Abstract
Purpose
Toxicity concerns have limited pelvic nodal prescriptions to doses that may be suboptimal for controlling microscopic disease. In a prospective trial, we tested whether image-guided IMRT can safely deliver escalated nodal doses while treating the prostate with hypofractionated radiotherapy in 5–1/2 weeks.
Methods and Materials
Pelvic nodal and prostatic image-guided IMRT was delivered to 53 NCCN high risk patients to a nodal dose of 56 Gy in 2 Gy fractions with concomitant treatment of the prostate to 70 Gy in 28 fractions of 2.5 Gy, and 50 of 53 patients received androgen deprivation for a median duration of 12 months.
Results
The median follow-up was 25.4 months (range 4.2–57.2). No early grade 3 (Gr3) RTOG or CTCAE v.3.0 GU or GI toxicities were seen. The cumulative actuarial incidence of Gr2 early GU toxicity (primarily alpha blocker initiation) was 38%. The rate was 32% for Gr2 early GI toxicity. None of the dose-volume descriptors correlated with GU toxicity, and only the volume of bowel receiving ≥30 Gy correlated with early GI toxicity (p=0.029). Maximum late grades 1,2 and 3 GU toxicities were seen in in 30%, 25% and 2%, respectively. Maximum late grade 1 and 2 GI toxicities were seen in 30% and 8% (rectal bleeding requiring cautery), respectively. The estimated 3-year biochemical control (nadir + 2) was 81.2 ± 6.6%. No patient manifested pelvic nodal failure, while two experienced para-aortic nodal failure outside the field. The 6 other clinical failures were distant only.
Conclusions
Pelvic IMRT nodal dose escalation to 56 Gy was delivered concurrently with 70 Gy of hypofractionated prostate radiotherapy in a convenient, resource-efficient and well-tolerated 28 fraction schedule. Pelvic nodal dose escalation may be an option in any future exploration of potential benefits of pelvic radiation therapy in high-risk prostate cancer patients.
Keywords: Pelvic Lymph Node Dose Escalation, Bowel Displacement Board, Rectal Balloon, Hypofractionated Radiation Therapy, Image-Guided Prostate IMRT
Introduction
The clinical benefit of pelvic nodal irradiation for prostate cancer has been difficult to establish and is uncertain. While progression free survival benefit has been reported in RTOG 94-13 at 5 year follow-up (1), an update of this study (2) and other other trials as well (3, 4). have either shown only trends or have failed to demonstrate benefit using doses of 45–50.4 Gy. Salvage radiation data indicate improved biochemical control with increasing doses to the 66–70 Gy range when targeting microscopic disease in the prostatic fossa (5, 6), but prescribed pelvic nodal doses have been limited by toxicity concerns. Beyond well-known difficulties in reliably selecting patients at high risk for nodal involvement, one hypothesis for such uncertain benefit from pelvic radiotherapy is that contemporary doses from 45–50.4 Gy are suboptimal for controlling microscopic disease.
Advances and techniques such as intensity modulated radiation therapy (IMRT), bowel displacement techniques (7), and bladder filling can better facilitate limiting the dose to small bowel while treating pelvic nodal basins. When using conventional fractionation for the pelvic nodes and the prostate, two separate 3D conformal or IMRT plans must be generated; however, one IMRT plan may direct the entire treatment if hypofractionated radiotherapy is delivered to the prostate gland via a simultaneous integrated boost. Early evidence also suggests that hypofractionated radiotherapy may confer some additional advantage by increasing the therapeutic ratio due to a hypothesized low α/β ratio for prostate cancer (8).
We therefore tested whether image-guided IMRT can safely deliver escalated nodal doses while treating the prostate simultaneously with a hypofractionated schedule. We present a dose-volume analysis of toxicities and preliminary biochemical control estimates in 53 high pelvic nodal risk patients treated to a nodal dose of 56 Gy in 2 Gy fractions with concomitant treatment of the prostate to 70 Gy in 2.5 Gy fractions, with the entire treatment delivered in 28 fractions.
Methods and Materials
Patients with high risk prostate adenocarcinoma with prediction of ≥ 10% pelvic lymph node involvement (9) were eligible for treatment on this phase I prospective protocol, approved by the institutional review board of the University of Wisconsin Hospital. In addition, any patient with radiographic evidence of pelvic lymph node involvement but absence of distal metastases could be enrolled on the protocol. All patients had no evidence of distal metastases on bone scan and CT of the abdomen and pelvis. Any patient with a history of pelvic radiotherapy or prior malignancy diagnosed within the past 5 years other than non-melanoma skin cancer was ineligible. Patients on full-dose anticoagulation or clopidogrel were ineligible, although patients taking aspirin could be enrolled. A total of 53 patients were enrolled.
