Abstract
BACKGROUND
This study aimed to determine the role and effects of baroreflexes during acute increase in blood pressure (BP) after severe and long-term infusion of morphine.
METHODS
This experimental study was conducted on male desert rats. They were assigned into 4 groups and the rats of the case group received morphine in the short and long term periods, whereas the control rats received normal saline for the same duration. Then, the rats were anesthetized, and their femoral artery and vein were cannulated for the injection of phenylephrine and naloxone, respectively. The injection of phenylephrine was performed by the device after a period of recording BP, mean arterial pressure (MAP), heart rate (HR) and baroreflex sensitivity (BRS) in order to induce acute hypertension before and after injecting naloxone. The Student t-test and analysis of variance (ANOVA) were used for statistical analysis.
RESULTS
The obtained results suggested that acute and chronic injections of morphine may cause significant reduction in systolic and diastolic arterial BP as well as the mean arterial pressure; moreover, it significantly increased the sensitivity of baroreflexes. Furthermore, the increased baroreflex sensitivity was observed after acute injection of morphine, whereas chronic morphine injection caused reduction in baroreflex sensitivity.
CONCLUSION
It seems that the details of the opiates' effects on the body including cardiovascular system depend on the type of opioids and consequently, on the type of stimulated receptor.
Keywords: Morphine, Baroreflex Sensitivity, Blood Pressure
Introduction
Long-term morphine use can impair the normal function of the central nervous system. These disruptions occur in a wide area of the brain; and cardiovascular control centers are not excluded from these changes and the function of these centers are also undergoing changes.1,2 Given the increasing prevalence of drug abuse in most societies, finding practical solutions for quitting the addiction is the subject of many common worldwide investigations. Current approaches used to treat addicts typically have not achieved much success and unfortunately, most addicts have turned to drug addiction after the quitting procedures again.3 Cardiovascular problems in addicted people are the most important factors in their deaths especially when the drug is suspended. Studies have shown that long-term consumption of morphine may change the performance of these centers. Acute morphine consumption as well as peripheral vasodilatation led to decrease in systemic blood pressure (BP). This effect was associated with inhibition of baroreceptor and respiratory depression reflexes and sometimes led to cardiac arrest and respiratory problems.4 Studies have shown that the intravenous (IV) acute injection of morphine in rabbits caused hypertension, bradycardia, and hyperglycemia.5,6 Moreover, injection of high doses of intrathecal morphine in dogs caused hypertension.7
In other studies, IV injection of dynorphin and beta-endorphins caused hypotension.8,9 The effect of hypotension along with bradycardia due to subcutaneous injection of morphine in the group of hypertensive rats can be seen more than those with normal pressure10–12. In addition to direct effects, there are other studies about the indirect effects of morphine during the quitting period. For instance, depending on the injection time of endomorphin 2, medial nucleus tractus solitarius (MNTS) can have a stimulating or inhibitory effect on baroreflex and as a result on the blood pressure.13 Another study also showed that opioids and gabaergic systems in the MNTS have an inhibitory effect on baroreflex.14,15 It is expected that endomorphins to have inhibitory effects on neurons and to increase blood pressure and heart rate.16–18 On the other handو it seems that the baroreflex sensitivity (BRS) will be decreased by activation of baroreceptor which causes the release of an opioids peptide in the brainstem and the increase of BRS, and also the activation of peripheral opioids possibly through preventing the release of norepinephrine from neurons of the heart.19 Therefore, because of existing controversies, and in order to find a solution to reduce the cardiovascular effects of morphine withdrawal syndrome, the current study was designed to determine the role and effects of baroreflexes during acute increase in BP after acute and long-term infusion of morphine.
Materials and Methods
This study had an experimental design. It was conducted on male wistar rats weighing 250±20 grams. The rats were assigned into 4 groups (ten pieces) by simple random sampling method. The first group received short-term morphine and the second group was recipient of long-term morphine while the third and the fourth group received normal saline in short and long term periods. After receiving the desired amount and during the predicted time, each group was anesthetized by intraperitoneal (IP) urethane injection with a dosage of 150 mg/100g. Then, tracheal intubation was performed. The femoral vein was cannulated in order to infuse phenylephrine and naloxone. The femoral artery was cannulated in order to record blood pressure and heart rate. After a recording period of BP, mean arterial pressure (MAP), heart rate (HR) and BRS, the injection of phenylephrine was performed to induce acute hypertension before and after injecting naloxone.
