Abstract
BACKGROUND
The association between epicardial fat and coronary in-stent restenosis has not been evaluated. The objective of the present study was to evaluate the relationship of echocardiographic epicardial fat thickness (EFT) with restenosis in drug eluting stents (DES).
METHODS
In this study, 117 patients who underwent coronary angiography due to recurrent clinical symptoms or findings of non-invasive cardiac tests one year after stent implantation were selected. According to angiographic results, they were divided into two groups, 65 patients with in-stent restenosis (case group) and 52 patients without such finding (control group). EFT was measured perpendicularly on the free wall of the right ventricle at the end of systole in two echocardiographic views (parasternal short and long axis) at least in three cardiac cycles. The average of obtained values was determined and recorded as EFT. Furthermore, the history of hypertension, smoking and diabetes, age and sex were investigated and body mass index (BMI) of each patient was also calculated.
RESULTS
There were no significant differences in the baseline characteristics (P=0.812). Patients with in-stent restenosis did not have statistically significant difference (4.6±1.8 mm) in EFT compared to subjects with patent stents (4.5±1.8 mm; P=0.88). The above results were also confirmed using multiple linear regressions. No significant correlation was found between EFT and other clinical variables (P >0.05).
CONCLUSION
In this selected population, the risk of restenosis was not correlated with epicardial fat thickness. Future studies for determining the role of epicardial fat in development of in-stent restenosis are warranted.
Keywords: Epicardial Fat Thickness, Echocardiography, In-Stent Restenosis (ISR)
Introduction
Development of intracoronary stents has profoundly influenced interventional cardiology. Coronary stents have emerged as the predominant form of percutaneous coronary intervention (PCI) and are currently used in more than 90 percent of PCI procedures worldwide.1
Although metallic stents conferred a significant advantage over balloon angioplasty both in reducing initial complications (such as abrupt vessel closure, emergency coronary artery bypass graft) and improving late clinical outcomes (by reducing rate of (BMS) placement restenosis remained a major obstacle due to wider application of percutaneous revascularization especially in complex lesions.2
While bare metal coronary stents reduce the incidence of angiographic and clinical restenosis compared to balloon angioplasty, angiographic restenosis (follow-up diameter stenosis >50 percent) still occurs in 20 to 30% of patients and clinical restenosis (recurrent angina due to restenosis in the treated coronary segment) develops in 10 to 15 percent of patients in the first year after treatment.3, 4
Excessive intimal hyperplasia with smooth muscle proliferation within the stent is the key mechanism of restenosis after coronary stent implantation.2
In-stent restenosis (ISR) has developed into a significant clinical problem, and is responsible for a large proportion of repeated PCI procedures. Among unselected patients undergoing PCI, repeated revascularization occurs in 14% and increases 1-year healthcare costs by >$19 000 per occurrence. These findings have important implications for the cost effectiveness of new treatments that substantially reduce in-stent restenosis.5
The most exciting strategy for restenosis prevention has been the drug-eluting stents scaffolding with local pharmacologic action.6, 7 The use of drug-eluting stents (DES) has lowered the incidence of ISR further, with clinical restenosis rates less than 10% in contemporary practice.7
Restenosis with drug eluting stents (DES) occurs more often in patients with history of restenosis or diabetes mellitus, small vessels (reference vessel diameter <2.5mm), long lesions (lesion length >20mm), chronic total occlusions and saphenous vein grafts.1 We searched the Cochrane Library, EMBASE, and Medline databases through June 2010 for studies investigating the association of epicardial fat and in-stent restenosis but did not find any article for such correlation.