Neoadjuvant, concurrent and adjuvant adcrogen deprivation therapy (ADT) using an LHRH agonist without concurrent anti-androgen therapy was delivered to all except 9 patients for between 6 and 24 month. Six patients refused androgen deprivation therapy or requested early termination, while 3 others received between 25 and 28 months of ADT. The resultant median duration of ADT was 12 months (Table 1).
Table 1.
Patient characteristics.
| Age | Number of Patients | |
| 49–59 | 4 | |
| 60–69 | 22 | |
| 70–80 | 27 | |
| Median Age (range) | 70 years (49 – 80) | |
| T stage | ||
| T1b | 1 | |
| T1c | 21 | |
| T2a | 9 | |
| T2b,c | 13 | |
| T3a,b | 7 | |
| T4 | 2 | |
| Gleason Score | ||
| 3 + 3 | 1 | |
| 3 + 4 and 4 + 3 | 14 | |
| 3 + 5 | 1 | |
| 4 + 4 | 12 | |
| 4 + 5 and 5 + 4 | 24 | |
| 5 + 5 | 1 | |
| PSA at Diagnosis | ||
| ≤10 | 18 | |
| 10.1 – 20 | 13 | |
| >20 | 22 | |
| Median PSA (range) | 15.4 ng/mL (1.6 – 150.2) | |
| N Stage | ||
| pN1 | 1 | |
| cN1 (radiographic) | 3 | |
| cN0 | 49 | |
| Androgen Deprivation median duration(range) | 12 months (0 – 28) | |
| Duration (mo.) | # pts | |
| 0 | 3 | |
| 3 – 4 | 3 | |
| 6–12 | 37 | |
| 15–24 | 7 | |
| 25 – 28 | 3
|
|
| 53 | ||
| Median Pretreatment IPSS Score (range) | 11 (0 – 27) | |
At simulation, patients were placed in the prone position in a bowel displacement custom mold (belly board) (7). The bladder was filled with 200 ml of diluted contrast after placement of a Foley catheter. A rectal balloon (Radiadyne™, Houston, TX) was subsequently placed to reduce prostatic motion and displace the posterior rectal wall from higher dose regions. Intravenous contrast was administered before 2.5 mm axial CT images were obtained. Linear accelerator step-and-shoot IMRT treatment plans (n=5) were created using the Pinnacle treatment-planning system (Philips Medical Systems, Andover, MA), while TomoTherapy treated patients (n=48) were planned using its associated treatment planning system (TomoTherapy, Inc., Middleton, Wisconsin).
Treatment consisted of 56 Gy delivered to pelvic lymph node basins with simultaneous integrated hypofractionated treatment to the prostate gland to 70 Gy, all delivered in 28 fractions over 5.5 weeks. The prostate was outlined on the planning CT scan, and a 5 mm expansion was added in all dimensions to generate the planning target volume (PTV), except that the posterior expansion was limited to 3 mm. The seminal vesicle bases were treated to 70 Gy, and the treating physician could elect to assign the superior seminal vesicles to either 56 Gy or 70 Gy depending on risk of involvement.
The nodal target volume was initially defined by a method of conformal avoidance described previously (10). Briefly, conformal avoidance target volumes were determined by including tissues normally included in the standard four field beam arrangement; however, small bowel, rectum, and bladder were contoured as avoidance structures. The superior border of the four-field was the L5-S1 interspace, and the inferior border was the ischial tuberosities. The AP and PA fields defined the lateral extent of the volume. Field edges were lateral to the SI joints and the obturator foramina and otherwise remained 2 cm outside the pelvic rim. The posterior border was behind the bony sacrum as inferiorly as S3 for coverage of presacral nodes, and the rectum was split below S3. The anterior block was defined by a line 1 cm anteriosuperior to an imaginary line from the sacral prominence to the superior pubic symphysis, allowing at least a 1.5 cm margin anterior to the common and external iliac vessels. The conformal avoidance nodal target volume was then contoured as the structure formed by the intersection of all four beams minus the small bowel, sigmoid colon, rectum, and bladder. Posteriorly, the contours were edited so that no volume extended more than 1 cm into the sacrum. Inferiorly, the contours were edited to not fall below the ischium. The space directly anterior to the bladder was also eliminated.