This study had an experimental design. It was conducted on male wistar rats weighing 250±20 grams. The rats were assigned into 4 groups (ten pieces) by simple random sampling method. The first group received short-term morphine and the second group was recipient of long-term morphine while the third and the fourth group received normal saline in short and long term periods. After receiving the desired amount and during the predicted time, each group was anesthetized by intraperitoneal (IP) urethane injection with a dosage of 150 mg/100g. Then, tracheal intubation was performed. The femoral vein was cannulated in order to infuse phenylephrine and naloxone. The femoral artery was cannulated in order to record blood pressure and heart rate. After a recording period of BP, mean arterial pressure (MAP), heart rate (HR) and BRS, the injection of phenylephrine was performed to induce acute hypertension before and after injecting naloxone.
The values of BP, MAP, HR and BRS were recorded before, during and after phenylephrine and naloxone injection. The BRS (ΔHR/ΔMAP) index was used to assess the performance of baroreceptors.20 In the first group, i.e., the group receiving short-term (acute) morphine, the IP morphine chloride solution was injected 3 times, 20, 40 and 60 mg per kg, respectively, at the time of testing. After each injection, changes in BP, MP, HR and BRS were measured. The mentioned factors were also studied in the sharp rise of BP and after receiving phenylephrine with the dosage of 6 µg/kg in two stages, after the injection of morphine and naloxone. In the second group, i.e., the group of long-term (chronic) morphine recipient, in 9 days IP morphine chloride solution was injected, 20 mg/kg in the first 3 days, 40 mg/kg in the second 3 days and 60 mg/kg in the third 3 days. On the tenth day BP, MAP, HR and BRS were recorded after anesthesia for comparing the changes with those of the acute group. These changes were recorded in order to assess the response to an acute increase in BP before and after the injection of phenylephrine in the phase of drug addiction before naloxone injection and in the phase of quitting, after naloxone injection.
An amount of 0.2 cc normal saline solution was injected to the third group (control group) rather than short-term injection of morphine chloride solution and the mentioned parameters were measured like the sample group. The rats injected by urethane with the dose of 150 mg/100g and were anesthetized with IP injection. After the skin incision and pushing aside the appeared fascia of skin, the femoral artery and vein were exposed and a tiny cut was created by using a pair of special scissors in an appropriate place of femoral artery under a stereomicroscope. Then, the artery was cannulated through a polyethylene tube with OD (outside diameter)=0.97 mm and the entry point of cannula into the artery was fixed with suture.
Then, the relevant cannula which was heparinized with a heparin concentration of 0.5/40 was connected to Power lab device after the bubble mark for recording HR, BP, MAP and BRS. Similarly, the femoral vein was cannulated and the respective cannula was also connected to an insulin syringe containing phenylephrine solution (6 mg/kg), for injecting to the rats. In the next step, to create the proper way to breathe during the test, the rat trachea connected to another special polyethylene pipe with an equal approximate diameter of the cannulated rat tracheal diameter. After fixing the endotracheal tube, the incision site of the artery and vein cannulation were stitched. It is worth mentioning that the rat body temperature was kept constant at 37±0.5 degrees of Celsius during all above stages. BP and HR were also recorded during the study period. Data were collected and calculated through the Power lab device and Chart 5 software by using the Student-t test and analysis of variance (ANOVA).
