Abstract
We have investigated the reproducibility of DiversiLab rep-PCR fingerprints between two laboratories with the aim of determining if the fingerprints and clustering are laboratory-specific or portable. One-hundred non-duplicate Acinetobacter baumannii isolates were used in this study. DNA isolation and rep-PCR were each performed separately in two laboratories and rep-PCR patterns generated in laboratory A were compared with those from laboratory B. Twelve A. baumannii isolates processed in laboratory A showed ≥98 % pattern similarity with the corresponding 12 isolates tested in laboratory B and were considered identical. Sixty-four isolates showed 95–97.9 % similarity with their corresponding isolates. Twenty-three isolates showed 90–94 % similarity with the corresponding isolates, while one isolate showed only 87.4 % similarity. However, intra-laboratory clustering was conserved: isolates that clustered in laboratory A also clustered in laboratory B. While clustering was conserved and reproducible at two different laboratories, demonstrating the robustness of rep-PCR, interlaboratory comparison of individual isolate fingerprints showed more variability. This comparison allows conclusions regarding clonality to be reached independent of the laboratory where the analysis is performed.
Introduction
A plethora of molecular methods are available to investigate the epidemiology of bacteria (Li et al., 2009; Singh et al., 2006). DiversiLab, which is one of those methods, is a commercial repetitive-sequence-based PCR (rep-PCR) typing system that amplifies strain-specific non-coding repetitive sequences. The system contains quality-controlled reagents in a kit format, automated detection and analysis using microfluidics with the corresponding information digitized in a software package that allows data archiving, retrieval and reporting (Healy et al., 2005). Rep-PCR libraries can therefore be easily assembled to enable, for example, the comparison of strains over time and probably across laboratories, with a view to charting the epidemiology of isolates. Outbreaks of Acinetobacter baumannii have been extensively studied using DiversiLab rep-PCR typing (Carretto et al., 2008; Fontana et al., 2008; Kohlenberg et al., 2009; Yan et al., 2010; Perez et al., 2010). Using DiversiLab, a snapshot emerged of the global epidemiology of carbapenem-resistant A. baumannii, where it was found that almost half of 492 isolates from a worldwide collection belonged to a single clonal lineage that clustered with European clone II (Higgins et al., 2010; Dijkshoorn et al., 1996). The majority of the remaining isolates grouped into seven distinct clonal clusters. Owing to their widespread distribution, these were termed worldwide clonal clusters 1–8 (WW1–8); WW1–WW3 corresponded to previously identified European clonal lineages 1–3 (Dijkshoorn et al., 1996; van Dessel et al., 2004). An in-house library representing the eight A. baumannii worldwide clusters, which is regularly used for our epidemiological investigations, is now established in Cologne.
To our knowledge, the interlaboratory reproducibility of rep-PCR patterns generated using the DiversiLab platform has not been demonstrated. In the present study, we investigated this by comparing rep-PCR fingerprints and clustering generated independently at two separate laboratories, in the hope that this knowledge will aid clonal investigations around the world.
Methods
Bacterial isolates.
One hundred non-duplicate, sporadic and epidemic A. baumannii clinical isolates were used in this study. These comprised 50 isolates each from the collections in Cologne (Germany) and Cleveland (Ohio, USA) and were numbered 1–50 (isolates supplied from Cologne) and 51–100 (isolates supplied from Cleveland) by an investigator who was unaware of the isolates’ original epidemiological characteristics (Higgins et al., 2010; Hujer et al., 2006). The isolates from Cologne were previously assigned to each of the eight worldwide (WW) clonal clusters using an in-house library (Higgins et al., 2010), and the Cleveland isolates were assigned to five clusters (Table 1).
Table 1. Cluster conservation (integrity) based on rep-PCR fingerprints generated at the two laboratories.