Mechanisms of in-stent Restenosis
It is hypothesized that restenosis is a manifestation of the general wound healing response expressed specifically in vascular tissue. The temporal response to injury occurs in three characteristic phases: inflammation, granulation, and extracellular matrix remodeling. The specific expression of these phases in the coronary artery leads to intimal hyperplasia at 1 to 4 months.8 The central role of autocrine and paracrine inflammatory mediators on smooth muscle proliferation at the site of vascular injury has been proposed.9
In the normal adults, epicardial fat is concentrated in the atrioventricular (AV) and interventricular (IV) grooves and along the major branches of the coronary arteries, and to a lesser extent, around the atria, over the free wall of the right ventricle (RV) and over the apex of the left ventricle (LV).10–12 However, fairly little is known about its physiologic and metabolic roles. A body of evidence shows that epicardial fat is an extremely active organ with various endocrine and paracrine functions, It is a source of several proinflammatory and proatherogenic cytokines (tumor necrosis factor-α, monocyte chemoattractant protein-1, interlukin-6, nerve growth factor, resistin, visfatin, omentin, leptin, plasminogen activator inhibitor-1, and angiotensinogen).13–18 By synthesizing and releasing these mediators, epicardial fat might implicated in the development of epicardial coronary atherosclerosis.19–21 Lack of any fascia between the adipocytes and myocardium allows direct interaction of vascular bed and the surrounding epicardial fat.11 In other words, no muscle fascia divides epicardial fat and myocardium, therefore, the two tissues share the same microcirculation. Secretion of adipokines out of adipocytes and diffusion into the coronary arteries has been demonstrated.22
In the vasculature, inflammatory signals are usually linked to blood-borne cells (e.g. macrophages and T cells) retained in the intima and activated resident cells of vascular origin.13 Interestingly, inflammatory mediators originating outside the coronary arteries are also capable of inducing compositional changes in the inner layer of intima.23, 24 There is growing evidence that the changes in or perivascular tissue surrounding epicardial coronary arteries could alter vascular homeostasis and contribute to endothelial dysfunction, amplification of vascular inflammation, intimal lesions, plaque progression by an “outside-to-inside” signaling mechanism. By the concept of such “outside-to-inside” cellular cross talk, inflammatory mediators outside the coronary artery, such as epicardial and adventitial inflammation, may contribute to intimal atherosclerotic lesions.23–26 In addition, some recent studies demonstrated augmented inflammatory responses, as reflected by the presence of several inflammatory mediators and inflammatory cell infiltrates, in subepicardial adipose tissue in patients with significant CAD.13,27
In this context, and on the basis of these pathophysiologic and clinical data, we suppose that the presence of metabolically active adipose stores that surround epicardial coronary arteries (epicardial fat) could intensify and contribute to the inflammatory burden at the site of vascular injury by stenting. The potential role of epicardial fat in the development of in-stent restenosis could be an attractive hypothesis and is the basis for our study. The association between subepicardial adipose tissue and epicardial CAD has not been evaluated. As far as we know, this is the first study to address the potential association between subepicardial adipose tissue and development de-novo intracoronary lesions at the site of stent deployment (in-stent restenosis)
The size of this metabolically active tissue (which could act as important source of inflammatory mediators) can be measured by magnetic resonance imaging (MRI), computed tomography and trans-thoracic echocardiography. MRI and CT scan are certainly precise, but they are expensive and cumbersome in clinical practice.28 Epicardial fat thickness (EFT) can be visualized and measured with two-dimensional (2D) echocardiography as first proposed and validated by Iacobellis et al.29, 30 Transthoracic echocardiography provides a reliable measurement of epicardial adipose tissue thickness and has been validated with MRI, one of the widely accepted reference standards to measure visceral adiposity.29 Echocardiographic epicardial fat measurement in both clinical and research scenarios has several advantages, including its low cost, easy accessibility, rapid applicability, and good reproducibility. Thus, the present study was designed to assess the association between epicardial fat measured by trans-thoracic echocardiography and the development of in-stent restenosis.
Materials and Methods
Study population
We studied patients who underwent coronary angiography at least 12 month after drug eluting stent (DES) implantation, as clinically indicated at the discretion of the referring cardiologist. This case-control study was conducted from January to May 2010 in the department of heart diseases of Chamran hospital in Isfahan, Iran (referral hospital, affiliates to Isfahan University of Medical Sciences). Patients with confirmed in-stent restenosis by angiography (case group) and persons with patent stent in angiography (control group) enrolled in the study.
Exclusion criteria included poor echo images, difficulties in differentiating epicardial, mediastinal and pericardial fat as well as pericardial effusion and history of in-stent restenosis. All participants were given informed written consent prior to the study, which was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki as revised in 2000.
After registering demographic data such as age, sex, weight and height, history of diabetes mellitus, smoking and hypertension, size and name of stent and coronary artery which stent was deployed, subjects underwent trans-thoracic echocardiography (vivid3, GE, USA). EFT was measured with standard technique in short and long axis views. The mean of EFT was compared between groups as a primary end point. The association of epicardial fat thickness with age and obesity (BMI <30) was the secondary end points.