Results
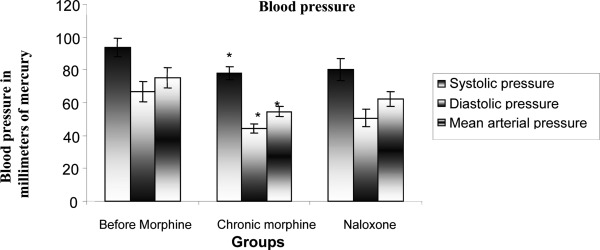
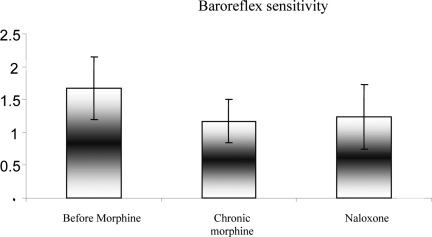
In this study, 40 rats were studied in four groups. The mean comparison of systolic and diastolic arterial pressure and mean arterial pressure during acute increase in blood pressure before receiving morphine and after receiving acute morphine and naloxone are shown in Figure 1. It was observed that the systolic and diastolic BP and MAP were decreased by receiving acute morphine and also after obtaining naloxone and the rate of decline in on all three cases were more than that in the other modes after receiving the acute morphine (P<0.05). But, the difference between systolic and diastolic BP and MAP was not statistically significant after receiving the naloxone (P>0.05). The mean BRS during increased acute BP was compared before receiving morphine and after receiving acute morphine and after receiving the naloxone in Figure 2. The figure is showing that the BRS declined after receiving the acute morphine and also obtaining the naloxone and this amount of reduction was more than that of the other mode after receiving the acute morphine but in neither of these two modes, the reduction in the mean BRS was statistically significant (P>0.05).
Figure 1.
Comparing changes in systolic and diastolic pressures and mean arterial pressure before and after morphine injection and after naloxone administration (0.3 mg/kg) in rats : P < 0.05*
Figure 2.
Comparison of changes in baroreflex sensitivity during the acute blood pressure before and after receiving morphine, after receiving chronic morphine and naloxone (0.3 mg/kg) in rats
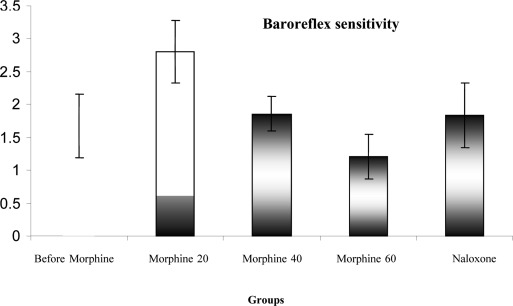
The average systolic and diastolic BP and MAP are shown during acute increase in blood pressure before receiving morphine and after obtaining acute morphine and also after receiving naloxone in Figure 3. The comparison diagram shows that systolic and diastolic arterial pressure and mean arterial pressure were decreased significantly after receiving the acute morphine compared with the case before obtaining the morphine (P <0.01). This diagram also shows that systolic and diastolic BP and MAP were increased after receiving the naloxone compared to the case before receiving the morphine but this increase was not statistically significant in the above cases (P>0.05). The comparison of mean BRS during acute BP increase is shown in Figure 4 before receiving morphine and after receiving acute morphine and also after receiving naloxone. It was observed that the BRS increased after receiving the acute morphine compared to the case before receiving the morphine (P<0.01). The figure also shows that the BRS increased after receiving the naloxone compared to the case before receiving the morphine, but this increase was not statistically significant (P>0.05).
Figure 3.
Comparison of changes in systolic and diastolic pressures and mean arterial pressure during acute increase in blood pressure before and after receiving morphine and acute morphine injection (20 mg/kg) and after naloxone administration (0.3 mg/kg) in rats
Figure 4.
Comparing changes in systolic and diastolic pressures and mean arterial pressure before and after morphine injection and after naloxone administration (0.3 mg/kg) in rats: P < 0.05*
Discussion
The results of this study showed that acute morphine injecting caused significant reduction in systolic and diastolic arterial BP and MAP, while it had not any strong effect on HR and BRS. Furthermore, the acute injection of morphine caused not only a significant reduction in systolic and diastolic arterial BP, MAP and HR, also a significant increase in BRS. Details of opioids effects on the body including the cardiovascular system are dependent on the type of opioid agent and consequently, the type of stimulated receptor. For instance, Fontana and colleagues showed that in the hypertensive patients, the endogenous opioids are responsible for high BP (including three main categories: encephalin: The Delta receptor agonist-Beta-endorphins: Mu and Kappa and Delta strong agonist receptors-dynorphin: Kappa strong receptor agonist and Mu relatively strong antagonist) by creating stimulatory effects.21 Comparison of the above results revealed that both acute and chronic morphine consumption significantly reduced the arterial pressure. Significant effect on increasing BRS was only observed in acute morphine injection cases and this effect was reverse in chronic morphine injection though, this reduction was not statistically significant.