The table shows the number of isolates from each centre that cluster ≥95 % similarity. Clusters A–E were assigned for isolates originating from Cleveland, and WW1–8 clustering originated from Cologne.
| Cluster | Testing laboratory and no. of isolates in cluster | |
| Cleveland | Cologne | |
| A | 5 | 5 |
| B | 4 | 2 |
| C | 2 | 2 |
| D | 4 | 5 |
| E | 29* | 23 and 6* |
| Unclustered | 6 | 7 |
| WW1 | 5 | 5 |
| WW2 | 8 | 8 |
| WW3 | 5 | 5 |
| WW4 | 4 | 4 |
| WW5 | 5 | 5 |
| WW6 | 5 | 5 |
| WW7 | 6 | 5 |
| WW8 | 5 | 5 |
| Unclustered | 7 | 8 |
Twenty-nine isolates clustered together from data generated in Cleveland. These same isolates formed two clusters of 6 and 23 isolates when rep-PCR was performed in Cologne.
Rep-PCR and analysis.
Rep-PCR typing was performed using the DiversiLab Acinetobacter kit (bioMérieux). DNA isolation and rep-PCR of the whole set of isolates were each performed separately in the two laboratories from isolates grown overnight on solid medium following the manufacturer’s instructions as previously reported (Perez et al., 2010; Endimiani et al., 2009; Kohlenberg et al., 2009; Higgins et al., 2010). However, owing to problems observed in the Cologne laboratory with both DNA yield and quality using the UltraClean Microbial DNA Isolation kit (MO BIO Laboratories) that is recommended by the manufacturer, DNA isolation in Cologne was performed using the Qiagen DNeasy kit (Qiagen) whereas the Cleveland laboratory used the MO BIO kit. As part of our initial evaluation of the DiversiLab system, we compared rep-PCR fingerprints generated with the DNA template prepared using functional MO BIO and Qiagen DNA isolation kits, respectively, and found that the DNA isolation method employed had no effect on the rep-PCR patterns (data not shown). The prefixes CL- and KO- were used to denote the city where rep-PCR was performed (Cleveland and Cologne, respectively). Thus, CL-1 is the same isolate as KO-1. For this study, these are termed an ‘isolate pair’. PCR was performed in a GeneAmp PCR system 9600 (Cologne) and an MJ Research Gradient Cycler model PTC 225 (Cleveland). Rep-PCR patterns generated in laboratory A (Cologne) were compared to those from laboratory B (Cleveland). The first step was to compare individual isolate patterns generated in laboratory A with their corresponding isolate patterns generated in laboratory B, i.e. isolate vs isolate (isolate pairs). In a second step we compared the clustering obtained independently at the two laboratories, i.e. if isolates that clustered together in laboratory A were the same isolates that clustered together independently in laboratory B (cluster integrity). In a third step, we compared clustering between study sites, i.e. if isolates run in laboratory A clustered with their corresponding isolates processed in laboratory B, in effect merging results from the two laboratories. The Pearson correlation (PC) and the modified Kullback–Leibler (KL) statistical methods, which are part of the analysis software, were employed for the analysis. These calculate similarity based on the relative intensity of each band; however, PC is more band-intensity based and KL is more band-presence based.
A cluster of closely related isolates was defined as isolates sharing ≥95 % similarity, and based on previous experience, for isolates to be identical a similarity of ≥98 % was used (Saeed et al., 2006; Kohlenberg et al., 2009). This ≥95 % similarity rule was strictly enforced, and isolates that showed ≤94.9 % similarity were classified as unrelated. DiversiLab has a function termed ‘classification’ whereby rep-PCR fingerprints are compared with either a pre-loaded library, or a user-generated library, to determine if an isolate clusters with a previously defined strain-type. In the fourth step, all rep-PCR patterns generated in this study were compared to the Cologne in-house library of worldwide clonal clusters to determine their epidemiological background.
Results
One hundred rep-PCR patterns generated in laboratory A were compared with the same number of patterns from laboratory B. Although we have previously used the KL method to identify worldwide clonal clusters (Higgins et al., 2010), in the present study we employed both the KL and PC statistical methods.