Over time, the nodal PTV was modified consistent with the Radiation Therapy Oncology Group’s consensus recommendations (11). The nodal target volume was created using vessel-based knowledge of lymph node drainage with inclusion of internal iliac, external iliac, obturator, presacral and distal common iliac nodes. The pelvic lymph node PTV began at the L5/S1 interspace at the level of the distal common iliac and proximal presacral lymph nodes. The presacral lymph nodes were included from S1 through S3, with the posterior border placed at the anterior sacrum and anterior border approximately 10 mm anterior to the anterior sacral bone. The external iliac artery and vein served as the reference for delineation of the external iliac basin, and a margin of 10 mm was allowed around the vessels to define the PTV. The external iliac PTV extended from the bifurcation of the common iliac vessels to the top of the femoral heads, which serve as the bony landmark for the inguinal ligament. The internal iliac vessels were contoured with a 10 mm expansion and both the internal and external iliac contours were connected on each slice, carving out bowel, bladder, and bone. The region between the expanded internal and external iliac vessels allowed for inclusion of the obturator nodes, and this volume was contoured to the top of the pubic symphysis. Bowel, bladder, and bone were carved out of the nodal PTV region.
Patients were followed at 1 month upon completion, every 3 months for one year post-treatment, every 4 months during years 2–3, every 6 months during years 4–5, and then annually. PSA values were obtained at each visit, along with assessments for toxicity. CT and bone scans during follow-up were not routinely performed, but only carried out in the context of rising PSA’s. Toxicity was graded per the NCI-CTC v.3.0 grading criteria Biochemical control was measured using the “nadir + 2” definition (12). Freedom from clinical failure accounted for any clinical recurrence including locoregional or distant recurrence, but death from other causes was censored. PSA failure alone was not considered an event in the actuarial analysis of freedom from clinical failure.
Early toxicities were defined as those occurring during or within 3 months of completing radiation therapy. The association between grade of early effects and dose-volume descriptors was tested using Spearman’s rank correlation coefficient. The Cox Proportional Hazards model was used to test associations between late effects and dose-volume descriptors. Two-tailed P values less than .05 were considered statistically significant. All data were examined using SPSS (version 16.0, SPSS, Inc, Chicago, IL).
Results
Between August 2004 and September 2008, a total of 53 patients were treated. Fifty patients received androgen deprivation for a median duration of 12 months (range 3–28 months), with three patients not so treated due to patient refusal. Patient characteristics are detailed in Table 1. The dosimetric parameters achieved using IMRT to spare organs at risk are detailed in Table 2. Median follow-up was 25.4 months (range 4.2–57.2 months).
Table 2.
Dosimetric parameters achieved with IMRT planning.
| Relative Dosimetric Parameter | Median % (25% Interquartile, 75% Interquartile) | Absolute Volume (cc) Dosimetric Parameter | Median cc (25% Interquartile, 75% Interquartile) |