Similarly, Lee and colleagues showed that IV acute form of morphine injection (3 mg/kg) in rabbits caused hypertension (HTN).14 In another study, the same researchers showed that ICV injection of morphine in rabbits would cause hypertension, bradycardia, hyperglycemia, respiratory depression and increased adrenaline while the IC injection of the same dose of morphine did not create significant increase in BP and adrenaline levels. IV injection of naloxone had no blocking effects on hypertension, hyperglycemia and increased catecholamine but it blocked the morphine-induced respiratory depression.15 High-dose intrathecal morphine injection caused hypertension in dogs.16 However, our results showed that the acute injection of morphine reduced the systolic and diastolic BP and MAP. The results are in line with the results of other studies which confirmed that the IV injection of dynorphin and beta-endorphins can cause hypotension.17,18 Another study on 144 ventilated neonates with severe asphyxia and severe intraventricular hemorrhage and major congenital anomalies showed no significant differences in MAP of the two groups receiving either morphine or placebo. Although the severity of hypotension in the group receiving morphine infusions was higher than that of the control group but, the use of blood volume enhancers and vasoconstrictor drugs did not establish a clear difference in the results of studied groups.22 However, intravenous injection of morphine in rats decreased HR, but had no any clear effect on arterial BP.23 We found both acute and chronic morphine injection created BP changes in order to reduce the pressure.
The involved mechanism is not properly known in BP changes following the acute and chronic injections of morphine. Many researches have been performed in this regard. For example the role of BRS in creating the cardiovascular effects induced by morphine has been investigated. The injection of endomorphin-2 which is an endogenous opioids to MNTS (cardiovascular control center in medulla oblongata and the afferent pathways entering the baroreflex network) in the rat would cause stimulatory effect on the baroreflex and creates bradycardia and decreases MAP subsequently, while if the baroreflex was stimulated prior to the injection of endomorphin-2 to MNTS, the injection of E-2 would reduce the response of baroreflex and the induced bradycardia and hypotension would be decreased. The activation of the Mu opioids receptors in MNTS would reduce the baroreflex response.24 So it seems that the chronic injection of morphine by activating the Mu opioids receptors reduced the BRS response which can justify the results of the present study. In a similar study, it was seen that opioids and gabaergic systems in the MNTS have the inhibitory effect on baroreflex25,26 whereas it is expected that endomorphins have inhibitory effects on neurons and may increase the blood pressure and heart rate.27,28 Probably the opioids effect on BRS is influenced by the type of stimulated receptor.
Endogenous and exogenous opioids regulate the performance of BRS through effects on central opioids receptors including Mu and Kappa.29 It is possible that the increase of the BRS which was observed in this study after an acute injection of morphine was due to the stimulation of Kappa and Sigma receptors and the chronic injection stimulates the Mu receptor, although the reduction of BRS in this regard was not significant. In another study, a kappa agonist increased the HR through its central and peripheral receptors but had a minimal effect on blood pressure which was the specific effect of Kappa agonist whereas the Mu agonist did not have this effect.23 It is anticipated that the rate of BRS stimulation also plays a role in the kind of impact. It appears that the activation of baroreceptor causes a release of peptide opioids in the brainstem which increases the BRS. Moreover, by activation of peripheral opioid system, the BRS may be reduced possibly by preventing the release of norepinephrine from the heart neurons.30
Conclusion
It seems that the details of opioids' effects on the body including the cardiovascular system are dependent onthe type of opioid agent and consequently, on the type of stimulated receptor. The endogenous opioids are responsible for high blood pressure (including three main categories: encephalin: The Delta receptor agonist-Beta-endorphins: Mu and Kappa and Delta strong agonist receptors-dynorphin: Kappa strong receptor agonist and Mu relatively strong antagonist) by creating stimulatory effects. Both acute and chronic morphine injection changed the blood pressure in order to decrease it properly.
Conflict of Interests
Authors have no conflict of interests.