Isolate pairs (step 1)
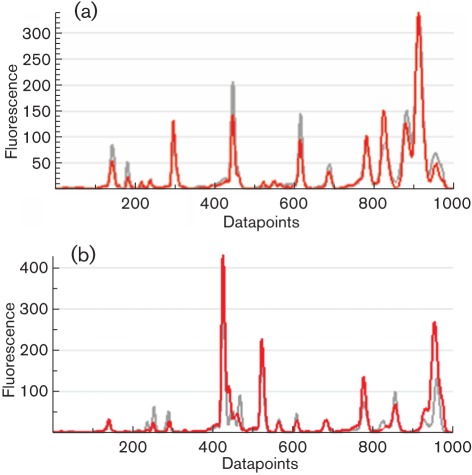
Analysis of isolate pairs demonstrated that the reproducibility of rep-PCR fingerprints was partially dependent on the statistical method employed. With the PC method, 12 isolate pairs were identical (≥98 % similarity) compared to 19 isolate pairs that were identical when analysed using the KL method (Table 2). However, the PC statistical method gave an overall greater strain-to-strain similarity, with 64 isolate pairs showing closely related fingerprint patterns versus 43 closely related patterns when the KL method was used. In addition, using the KL method, a higher incidence of isolate pairs showing 90–94.9 % similarity (32 vs 23) and ≤89.9 % similarity (6 vs 1) was observed (Table 2). Therefore few rep-PCR fingerprints were identical. Discrepancies occurred due to variability in rep-PCR fingerprints and were clearly evident as a combination of differences in band intensity and/or missing bands. For example, Fig. 1(a) shows fingerprints of isolate 17 generated at both sites. The banding patterns are nearly identical and the samples differ only in the intensity of the peaks. However, in Fig. 1(b), isolate 27 shows differences not only in band intensity but also in bands that are absent.
Table 2. Number of isolate pairs showing similarity between laboratories using the PC and KL methods.
| Percentage similarity between isolate pairs | No. of isolate pairs | Interpretation | |
| PC method | KL method | ||
| ≥98 | 12 | 19 | Identical |
| ≥95–97.9 | 64 | 43 | Closely related |
| 90–94.9 | 23 | 32 | Unrelated |
| ≤89.9 | 1 | 6 | Unrelated |
Fig. 1.
Comparison of fingerprints using the PC and KL statistical methods. (a) Comparison between CL-17 (red line) and KO-17 (grey line). By the PC method the isolates show 96.6 % similarity and by the KL method 94.9 % similarity. (b) Comparison between CL-27 (red line) and KO-27 (grey line). By the PC method the isolates show 95 % similarity and by the KL method 90.9 % similarity.
Interlaboratory clustering (step 2)
To determine if interlaboratory clustering was conserved, we compared clustering (groups of isolates showing ≥95 % similarity) generated from data in laboratory A with clustering generated in laboratory B. Table 1 summarizes cluster conservation between the two laboratories. For this comparison, rep-PCR data were separated into four separate datasets based on origin of the isolates and where the rep-PCR was performed. Each dataset therefore contained 50 rep-PCR fingerprints. We employed the PC statistical method for this analysis. Isolates that originated from Cleveland were represented by five clusters (termed A–E). Comparison of rep-PCR patterns of these strains amplified in Cleveland and Cologne showed that on the whole, clustering was conserved. For example, cluster A consisted of five isolates when tested in Cleveland, and these same isolates also clustered when rep-PCR was performed in Cologne. The only major difference was cluster E, which was not wholly conserved between laboratories: rep-PCR patterns from Cleveland had 29 isolates clustering at ≥95 % similarity but when these isolates were tested in Cologne they formed two separate clusters of 23 and 6 isolates. Rep-PCR patterns from the isolates originating from Cologne showed a similar degree of clustering (Table 1). Therefore, cluster integrity was maintained.
