGF (Fig. 1d). Although the lengths (Fig. 1f) of leptin- and VEGF-stimulated blood vessels were similar, the clock hours (Fig. 1g) and areas (Fig. 1h) of VEGF-induced vessels were significantly greater compared with the leptin-stimulated angiogenesis. In FGF-2-implanted corneas, new microvessels crossed the cornea from the limbus toward the pellet, occasionally penetrating into the pellet (Fig. 1c). In addition, microvessels induced by all three factors were dilated (Fig. 1 b–d). The angiogenic response stimulated by VEGF was intense, with a high density of capillary sprouts that seemed to be leaky and tended to form capillary blobs at the growing edge (Fig. 1d and Fig. 5c). We should emphasize that 160 ng of leptin, 80 ng of FGF-2, or 160 ng of VEGF was used for implantation into each cornea. According to other published results and our growth factor titration experiments, the amounts of FGF-2 and VEGF used here are minimal doses that produce maximal effects of corneal angiogenesis in C57BL/6J mice (10). We have found that, similar to VEGF, 160 ng of leptin induced the maximal growth of corneal angiogenesis and that higher doses did not significantly increase the angiogenic potency.
Figure 5.
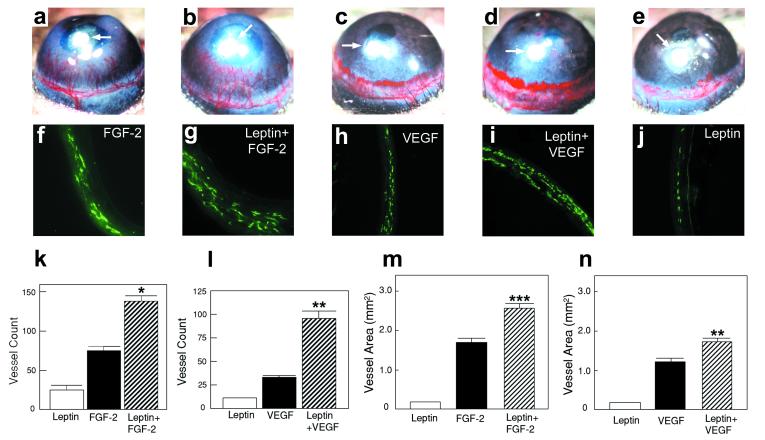
(a–e) Approximately 80 ng of leptin (e), 40 ng of FGF-2 (a), 80 ng of VEGF (c), and the same amounts of leptin plus FGF-2 (b) and leptin plus VEGF (d) were implanted into each corneal micropocket of mice. Corneas were photographed by a stereomicroscope on day 5 after growth factor implantation; positions of implanted pellets are indicated by white arrows in a–e. The area of neovascularization (m and n) was calculated as described (28, 29) and presented as mean determinants (±SEM) of 10–12 corneas in each group. (f–j) Corneal sections were incubated with an anti-CD31 antibody and stained with an FITC-conjugated secondary antibody. Immunohistological sections of the leptin-implanted cornea (j), FGF-2-implanted cornea (f), VEGF-implanted cornea (h), leptin plus FGF-2-implanted cornea (g), and leptin plus VEGF-implanted cornea (i). Corneal microvessels are revealed in green color. Vessel counts (k and l) are presented as mean determinants (±SEM) of 6–8 corneas in each group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Capillary Fenestrations of Newly Formed Blood Vessels.