|---|---|---|---|
| Bowel V30 | 23.9 % (42.0, 51.3) | Bowel cc ≥ 30 Gy | 372.4 cc (210.9, 610.2) |
| Bowel V40 | 20.0 % (10.5, 27.8) | Bowel cc ≥ 40 Gy | 174.6 cc (83.1, 278.4) |
| Bowel V45 | 15.0 % (6.2, 21.0) | Bowel cc ≥ 45 Gy | 116.9 cc (47.6, 219.0) |
| Bowel V50 | 9.0 % (2.2, 15.5) | Bowel cc ≥ 50 Gy | 65.1 cc (19.4, 141.3) |
| Bowel V60 | 0.5 % (0, 1.3) | Bowel cc ≥ 60 Gy | 2.4 cc (0, 13.1) |
| Rectum V50 | 25.0 % (20.5, 29.0) | Rectum cc ≥ 50 Gy | 14.9 cc (8.7, 20.6) |
| Rectum V60 | 15.0 % (12.5, 18.3) | Rectum cc ≥ 60 Gy | 9.0 cc (5.3, 11.9) |
| Rectum V70 | 7.5 % (5.8, 10.0) | Rectum cc ≥ 70 Gy | 4.5 cc (2.7, 6.9) |
| Bladder V45 | 34.0 % (30.0, 37.0) | Bladder cc ≥ 45 Gy | 89.5 cc (65.4, 109.7) |
| Bladder V60 | 14.0 % (9.9, 17.3) | Bladder cc ≥ 60 Gy | 33.6 cc (24.9, 45.4) |
| Bladder V70 | 4.0 % (2.0, 6.0) | Bladder cc ≥ 70 Gy | 9.6 cc (5.9, 15.6) |
Acute Toxicity
Acute GU toxicities were grade 0 in 6 (11%), grade 1 in 27 (51%), and grade 2 in 20 patients (38%). All grade 2 GU toxicities involved initiation of either alpha blocker or anti-cholinergic medications. Pearson and Spearman’s correlations were performed for correlation of GU toxicity with absolute volume and percent of bladder receiving ≥ 45 Gy, ≥ 60 Gy, and ≥ 70 Gy. Early GU toxicity demonstrated no statistical correlation with these dosimetric parameters. Acute GI toxicities were grade 0 in 9 (17%), grade 1 in 27 (51%), and grade 2 in 17 patients (32%). Grade 2 GI toxicities related to increased bowel frequency requiring anti-diarrheal medications. Pearson and Spearman’s correlations were performed for correlation of GI toxicity with absolute volume and percent small bowel receiving ≥ 30 Gy, ≥ 40 Gy, ≥ 45 Gy, ≥ 50 Gy, and ≥ 60 Gy as well as absolute volume and percent rectum receiving ≥ 50 Gy, ≥ 60 Gy, and ≥ 70 Gy. Early GI toxicity demonstrated statistically significant correlation only with the absolute volume of small bowel receiving ≥ 30 Gy (r=0.322, p=0.029; ρ =0.335, p=0.023). Mean weight loss during treatment was 0.6% (range 0–6.4%). No patient developed grade 3 or greater acute toxicity.
Late Toxicity
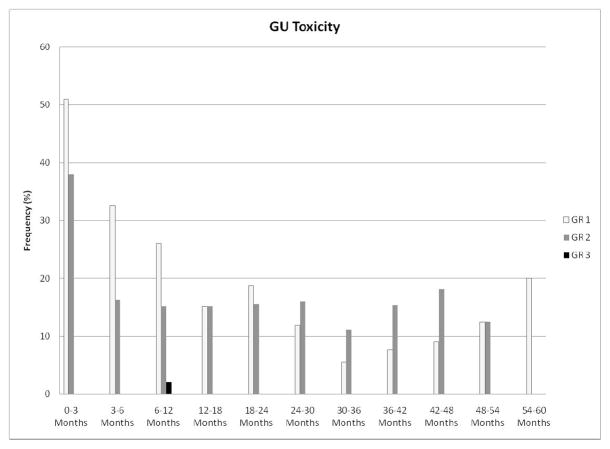
Maximum late GU toxicities were grade 0 in 23 (43%), grade 1 in 16 (30%), grade 2 in 13 (25%), and grade 3 in 1 patient (2%). The single late grade 3 GU toxicity occurred in a patient who developed urinary retention 7 months after completion of radiotherapy, requiring Foley catheter placement for 26 days with acute renal failure. His obstruction subsequently resolved with alpha-blocker maintenance, and his renal function normalized. As shown in Figure 1, the prevalence of late GU toxicity ≥ grade 2 was lower than 20% over the duration of follow-up. Maximum late GI toxicities were grade 0 in 33 (62%), grade 1 in 16 (30%), and grade 2 in 4 patients (8%). The prevalence of late GI toxicity ≥ grade 2 was lower than 10% over the duration of follow-up, as demonstrated in Figure 2. All late grade 2 GI toxicities consisted of rectal bleeding requiring no more than 2 argon laser procedures. There was no statistical correlation of the dosimetric parameters included in Table 2 with late GU or late GI toxicity.
Figure 1.
Prevalence of genitourinary toxicity following initiation of radiotherapy.
Figure 2.