Merging data from two laboratories (step 3)
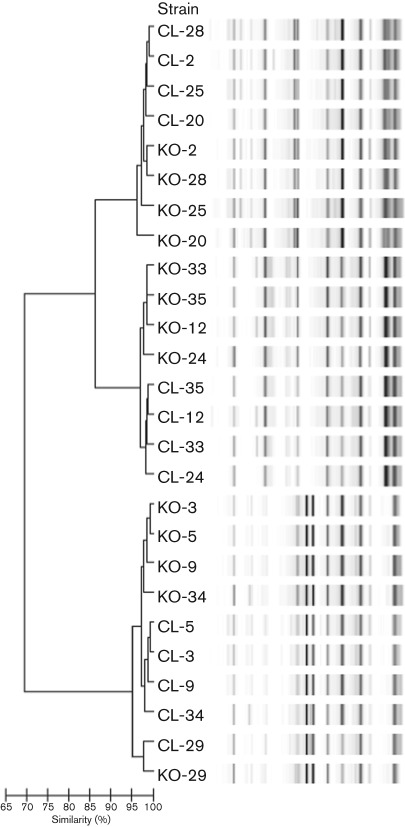
When rep-PCR fingerprints generated in both laboratories were merged, clustering was found to be partly laboratory-specific (Fig. 2). For example in Fig. 2 using the PC method of analysis, isolates CL-28, CL-2, CL-25 and CL-20 (rep-PCR fingerprints generated in Cleveland) cluster together and the corresponding fingerprints generated in Cologne are adjacent to this cluster, i.e. they are not intermingled. Taken together, these eight fingerprints still form a cluster where there is ≥95 % similarity between the samples, but the CL fingerprints show greater similarity with one another than to KO fingerprints. With few exceptions, CL clusters were adjacent to their corresponding KO clusters, irrespective of the statistical method used for analysis (Fig. 2).
Fig. 2.
Rep-PCR analysis. Dendrogram and computer-generated image of rep-PCR banding patterns showing clustering between fingerprints of corresponding isolate pairs generated in Cleveland (CL) and Cologne (KO) using the PC statistical method.
Comparison with library (step 4)
One further analysis was performed with these merged data. We used the classification report of DiversiLab with the ‘top match’ function using our in-house library of WW clusters. This revealed that 84 isolate pairs were in agreement, with both isolates clustering in the same epidemiological group with a similarity of ≥95 % using the PC method (Table 3). If this threshold was lowered to ≥93.5 % a further 11 isolate pairs clustered in the same epidemiological group. The KL method showed less agreement.
Table 3. Number of isolate pairs clustering within the same worldwide clonal cluster using the PC and KL methods in the centrally performed pattern analysis.
| Percentage similarity | No. of isolate pairs | |
| PC method | KL method | |
| ≥95 | 84 | 62 |
| 93.5–94.9 | 11 | 22 |
| ≤93.4 | 5 | 16 |
Discussion
Our results show that although DiversiLab fingerprints are fairly well conserved, they were not identical between laboratories. Discrepancies in banding patterns are most likely related to the technology employed, although factors such as DNA template concentration could also play a role. While it could be argued that differences in the DNA extraction methods may also play a part, we have previously found that there was little or no difference in rep-PCR fingerprints when we compared DNA prepared using functional Qiagen and MO BIO kits. The DiversiLab system uses a very precise protocol and standardized reagents to reduce the effect of outside influences (e.g. primer and dNTP concentration) that have the potential to affect rep-PCR fingerprints. However, amplification of PCR products is also dependent upon annealing temperature, and differences in the heating block between PCR machines may lead to higher or lower numbers of amplicons, or in some cases loss of amplicon. As part of our initial evaluation of DiversiLab we tested the effect of different PCR machines on rep-PCR patterns with A. baumannii and Staphylococcus aureus. Using the same template DNA and PCR reagents, samples were amplified in three different PCR machines and we found that for some strains, similarities were as low as 95 % (unpublished data). Therefore it is highly likely that differences in rep-PCR fingerprints reported here result primarily from the use of different PCR machines and not from other factors.