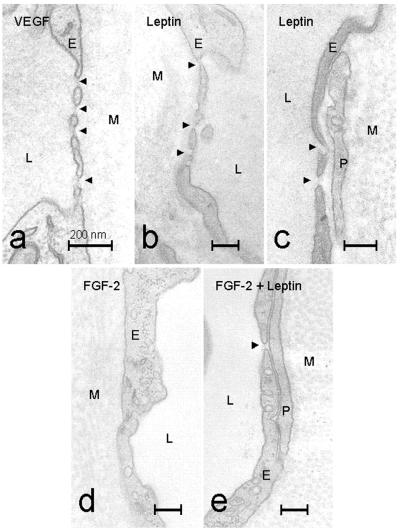
The differences in the corneal angiogenesis responses induced by leptin, FGF-2, and VEGF prompted us to study the ultrastructure of these microvessels. Corneal vessels were examined by electron microscopy and were found to be completely capillary in nature. They consisted of single layers of endothelial cells occasionally surrounded by pericytes (Fig. 2 c and e). No smooth muscle cells were evident, and in most locations, the endothelium was in direct contact with the dense collagenous matrix of the cornea. The formation of endothelial fenestrations was found in leptin-induced capillaries (Fig. 2 b and c arrowheads). Although their frequency of occurrence varied, fenestrations could often be found in the thinnest parts of the capillary walls. Notably, VEGF-induced capillaries demonstrated numerous fenestrations that had diameters of about 50 nm and usually contained fine diaphragms (Fig. 2a arrowheads). Their frequency of occurrence varied, but up to 2 or 3 fenestrations could be found per μm of blood vessel cross section. In striking contrast to the above findings, no fenestrations were detected in capillaries induced by FGF-2 (Fig. 2d). Interestingly, leptin facilitated the formation of a few endothelial fenestrations in microvessels induced by FGF-2 when both factors were used to stimulate angiogenesis (Fig. 2e). These data demonstrate that leptin and VEGF, but not FGF-2, induce vascular fenestrations in newly formed blood vessels.
Figure 2.
Thin parts of the walls of microvessels growing into the mouse cornea after stimulation with VEGF (a), leptin (b and c), FGF-2 (d) and FGF-2 + leptin (e). Arrowheads mark endothelial fenestrations. L, capillary lumen; M, collagenous matrix of the cornea; P, perivascular cell; E, endothelial cell. (Bars = 0.2 μm.)
Capillary Fenestrations in Adipose Tissues.
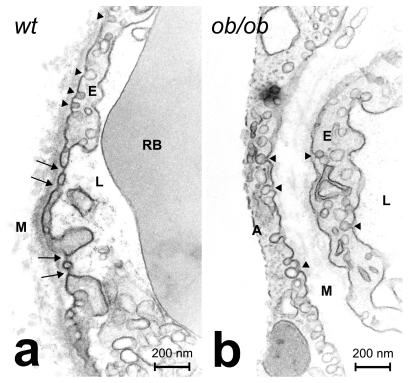
Capillary fenestrations were examined using subcutaneous adipose tissue derived from wild-type and ob/ob C57BL/6J mice. In general, the microvessel density in the adipose tissue seemed to be lower in ob/ob mice than in wild-type mice. The reduction of microvessel density could be caused by the increased size of adipocytes in ob/ob mice. In wild-type mice, a small number of fenestrated capillaries were detected. In some cases, several fenestrations were present in a single endothelial cell section (Fig. 3). When the thickness of the sections was taken into account (50 nm per section), one or more fenestrations were estimated to occur every 1.5–2 μm along the length of the capillaries. This frequency seemed to be lower than that observed in the corneal vessels. However, this fact was not surprising, because the corneal blood vessels were exposed to higher doses of leptin stimulation than were the capillaries of the normal adipose tissue. In contrast to the wild-type mice, no fenestrations were found in the adipose tissue of ob/ob mice. These data indicate that leptin plays a significant role in the maintenance of vascular fenestrations in the adipose tissue under physiological conditions.
Figure 3.
Details of capillary structures in adipose tissue from (a) wild-type (wt) and (b) ob/ob mice. At least 200 capillary sections were examined in each group. Black arrows point to endothelial fenestrations. Arrowheads mark caveolae. A, adipocyte; L, capillary lumen; RB, red blood cell; E, endothelial cell; M, collagenous matrix of the cornea. (Bars = 0.2 μm.)
Vascular Permeability.
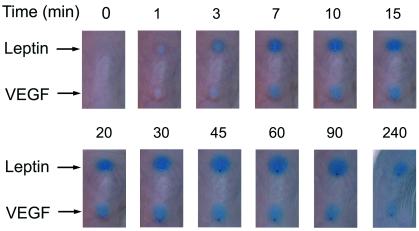
Vascular fenestration is one of the structural bases that cause capillary permeability. In an effort to study vascular permeability, we carried out a modified Miles assay in mice. Leptin and VEGF, but not FGF-2 or BSA (data not shown), induced a rapid vascular permeability response as measured by the extravasation of Evans blue dye. The leptin-induced permeability was detectable 3 min after injection and reached a maximum after ≈60 min (Fig. 4). This effect was almost comparable to that induced by VEGF. Our data show that leptin acts as a potent vascular permeability factor.