References
- 1.Dongier M, Schwartz G. The feasibility of effective psychopharmacological treatments for alcoholism. Br J Addict. 1989;84(2):227–8. doi: 10.1111/j.1360-0443.1989.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 2.Katzung BG. 8th ed. New York: McGraw Hill Medical; 2005. Basic and clinical pharmacology. [Google Scholar]
- 3.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 4.Smart D, Lambert DG. The stimulatory effects of opioids and their possible role in the development of tolerance. Trends Pharmacol Sci. 1996;17(7):264–9. doi: 10.1016/0165-6147(96)10023-7. [DOI] [PubMed] [Google Scholar]
- 5.May CN, Ham IW, Heslop KE, Stone FA, Mathias CJ. Intravenous morphine causes hypertension, hyperglycaemia and increases sympatho-adrenal outflow in conscious rabbits. Clin Sci (Lond) 1988;75(1):71–7. doi: 10.1042/cs0750071. [DOI] [PubMed] [Google Scholar]
- 6.May CN, Whitehead CJ, Dashwood MR, Mathias CJ. Investigation of the central sites at which morphine acts to cause hypertension in conscious rabbits. Br J Pharmacol. 1989;97(3):873–81. doi: 10.1111/j.1476-5381.1989.tb12027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Cunha AF, Carter JE, Grafinger M, Montgomery H, Marks SL, Posner LP, et al. Intrathecal morphine overdose in a dog. J Am Vet Med Assoc. 2007;230(11):1665–8. doi: 10.2460/javma.230.11.1665. [DOI] [PubMed] [Google Scholar]
- 8.Xie CW, Yin LY, Xie XZ, Gao XM, Xia ZQ, Chang JK, et al. A dynorphin peptide induces hypotension by stimulating the release of atrial natriuretic peptide from rat atrium. Life Sci. 1988;42(11):1117–22. doi: 10.1016/0024-3205(88)90605-4. [DOI] [PubMed] [Google Scholar]
- 9.Catlin DH, Gorelick DA, Gerner RH. Clinical pharmacology of a-endorphin in depression and schizophrenia. In: Verebey K, editor. Opioids in mental illness: theories, clinical observations, and treatment possibilities. New York: New York Academy of Sciences; 1982. pp. 434–47. [DOI] [PubMed] [Google Scholar]
- 10.Mahinda TB, Lovell BM, Taylor BK. Morphine-induced analgesia, hypotension, and bradycardia are enhanced in hypertensive rats. Anesth Analg. 2004;98(6):1698–704. doi: 10.1213/01.ANE.0000115148.03515.56. table. [DOI] [PubMed] [Google Scholar]
- 11.Simons SH, Roofthooft DW, Van Dijk M, Van Lingen RA, Duivenvoorden HJ, Van den Anker JN, et al. Morphine in ventilated neonates: its effects on arterial blood pressure. Arch Dis Child Fetal Neonatal Ed. 2006;91(1):F46–F51. doi: 10.1136/adc.2004.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delle M, Ricksten SE, Thoren P. Renal sympathetic nerve activity during morphine abstinence in sino-aortic baroreceptor-denervated rats. Acta Physiol Scand. 1988;134(4):479–91. doi: 10.1111/j.1748-1716.1998.tb08522.x. [DOI] [PubMed] [Google Scholar]
- 13.Viard E, Sapru HN. Endomorphin-2 in the medial NTS attenuates the responses to baroreflex activation. Brain Res. 2006;1073-1074:365–73. doi: 10.1016/j.brainres.2005.12.102. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Li P. Inhibition of baroreflex following microinjection of GABA or morphine into the nucleus tractus solitarii in rabbits. J Auton Nerv Syst. 1988;25(2-3):165–72. doi: 10.1016/0165-1838(88)90021-5. [DOI] [PubMed] [Google Scholar]
- 15.Kasamatsu K, Chitravanshi VC, Sapru HN. Depressor and bradycardic responses to microinjections of endomorphin-2 into the NTS are mediated via ionotropic glutamate receptors. Am J Physiol Regul Integr Comp Physiol. 2004;287(4):R715–R728. doi: 10.1152/ajpregu.00642.2003. [DOI] [PubMed] [Google Scholar]