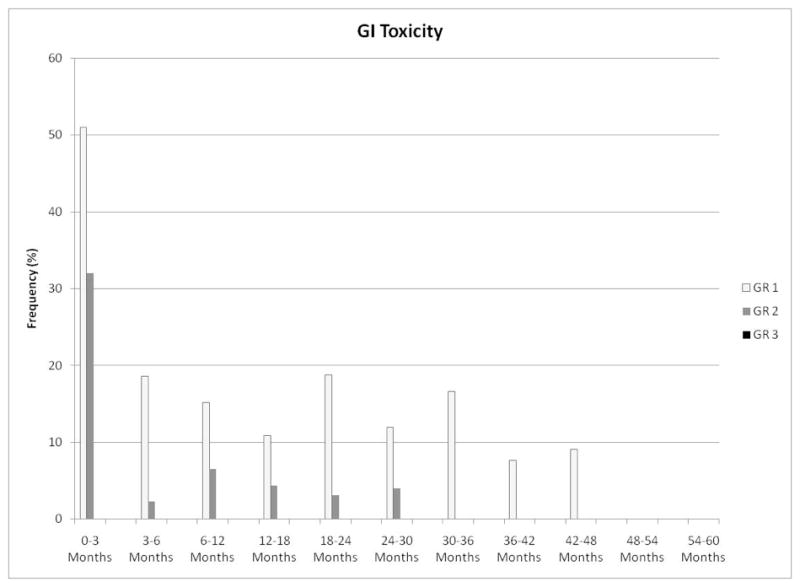
Prevalence of gastrointestinal toxicity following initiation of radiotherapy.
Patterns of Failure
The preliminary biochemical control estimate using the nadir + 2 definition was 81.2% at 3 years (95% CI: 74.6%–87.8%), as shown in Figure 3. At a median follow-up of 25.4 months, three deaths have occurred, all from other malignancies including pancreatic cancer, bladder cancer, and multiple myeloma. No patient developed pelvic nodal failure, although two patients manifested peri-aortic nodal failure outside the treatment volumes. One of the patients with peri-aortic nodal failure had pre-treatment radiographic evidence of pelvic nodal metastases (clinical stage T3b N1 M0); he manifested concurrent seminal vesicle failure in a region of initial disease but had no evidence of pelvic nodal failure. The other 3 patients with N1 disease have not clinically failed. There were no additional local failures detected either by digital rectal exam or by imaging. Six patients developed bone metastases. The estimated freedom from clinical failure at 3 years was 83.1% (95% CI: 76.7%–89.5%).
Figure 3.
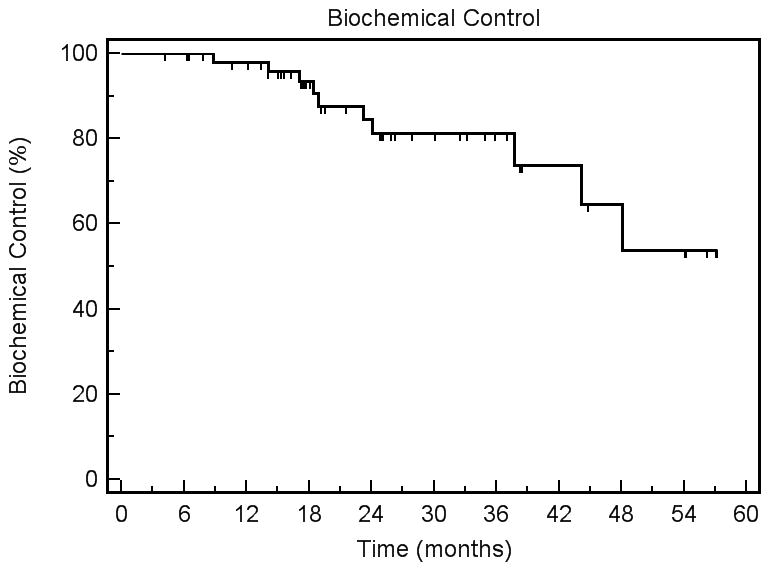
Biochemical control using the nadir + 2 definition of PSA failure.
Discussion
Pelvic nodal irradiation, delivered with various schedules of androgen deprivation therapy, has been the historic standard for locally advanced prostate cancer (13–16). The two minimum requirements for clinical benefit from pelvic radiotherapy are the selection of patients at sufficiently high risk for nodal metastatic involvement and the safe delivery of radiation doses sufficiently high to sterilize nodal metastases.
There are a number of models that estimate the risk of pelvic nodal involvement (17–21), many of which may underestimate such risk, given their reliance on nodal pathology from limited rather than extended lymph node dissections. Extended pelvic lymph node dissections have revealed that nodal involvement is often not contiguous (22, 23), whereas limited lymphadenectomy usually results in sampling of only obturator and external iliac lymph nodes. Higher rates of nodal positivity have been reported with extended lymph node dissections compared to historical standard node dissections (23, 24), leading some to recommend extended dissection including internal iliac, external iliac, obturator, and presacral regions in patients with clinical parameters that place them at higher risk (22–26). While one randomized trial failed to demonstrate increased nodal positivity when comparing extended to standard dissection, the study included largely low risk patients, and only 6.5% of all patients were found to have pathologically involved nodes (27).