Figure 4.
Evans blue was injected intravenously into the tail veins of BALB/c mice. After 5 min, 50 ng of leptin or VEGF was administered intradermally in 20 μl of PBS. The same amounts of FGF-2 and BSA were used as negative controls. The extravasation of Evans blue was recorded with a digital camera system at various time points (min).
Synergistic Angiogenesis.
Leptin has been found to be coexpressed with other angiogenic factors (including FGF-2 and VEGF) in adipose, placental, and fetal tissues, suggesting that leptin might modulate the angiogenic activity of these factors (3, 7, 11). To test this possibility, low doses of leptin and FGF-2 or VEGF were coimplanted into mouse corneas. At 80 ng per pellet, leptin alone displayed only a weak stimulation of corneal angiogenesis (Fig. 5e) as measured by vessel density (Fig. 5 j–l) and area (Fig. 5 m and n). FGF-2 (Fig. 5a) and VEGF (Fig. 5c) at a similar low dose induced a more intense corneal neovascularization (Fig. 5 f, h, and k–n). However, a remarkable synergistic effect on the stimulation of corneal blood vessel growth was observed when leptin was implanted together with FGF-2 (Fig. 5b) or VEGF (Fig. 5d). The vessel length, density, and area were all distinctly increased in leptin and FGF-2 coimplanted corneas (Fig. 5 b, g, k, and m) or leptin and VEGF coimplanted corneas (Fig. 5 d, i, l, and n), as compared with those stimulated by leptin alone (Fig. 5 e and j–n), FGF-2 alone (Fig. 5 a, f, k, and m), or VEGF alone (Fig. 5 c, h, l, and n). The measured area and density of neovascularization induced by leptin and FGF-2, or leptin and VEGF, were statistically greater than the sum of the effects obtained with these factors used alone. Thus, leptin stimulates angiogenesis synergistically with FGF-2 and VEGF. The calculated P values for vessel area were P < 0.001 in FGF-2 and leptin coimplanted corneas vs. the sum of FGF-2 alone plus leptin alone, and P < 0.01 in VEGF and leptin coimplanted corneas vs. the sum of VEGF alone plus leptin alone. The calculated P values for vessel density were P < 0.05 in FGF-2 and leptin coimplanted corneas vs. the sum of FGF-2 alone plus leptin alone, and P < 0.01 in VEGF and leptin coimplanted corneas vs. the sum of VEGF alone plus leptin alone.
Discussion
The angiogenic function of leptin demonstrates that this endocrine hormone acts as a paracrine growth factor for the vascular system. By stimulating local angiogenesis, leptin may promote its own release into the circulatory system to regulate the hypothalamus-mediated satiety effect that partially contributes to the maintenance of body-weight homeostasis (12). In addition, leptin-induced angiogenesis may increase fatty acid oxidation and maintain an appropriate balance between blood supply and fat deposits in adipose tissues. The maintenance of such a balance requires the exchange of molecules between the blood stream and adipose tissues. Adipose tissues, like most other endocrine organs, have to secrete hormones into the blood stream. The release of hormones into the circulatory system may require hyperpermeable vessels that may contain fenestrated capillaries. The different responses of corneal angiogenesis induced by leptin, FGF-2, and VEGF imply that different, although overlapping, signaling pathways in endothelial cells could be involved in the angiogenic responses stimulated by these factors. The fact that vascular fenestrations are present in both leptin- and VEGF-induced, but not in FGF-2-induced new blood vessels, suggests that leptin and VEGF could be involved in the maintenance of fenestrated endothelium in adipose tissue and endocrine organs. VEGF was discovered as a potent vascular permeability factor, and its permeability action has been correlated with the increase of endothelial fenestrations (5, 13). The energy expenditure and hormone release of leptin in adipose tissues require permeable blood vessels. Thus, vascular permeability induced by leptin in tissues might provide a mechanism by which the excess amounts of leptin would be exported into the circulation. Thus, the regulation of vascular permeability could act as a gatekeeper to control the actions of leptin as an endocrine hormone or as an angiogenic factor.