Height and weight were measured by using standardized procedures at both surveys, and we computed body mass index (BMI) as weight in kilogram divided by the square of height (m2). Hypertension was defined as systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg, or requirement for antihypertensive medication. Diabetes mellitus was defined according to the criteria of the American Diabetes Association, or requirement for insulin or oral hypoglycemic drugs.31
Coronary angiographic data
Angiographic restenosis was defined as diameter stenosis ≥ 50% by quantitative coronary angiography (QCA) in the stent and in the stented segment (defined as the stent plus the 5mm edges proximal and distal to the stent) during diastole. In the cases that overlapping stents were placed, a single in-stent value was measured and the segment was considered as the entirely stented segment plus the 5mm proximal to the more proximal stent and the 5mm distal to the more distal stent implanted. Coronary arteries were visualized at least in two orthogonal views and reviewed and interpreted by two expert interventional cardiologists.
Echocardiographic measurements
A-Technical tips:
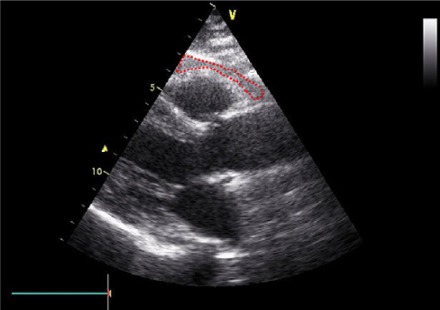
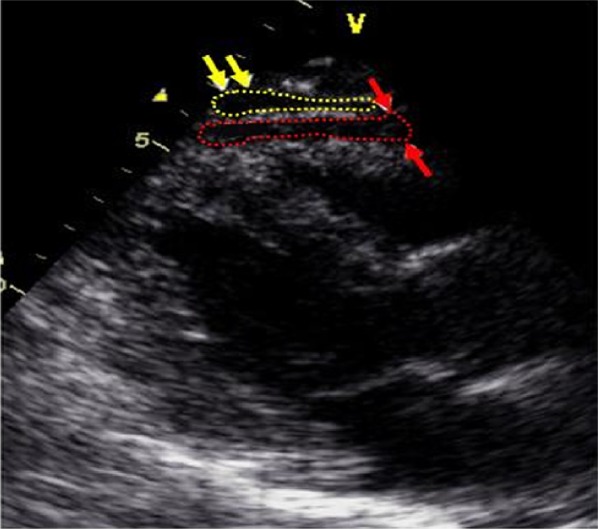
Epicardial fat was identified as an echo-free space in the pericardial layers on the 2-dimensional echocardiography (Figure 1). Mediastinal fat presenting as an echo-lucent area above the parietal pericardium was not included in the measurement of epicardial fat. Pericardial fat was identified as the hypoechoic space anterior to the epicardial fat and parietal pericardium (Figure 2). It usually is not deformed substantially with cardiac cycles and does not appear hyperechoic.
Figure 1.
Echocardiographic epicardial fat thickness. Epicardial fat thickness (within red dashed shape) is identified as the echo-free space between the outer wall of the myocardium and the visceral layer of pericardium in the parasternal long-axis view. Epicardial fat thickness is measured during end-systole at the point on the free wall of the right ventricle along the midline of the ultrasound beam, with the best effort to be perpendicular to the aortic annulus, used as an anatomic landmark.
Figure 2.
Epicardial versus pericardial fat thickness. Pericardial fat (within yellow arrows and yellow dashed shape) can be identified as the hypoechoic space anterior to the epicardial fat (within red arrows and red dashed shape). Pericardial fat usually does not deform substantially with cardiac cycles and does not appear as hyperechoic space. Modified parasternal long-axis view.
Pericardial effusion was delineated as a more homogeneous echo-free space with clear visualization of both pericardial layers on M-mode echocardiography. In contrast, compared with pericardial effusion, epicardial fat presented as a somewhat inhomogeneous, whitish-speckled, echo-lucent area. In most patients, we could differentiate pericardial effusion from epicardial fat using M-mode echocardiography.32
B-2D measurements:
Two-dimensional transthoracic echocardiography was performed with a VIVID 3 (GE, 2.5 MHz transducer, USA) instrument according to standard techniques, with subjects in the left lateral decubitus position. Echocardiographic images were recorded in a computer database. The off-line measurement of epicardial adipose tissue was performed by an echocardiographer who was unaware of clinical and angiographic data.