To our knowledge, studies have not been done on the interlaboratory reproducibility of DiversiLab rep-PCR typing. In a recent publication Carretto et al. (2011) found intralaboratory reproducibility to be 98.6 % when the procedure was tested in triplicate, but it was not described how their replicates were performed: three independent DNA samples, three independent rep-PCRs or three different DNA chips. Recent comparisons of DiversiLab rep-PCR typing have been made against multi-locus sequence typing, PFGE and spa-typing (Church et al., 2011; Brolund et al., 2010; Ben-Darif et al., 2010), with the authors concluding that DiversiLab rep-PCR typing is a useful tool for identifying outbreaks. However, using isolates with previously determined Salmonella enterica serotypes, Ben-Darif et al. (2010) found that 10 % of their isolates failed to cluster with the correct serotype in the DiversiLab Salmonella serotype library. This may mean that there is a small but significant problem with comparing fingerprints generated in different laboratories.
In summary, we have shown that rep-PCR clustering using DiversiLab is reproducible, demonstrating the robustness and broad applicability of the method. However, given that a small but significant proportion of isolates did not cluster when compared to their corresponding fingerprints generated in another laboratory, care should be exercised in the interpretation of every isolate. We recommend that individual rep-PCR libraries should also be generated in house to serve as reference standards for local analysis of outbreaks since centrally hosted libraries are probably not able to correctly assess all strain identities, e.g. for outbreak delineation. Despite these limitations, our data show that conclusions regarding clonal relatedness, while dependent on the statistical method used, can be reached independent of the laboratory where the analysis was performed.
Acknowledgements
The contribution of P. G. H. and H. S. was supported by a grant from the Bundesministerium für Bildung und Forschung (BMBF), Germany, Klinische Forschergruppe Infektiologie (grant number 01KI0771). This work was supported in part by the Veterans Affairs Merit Review Program (R. A. B.), the National Institutes of Health (grants AI072219-05 and AI063517-07 to R. A. B.) and the Geriatric Research Education and Clinical Center VISN 10 (R. A. B.). DiversiLab Acinetobacter kits were kindly provided by bioMérieux. Part of this work has previously been presented at the 21st ECCMID/27th ICC: Milan, Italy, 7–10 May 2011 (poster no. 1706).
Abbreviations:
- KL
Kullback–Leibler
- PC
Pearson correlation
- rep-PCR
repetitive-sequence-based polymerase chain reaction
References
- Ben-Darif E., De Pinna E., Threlfall E. J., Bolton F. J., Upton M., Fox A. J. (2010). Comparison of a semi-automated rep-PCR system and multilocus sequence typing for differentiation of Salmonella enterica isolates. J Microbiol Methods 81, 11–16 10.1016/j.mimet.2010.01.013 [DOI] [PubMed] [Google Scholar]
- Brolund A., Hæggman S., Edquist P. J., Gezelius L., Olsson-Liljequist B., Wisell K. T., Giske C. G. (2010). The DiversiLab system versus pulsed-field gel electrophoresis: characterisation of extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Methods 83, 224–230 10.1016/j.mimet.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Carretto E., Barbarini D., Farina C., Grosini A., Nicoletti P., Manso E., APSI ‘Acinetobacter Study Group’, Italy (2008). Use of the DiversiLab semiautomated repetitive-sequence-based polymerase chain reaction for epidemiologic analysis on Acinetobacter baumannii isolates in different Italian hospitals. Diagn Microbiol Infect Dis 60, 1–7 10.1016/j.diagmicrobio.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Carretto E., Barbarini D., Dijkshoorn L., van der Reijden T. J. K., Brisse S., Passet V., Farina C., APSI Acinetobacter Study Group (2011). Widespread carbapenem resistant Acinetobacter baumannii clones in Italian hospitals revealed by a multicenter study. Infect Genet Evol 11, 1319–1326 10.1016/j.meegid.2011.04.024 [DOI] [PubMed] [Google Scholar]