The leptin receptor consists of five spliced isoforms (14). The longest form, Ob-Rb, contains an active signaling site in the cytoplasmic domain. In db/db mice, a premature stop codon is inserted into the cytoplasmic region, resulting in obesity. The Ob-Rb is primarily expressed in neuronal tissue (15). Other spliced short forms of leptin receptors are expressed in many tissues. It is not known which isoform of receptors mediates vascular permeability. However, according to previous receptor studies, certain short isoforms of receptors (including Ob-Ra) could mediate biological signals (16). Thus, it is possible that the short form of leptin receptor could mediate vascular permeability. This hypothesis is supported by the fact that high levels of leptin have been detected in the plasma of db/db mice as compared with wild-type mice (17). This observation supports our hypothesis that leptin could act as a gatekeeper for its own release. In other words, the more leptin that is synthesized, the greater is the amount released into the circulation.
The molecular mechanisms of the control of vascular fenestrations are poorly understood. Interestingly, VEGF is also produced by adipocytes (18). Thus, the regulation of vascular fenestrations in adipose tissue seems to be complex and involves several factors. The presence of capillary fenestrations in adipose tissue in normal mice, but not in ob/ob mice, suggests that leptin, but not VEGF, is the key molecule in the maintenance of capillary fenestrations in the adipose tissue.
Another site (in addition to adipose tissue) where leptin is produced at a high level is the placenta, a highly angiogenic tissue (19). The biological function of leptin in the placenta is not known, but our data suggest that in addition to its angiogenic effect, leptin may increase the exchange of small molecules between the maternal circulation and the fetus by the induction and maintenance of vascular permeability in the placenta. Thus, leptin may have dual roles in the placenta, i.e., stimulation of angiogenesis and maintenance of the appropriate vascular structure.
It seems that leptin may not be the only endocrine hormone that stimulates angiogenesis and regulates vascular permeability. For example, the growth hormone family has been found to have angiogenic properties (20, 21). Corticotropin-releasing hormone (CRH) has been reported to act as a proangiogenic molecule (22). In contrast to leptin, CRH reduces vascular permeability (22–24). In addition, gonadotropin, another endocrine hormone, also reduces vascular permeability (25). Thus, it is possible that vascular permeability in an organ represents the balanced status controlled by both vascular permeability enhancing and inhibitory factors.
In this paper, we also provide evidence that leptin modulates angiogenic responses induced by FGF-2 and VEGF. Leptin and FGF-2 or VEGF could produce synergistic effects in stimulation of blood vessel growth in tissues where these factors are coexpressed. For example, all three of these factors have been found to be expressed at high levels in the developing placenta and in several regions of the fetus, including heart and bone (3). The signaling pathways stimulated by leptin in endothelial cells support its angiogenic synergism with FGF-2 and VEGF. Leptin-stimulated angiogenesis is mediated through the Jak-STAT (Janus-tyrosine kinase–signal transducers and activators of transcription) pathway, whereas VEGF-induced angiogenesis is most likely mediated by the protein kinase C pathway, and FGF-2-induced angiogenic response is transduced by mitogen-activated protein kinases-regulated pathways (1, 26, 27).
We show in this paper that leptin-induced new blood vessels are fenestrated and permeable, and that leptin displays a synergistic effect with FGF-2 or VEGF in the stimulation of blood-vessel growth. Our data demonstrate that several angiogenic factors are more effective than a single molecule in stimulation of angiogenesis.
Acknowledgments
We thank Duojia Cao for her valuable technical assistance and for discussions. We thank Niina Veitonmaki and Anna Eriksson for reading the manuscript and for discussions. This work was supported by the Swedish Cancer Foundation Grant 3811-B00–05XAC (to Y.C.), by a grant from the Human Frontier Science Program (to Y.C.), by the Karolinska Institute Foundation, the Magnus Bergvalls Foundation, the Ake Wibergs Foundation, Pharmacia, and Upjohn. R.C. is supported by Pharmacia and Upjohn and the David and Astrid Hagelens Foundation. J.T. is supported by Grant 06537 from the Swedish Medical Research Council, by the Swedish Heart Lung Foundation, and by the King Gustaf V 80th Birthday Fund.