- 16.Dun NJ, Dun SL, Wu SY, Williams CA, Kwok EH. Endomorphins: localization, release and action on rat dorsal horn neurons. J Biomed Sci. 2000;7(3):213–20. doi: 10.1007/BF02255468. [DOI] [PubMed] [Google Scholar]
- 17.Guyenet PG, Stornetta RL, Schreihofer AM, Pelaez NM, Hayar A, Aicher S, et al. Opioid signalling in the rat rostral ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002;29(3):238–42. doi: 10.1046/j.1440-1681.2002.03636.x. [DOI] [PubMed] [Google Scholar]
- 18.Sapru HN. Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol. 2002;29(5-6):491–6. doi: 10.1046/j.1440-1681.2002.03661.x. [DOI] [PubMed] [Google Scholar]
- 19.Weksler-Zangen S, Chorev M, Weinstock M. Contrasting influences of central and peripheral opioids on cardiac baroreflex sensitivity in rabbits. J Cardiovasc Pharmacol. 1992;20(5):688–93. [PubMed] [Google Scholar]
- 20.Valenti VE, Ferreira C, Meneghini A, Ferreira M, Murad N, Ferreira Filho C, et al. Evaluation of baroreflex function in young spontaneously hypertensive rats. Arq Bras Cardiol. 2009;92(3):205–15. doi: 10.1590/s0066-782x2009000300009. [DOI] [PubMed] [Google Scholar]
- 21.Petty MA, Reid JL. The effect of opiates on arterial baroreceptor reflex function in the rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1982;319(3):206–11. doi: 10.1007/BF00495866. [DOI] [PubMed] [Google Scholar]
- 22.Schindler CW, Graczyk Z, Gilman JP, Negus SS, Bergman J, Mello NK, et al. Effects of kappa opioid agonists alone and in combination with cocaine on heart rate and blood pressure in conscious squirrel monkeys. Eur J Pharmacol. 2007;576(1-3):107–13. doi: 10.1016/j.ejphar.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto S, Liang CS. Opiate receptor inhibition improves the blunted baroreflex function in conscious dogs with right-sided congestive heart failure. Circulation. 1989;80(4):1010–5. doi: 10.1161/01.cir.80.4.1010. [DOI] [PubMed] [Google Scholar]
- 24.Himura Y, Liang CS, Imai N, Delehanty JM, Woolf PD, Hood WB. Short-term effects of naloxone on hemodynamics and baroreflex function in conscious dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 1994;23(1):194–200. doi: 10.1016/0735-1097(94)90520-7. [DOI] [PubMed] [Google Scholar]
- 25.Buccafusco JJ, Zhang LC, Shuster LC, Jonnala RR, Gattu M. Prevention of precipitated withdrawal symptoms by activating central cholinergic systems during a dependence-producing schedule of morphine in rats. Brain Res. 2000;852(1):76–83. doi: 10.1016/s0006-8993(99)02197-6. [DOI] [PubMed] [Google Scholar]
- 26.Liskow BI, Goodwin DW. Pharmacological treatment of morphine intoxication, withdrawal and dependence: A critical review. J Stud Alcohol. 1987;48:356–70. doi: 10.15288/jsa.1987.48.356. [DOI] [PubMed] [Google Scholar]
- 27.Meyer RE. Prospects for a rational pharmacotherapy of alcoholism. J Clin Psychiatry. 1989;50(11):403–12. [PubMed] [Google Scholar]
- 28.Sapru HN. Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol. 2002;29(5-6):491–6. doi: 10.1046/j.1440-1681.2002.03661.x. [DOI] [PubMed] [Google Scholar]
- 29.Thurston CL, Starnes A, Randich A. Changes in nociception, arterial blood pressure and heart rate produced by intravenous morphine in the conscious rat. Brain Res. 1993;612(1-2):70–7. doi: 10.1016/0006-8993(93)91645-9. [DOI] [PubMed] [Google Scholar]
- 30.Fontana F, Bernardi P, Spampinato S, Boschi S, De Iasio R, Grossi G. Pressor effects of endogenous opioid system during acute episodes of blood pressure increases in hypertensive patients. Hypertension. 1997;29(1 Pt 1):105–10. doi: 10.1161/01.hyp.29.1.105. [DOI] [PubMed] [Google Scholar]