Echocardiographically, epicardial fat is identified as the relatively echo-free space between the outer wall of the myocardium and the visceral layer of pericardium. Its thickness is measured perpendicularly on the free wall of the right ventricle at end-systole in 3 cardiac cycles (Figure 1), according to the criteria of the American Society of Echocardiography.33 The measurement of EFT on the RV free wall was suggested in previous studies because this area has the maximum absolute thickness of epicardial fat and these two views allow optimum curser-beam orientation.34 A normal upper-limit value for EFT has not been established yet. Echocardiographic EFT ranges from a minimum of 1mm to a maximum of almost 23mm.30
Because epicardial fat is compressed during diastole, EFT is best measured at end-systole at the point on the free wall of the right ventricle at which the ultrasound beam is oriented in a perpendicular manner, using the aortic annulus as an anatomic landmark.29 Maximum EFT is measured from 2D parasternal long-axis images at the point on the free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the aortic annulus, used as an anatomic landmark for this view.
For midventricular parasternal short-axis assessment, maximum EFT is measured from 2D images on the right ventricular free wall along the midline of the ultrasound beam perpendicular to the interventricular septum at midchordal and tip of the papillary muscle level, as the anatomic landmarks. The average value of 3 cardiac cycles from each echocardiographic view was determined. The average value of two images (parasternal long-axis and short-axis views) was obtained and used as the EFT of each patient for statistical analysis.
The echocardiographically measurable amount of epicardial fat was defined as adipose tissue thickness >1mm in at least one echocardiographic view. In our echo laboratory, the intra-observer correlation coefficient of EFT measurement were 0.92, and intra-observer variability of EFT measurement yielded differences in repeated measurements that was very low (0.5+0.3 mm), indicating good reproducibility and reliability. Concordance of long-axis and short-axis average EFT measurement was also excellent at 0.96 (95% confidence interval, 0.96–0.97).
Statistical analyses
SPSS version 18.0 (SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis. All continuous variables were expressed as mean ± standard deviation; categorical variables were expressed as number and percentage. To determine the associations, independent sample t-test and chi-square test were used. To remove the possible confounding factors, multiple linear regression (MLR) test was used. A P-value <0.05 indicated statistical significance.
Results
In this study, 117 patients with mean age of 57.6±9.6 years were enrolled. The youngest patient was 36 years old and the oldest was 75 years old. Seventy-one of the study subjects (60.7%) were males. There was no statistically significant difference in terms of demographic variables such as age, sex, BMI, smoking status, blood pressure and diabetes between two groups (Table 1).
Table 1.
Baseline characteristics
| Demographic data | Group | P-value* | ||
|---|---|---|---|---|
| Control (n=52) | Case (n=65) | |||
| Age (year) | 57.8±9.7 | 57.4±9.7 | 0.8 | |
| Sex (male) | 29 (55.8) | 42 (64.6) | 0.4 | |
| BMI (Kg/m2) | 27.4±2.5 | 27.7±2.6 | 0.5 | |
| Smoking | 9 (17.3) | 10 (15.4) | 0.6 | |
| Hypertension | 14 (26.9) | 24 (36.9) | 0.3 | |
| Diabetes mellitus | 5 (9.6) | 12(18.5) | 0.3 | |
| Epicardial fat Thickness (mm) PSLX** | 4.4±1.8 | 4.52±1.9 | 0.8 | |
| Epicardial fat Thickness (mm) PSSX*** | 4.73±1.9 | 4.75±1.9 | 0.9 | |
| Mean epicardial Fat Thickness (mm) | 4.58±1.9 | 4.63±1.9 | 0.9 | |
| Stent diameter (mm) | 2.3±0.2 | 2.9±0.16 | 0.08 | |
| Stent Length (mm) | 26.4±5.6 | 31.3±5.6 | <0.0001 | |
| Stented coronary vessels | LAD 1 | 23 (44.2) | 41 (63.1) | 0.1 |
| CX 2 | 15 (28.8) | 13 (20) | ||
| RCA 3 | 14 (26.9) | 11 (16.9) | ||
Numerical characteristics are presented as mean ± SD and categorical one as number (%)
P-values were calculated with student's t test and chi-square test
Parasternal Long Axis view
Parasternal Short Axis view
LAD stands for left anterior descending artery, CX for circumflex artery and RCA for right coronary artery
The results of multiple linear regression test showed that even after controlling the possible confounding variables, there was no association between ISR and epicardial adipose tissue thickness (adjusted Beta=0.039, P=0.572).