Abbreviations
- VEGF
vascular endothelial growth factor
- FGF
fibroblast growth factor
- BCE
bovine capillary endothelial
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sierra-Honigmann M R, Nath A K, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa W C, Madge L A, Schechner J S, Schwabb M B, Polverini P J, Flores-Riveros J R. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 2.Bouloumie A, Drexler H C, Lafontan M, Busse R. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 3.Trayhurn P, Hoggard N, Mercer J G, Rayner D V. Int J Obes Relat Metab Disord. 1999;1:22–28. doi: 10.1038/sj.ijo.0800791. [DOI] [PubMed] [Google Scholar]
- 4.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. J Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts W G, Palade G E. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Alitalo K. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 7.Dvorak H F, Nagy J A, Feng D, Brown L F, Dvorak A M. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 8.Cao R, Wu H L, Veitonmaki N, Linden P, Farnebo J, Shi G Y, Cao Y. Proc Natl Acad Sci USA. 1999;96:5728–5733. doi: 10.1073/pnas.96.10.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Cao R. Nature (London) 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 10.Kenyon B M, Voest E E, Chen C C, Flynn E, Folkman J, D'Amato R J. Invest Ophthalmol Visual Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 11.Fu Y M, Spirito P, Yu Z X, Biro S, Sasse J, Lei J, Ferrans V J, Epstein S E, Casscells W. J Cell Biol. 1991;114:1261–1273. doi: 10.1083/jcb.114.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman J M. Nature (London) 2000;404:632–634. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- 13.Senger D R, Galli S J, Dvorak A M, Perruzzi C A, Harvey V S, Dvorak H F. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 14.Friedman J, Halaas J L. Nature (London) 1998;395:763–769. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 15.Lee G-H, Proenca R, Montez J M, Carroll K M, Darvishzadeh J G, Lee J I, Friedman J. Nature (London) 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 16.Bjorbaek C, Uotani S, da Silva B, Flier J S. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 17.Frederich R C, Löllmann B, Hamann A, Napolitano-Rosen A, Kahn B B, Lowell B B, Flier J S. J Clin Invest. 1995;96:1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q-X, Magovern C, Mack C, Budenbender K, Ko W, Rosengart T. J Surg Res. 1997;67:147–154. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 19.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 20.Gould J, Aramburo C, Capdevielle M, Scanes C G. Life Sci. 1995;56:587–594. doi: 10.1016/0024-3205(94)00491-a. [DOI] [PubMed] [Google Scholar]
- 21.Jackson D, Volpert O V, Bouck N, Linzer D I. Science. 1994;266:1581–1584. doi: 10.1126/science.7527157. [DOI] [PubMed] [Google Scholar]
- 22.Arbiser J L, Karalis K, Viswanathan A, Koike C, Anand-Apte B, Flynn E, Zetter B, Majzoub J A. J Invest Dermatol. 2000;113:838–842. doi: 10.1046/j.1523-1747.1999.00760.x. [DOI] [PubMed] [Google Scholar]
- 23.Wei E T, Kiang J G, Buchan P, Smith T W. J Pharmacol Exp Ther. 1986;238:783–787. [PubMed] [Google Scholar]
- 24.Kelley D M, Lichtenstein A, Wang J, Taylor A N, Dubinett S M. Immunopharmacol Immunotoxicol. 1994;16:139–148. doi: 10.3109/08923979409007086. [DOI] [PubMed] [Google Scholar]
- 25.Kramer B. Anat Rec. 1997;247:20–24. doi: 10.1002/(SICI)1097-0185(199701)247:1<20::AID-AR3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Yoshiji H, Kuriyama S, Ways D K, Yoshii J, Miyamoto Y, Kawata M, Ikenaka Y, Tsujinoue H, Nakatani T, Shibuya M, Fukui H. Cancer Res. 1999;59:4413–4418. [PubMed] [Google Scholar]
- 27.Giuliani R, Bastaki M, Coltrini D, Presta M. J Cell Sci. 1999;112:2597–2606. doi: 10.1242/jcs.112.15.2597. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi J H, Claesson-Welsh L, Alitalo K. Proc Natl Acad Sci USA. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao R, Farnebo J, Kurimoto M, Cao Y. FASEB J. 1999;13:2195–2202. doi: 10.1096/fasebj.13.15.2195. [DOI] [PubMed] [Google Scholar]