- Church D. L., Chow B. L., Lloyd T., Gregson D. B. (2011). Comparison of automated repetitive-sequence-based polymerase chain reaction and spa typing versus pulsed-field gel electrophoresis for molecular typing of methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis 69, 30–37 10.1016/j.diagmicrobio.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Dijkshoorn L., Aucken H., Gerner-Smidt P., Janssen P., Kaufmann M. E., Garaizar J., Ursing J., Pitt T. L. (1996). Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol 34, 1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endimiani A., Depasquale J. M., Forero S., Perez F., Hujer A. M., Roberts-Pollack D., Fiorella P. D., Pickens N., Kitchel B. & other authors (2009). Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J Antimicrob Chemother 64, 1102–1110 10.1093/jac/dkp327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana C., Favaro M., Minelli S., Bossa M. C., Testore G. P., Leonardis F., Natoli S., Favalli C. (2008). Acinetobacter baumannii in intensive care unit: a novel system to study clonal relationship among the isolates. BMC Infect Dis 8, 79. 10.1186/1471-2334-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy M., Huong J., Bittner T., Lising M., Frye S., Raza S., Schrock R., Manry J., Renwick A. & other authors (2005). Microbial DNA typing by automated repetitive-sequence-based PCR. J Clin Microbiol 43, 199–207 10.1128/JCM.43.1.199-207.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. G., Dammhayn C., Hackel M., Seifert H. (2010). Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 65, 233–238 10.1093/jac/dkp428 [DOI] [PubMed] [Google Scholar]
- Hujer K. M., Hujer A. M., Hulten E. A., Bajaksouzian S., Adams J. M., Donskey C. J., Ecker D. J., Massire C., Eshoo M. W. & other authors (2006). Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother 50, 4114–4123 10.1128/AAC.00778-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlenberg A., Brümmer S., Higgins P. G., Sohr D., Piening B. C., de Grahl C., Halle E., Rüden H., Seifert H. & other authors (2009). Outbreak of carbapenem-resistant Acinetobacter baumannii carrying the carbapenemase OXA-23 in a German university medical centre. J Med Microbiol 58, 1499–1507 10.1099/jmm.0.012302-0 [DOI] [PubMed] [Google Scholar]
- Li W. J., Raoult D., Fournier P. E. (2009). Bacterial strain typing in the genomic era. FEMS Microbiol Rev 33, 892–916 10.1111/j.1574-6976.2009.00182.x [DOI] [PubMed] [Google Scholar]
- Perez F., Endimiani A., Ray A. J., Decker B. K., Wallace C. J., Hujer K. M., Ecker D. J., Adams M. D., Toltzis P., et al. (2010). Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 65, 1807–1818 10.1093/jac/dkq191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S., Fakih M. G., Riederer K., Shah A. R., Khatib R. (2006). Interinstitutional and intrainstitutional transmission of a strain of Acinetobacter baumannii detected by molecular analysis: comparison of pulsed-field gel electrophoresis and repetitive sequence-based polymerase chain reaction. Infect Control Hosp Epidemiol 27, 981–983 10.1086/507286 [DOI] [PubMed] [Google Scholar]
- Singh A., Goering R. V., Simjee S., Foley S. L., Zervos M. J. (2006). Application of molecular techniques to the study of hospital infection. Clin Microbiol Rev 19, 512–530 10.1128/CMR.00025-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dessel H., Dijkshoorn L., van der Reijden T., Bakker N., Paauw A., van den Broek P., Verhoef J., Brisse S. (2004). Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res Microbiol 155, 105–112 10.1016/j.resmic.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Yan Z. Q., Shen D. X., Cao J. R., Chen R., Wei X., Liu L. P., Xu X. L. (2010). Susceptibility patterns and molecular epidemiology of multidrug-resistant Acinetobacter baumannii strains from three military hospitals in China. Int J Antimicrob Agents 35, 269–273 10.1016/j.ijantimicag.2009.10.016 [DOI] [PubMed] [Google Scholar]