Mean EFT ranged from 2.7 through 6.6 mm. As indicated in the table 1, EFT (in parasternal short and long axis views and mean of thicknesses) was not statistically significant between two groups (patients with and without ISR). EFT in females and males was 4.98±1.8 and 4.36±1.8 mm, respectively, which although EFT was higher in females but it was not statistically significant (P=0.08). In addition, there was no association between age and EFT (r=0.05, P=0.6) and between BMI and EFT (r=0.03, P=0.64). There was not any difference for restenosis in major coronary vessels (P<0.1). Stent length statistically was higher (P-value <0.0001) in patients with restenosis (case group).
Discussion
The main objective of the present study was to assess the association between epicardial fat thickness and restenosis inside the drug eluting stents (DES) in coronary arteries. As far as we know, this was the first study to evaluate the potential association between sub EFT and re-stenosis of the drug eluting stents.
Several studies have shown the association between echocardiographic EFT and the presence and severity of coronary artery disease.27 However, there have been no reports of the correlation between EFT and restenosis of DES in the coronary arteries. Our study demonstrated that there was not any association between EFT and restenosis inside the drug eluting stent which was deployed in the coronary arteries.
The present study has several limitations. First, the study group was highly selected, since it involved only limited number of Asian patients pre-selected by cardiologist to undergo repeated coronary angiography. Second, EFT on the right ventricular free wall does not exactly represent the amount of total epicardial fat. In some subjects, there is also abundant epicardial fat on the left ventricular apex.35 In addition, as epicardial fat has a 3-dimensional distribution, two-dimensional echocardiography may not completely assess the total amount of epicardial fat. Moreover, the design of our study was cross-sectional. Further studies which include large number of patients are necessary.
In conclusion, measuring EFT using transthoracic echocardiography is simple, feasible and well validated. The present study indicated that the EFT measured with trans-thoracic echocardiography was not correlated with in-stent restenosis in drug eluting stents. The echocardiographic measurement of EFT may provide additional information for estimating restenosis risk and predicting the presence of coronary in-stent restenosis.
Acknowledgments
We are indebted to Mr. Akbari for statistical assistance, Dr. S.A Mirdamadi in echo Lab for measurement of epicardial fat thickness and personnel of Chamran heart center for their advocated co-operation.
Conflict of Interests
Authors have no conflict of interests.
References
- 1.Libby P, Bonow RO, Zipes DP, Mann DL. Braunwald's heart disease: a textbook of cardiovascular medicine. 8th ed. Philadelphia: Elsevier-Health Sciences Division; 2007. pp. 1430–31. [Google Scholar]
- 2.Fuster V, Walsh RA, O'Rourke RA, Poole-Wilson P. Hurst's the heart. 12nd ed. New York: McGraw-Hill Medical; 2008. pp. 1431–32. [Google Scholar]
- 3.Cutlip DE, Chauhan MS, Baim DS, Ho KK, Popma JJ, Carrozza JP, et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002;40(12):2082–9. doi: 10.1016/s0735-1097(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 4.Cutlip DE, Chhabra AG, Baim DS, Chauhan MS, Marulkar S, Massaro J, et al. Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation. 2004;110(10):1226–30. doi: 10.1161/01.CIR.0000140721.27004.4B. [DOI] [PubMed] [Google Scholar]
- 5.Clark MA, Bakhai A, Lacey MJ, Pelletier EM, Cohen DJ. Clinical and economic outcomes of percutaneous coronary interventions in the elderly: an analysis of medicare claims data. Circulation. 2004;110(3):259–64. doi: 10.1161/01.CIR.0000135589.85501.DB. [DOI] [PubMed] [Google Scholar]
- 6.Serruys PW, Kutryk MJ, Ong AT. Coronary-Artery Stents. N Engl J Med. 2006;354:483–95. doi: 10.1056/NEJMra051091. [DOI] [PubMed] [Google Scholar]
- 7.Sousa JE, Serruys PW, Costa MA. New frontiers in cardiology: drug-eluting stents: Part I. Circulation. 2003;107(17):2274–9. doi: 10.1161/01.CIR.0000069330.41022.90. [DOI] [PubMed] [Google Scholar]
- 8.Forrester JS, Fishbein M, Helfant R, Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991;17(3):758–69. doi: 10.1016/s0735-1097(10)80196-2. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Schwartz D, Brogi E, Tanaka H, Clinton SK. A cascade model for restenosis. A special case of atherosclerosis progression. Circulation. 1992;86(6) Suppl:III47–III52. [PubMed] [Google Scholar]
- 10.Gray H, Williams PL, Bannister LH. Gray's anatomy: the anatomical basis of medicine and surgery. 38th ed. London: Churchill Livingstone; 1995. [Google Scholar]
- 11.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2(10):536–43. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 12.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153(6):907–17. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 14.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaldakov GN, Fiore M, Stankulov IS, Manni L, Hristova MG, Antonelli A, et al. Neurotrophin presence in human coronary atherosclerosis and metabolic syndrome: a role for NGF and BDNF in cardiovascular disease? Prog Brain Res. 2004;146:279–89. doi: 10.1016/S0079-6123(03)46018-4. [DOI] [PubMed] [Google Scholar]
- 16.Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91(11):4620–7. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 17.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32(2):268–74. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 18.Fain JN, Sacks HS, Buehrer B, Bahouth SW, Garrett E, Wolf RY, et al. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond) 2008;32(5):810–5. doi: 10.1038/sj.ijo.0803790. [DOI] [PubMed] [Google Scholar]
- 19.Aldhahi W, Hamdy O. Adipokines, inflammation, and the endothelium in diabetes. Curr Diab Rep. 2003;3(4):293–8. doi: 10.1007/s11892-003-0020-2. [DOI] [PubMed] [Google Scholar]
- 20.Funahashi T, Nakamura T, Shimomura I, Maeda K, Kuriyama H, Takahashi M, et al. Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Intern Med. 1999;38(2):202–6. doi: 10.2169/internalmedicine.38.202. [DOI] [PubMed] [Google Scholar]
- 21.Chaldakov GN, Stankulov IS, Aloe L. Subepicardial adipose tissue in human coronary atherosclerosis: another neglected phenomenon. Atherosclerosis. 2001;154(1):237–8. doi: 10.1016/s0021-9150(00)00676-6. [DOI] [PubMed] [Google Scholar]
- 22.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16(9):1057–63. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 23.Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, et al. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol. 2000;20(11):2351–8. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- 24.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, et al. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest. 1996;97(3):769–76. doi: 10.1172/JCI118476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci U S A. 1997;94(26):14483–8. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, et al. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82(7):810–8. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 27.Chaowalit N, Somers VK, Pellikka PA, Rihal CS, Lopez-Jimenez F. Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis. 2006;186(2):354–9. doi: 10.1016/j.atherosclerosis.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Iacobellis G. Imaging of visceral adipose tissue: an emerging diagnostic tool and therapeutic target. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5(4):345–53. doi: 10.2174/1568006054553408. [DOI] [PubMed] [Google Scholar]
- 29.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11(2):304–10. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 30.Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring) 2008;16(4):887–92. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- 31.Statements AD. Summary of Revisions for the 2008 Clinical Practice Recommendations. Diabetes Care. 2008;31(Suppl1):S3–S4. [Google Scholar]
- 32.Kanna B, Osorio F, Dharmarajan L. Pericardial fat mimicking pericardial effusion on two-dimensional echocardiography. Echocardiography. 2006;23(5):400–2. doi: 10.1111/j.1540-8175.2006.00229.x. [DOI] [PubMed] [Google Scholar]
- 33.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22(12):1311–9. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2(10):536–43. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 35.Schejbal V. Epicardial fatty tissue of the right ventricle--morphology, morphometry and functional significance. Pneumologie. 1989;43(9):490–9. [PubMed] [Google Scholar]