Conversely, there is evidence for model overestimation as well. An analysis of the Surveillance, Epidemiology, and End Results Registry (SEER) 2004 database suggested that the Roach formula overestimates pelvic lymph node risk by a factor of 16 for patients with Roach score ≤ 10%, 7 for Roach score 10–20%, and 2.5 for Roach score > 20%. This analysis, however, was based on a population of almost exclusively cT1/2 patients who had a median of only 5 nodes surgically removed, and the overall rate of involved nodes was only 3.29% (28).
Thus, failure of randomized trials to demonstrate long-term efficacy of nodal irradiation (2–4) could relate, in some part, to the inability to select and study patients at sufficiently high risk of harboring nodal metastases. Lymphotropic nanoparticle enhanced MRI is one approach that may offer potential to better select patients at risk for nodal metastases. MRI with lymphotropic superparamagnetic nanoparticles has in one study demonstrated an overall sensitivity of 90.5% and specificity of 90.4% on a node-by-node basis for detection of nodal metastases (29). This diagnostic tool, should it ever become widely available, could, in addition to facilitating a decision regarding pelvic radiation, also help to identify patients better treated with pelvic nodal and prostate irradiation rather than prostatectomy. Specifically, Heesakkers et al. reported that 41% of patients with histologically confirmed nodes detected by MR lymphography had nodal metastases in areas outside of the region of routine pelvic lymph node dissection; additionally, 41% of patients had positive nodes both inside and outside the routine dissection area (30).
Toxicity related dose limitations may also have decreased the ability to demonstrate a clinical benefit from pelvic radiation therapy. Pelvic nodal doses of 45–46.8 Gy have not demonstrated consistent benefit in randomized trials compared with treatment to only the prostate (2–4). In most other malignant diseases, a dose of 45 Gy would be considered suboptimal to sterilize microscopic disease in the absence of chemotherapy. Head and neck squamous cell carcinomas are typically regarded as radiocurable, and the draining neck lymphatics are often treated to 54 Gy using conventional fractionation, with or without chemotherapy (31). Furthermore, there is evidence for dose response in prostate cancer not only in the definitive setting but also for presumptive microscopic disease in the post-prostatectomy setting. King et al. reported 5-year biochemical control of 25% for a prostate bed dose of 60 Gy compared to 58% for a dose of 70 Gy (p<0.0001) (6). A subsequent analysis of salvage trials reported a gain in tumor control probability per additional gray within the steep portion of the probability curve of 3.8%/Gy (5). By this measure, the increase in dose in our study from the 45–50.4 Gy range to 56 Gy could result in significantly increased rates of sterilization of microscopic nodal disease. Unknown at this point is the potential dose modifying effect that concurrent androgen deprivation therapy might have on dose response.
The escalation of nodal dose to 56 Gy in our series does not appear to increase either acute or late GU or GI toxicity when compared to preliminary toxicity reporting from a series from the University of Colorado delivering 50.4 Gy to the nodal basin concurrently with 70 Gy to the prostate in 28 fractions (33). Interestingly, the median volume of small bowel receiving ≥ 45 Gy in our study was only 116.9 cc, below the recommended limit of 195 cc from the recent QUANTEC (Quantitative Analyses of Normal Tissue Effects in the Clinic) series when the entire peritoneal potential space of bowel is included (34). Thirteen patients in our series marginally exceed the 195 cc limit (maximum 549cc), but only 2 of these 13 developed grade 2 acute GI toxicities, neither of whom subsequently experienced scorable late GI toxicity. As indicated earlier, none of the dose-volume descriptors correlated with early GU toxicity, and only the volume of bowel receiving ≥ 30 Gy correlated with early GI toxicity (p=0.029).
Potential differences in patient characteristics and the preliminary nature of the biochemical control estimates of our study preclude detailed comparisons between these results and those of other studies that involved high risk patients treated with androgen deprivation and pelvic radiotherapy. However, with a median of 12 months of androgen deprivation therapy, this study’s estimated 3 year biochemical disease free survival of 81.2% ± 6.6%, does appear consistent with the RTOG 9413 reported 4-year biochemical control rate of 70% with 4 months of neoadjuvant/concurrent androgen deprivation (2). and with the RTOG 9202 reported 5-year biochemical control rate of 66.6% with 28 months total androgen deprivation (38).
The hypofractionated regimen of 70 Gy in 28 fractions of 2.5 Gy delivered to the prostate in this present trial is familiar and potentially attractive. This is both because prostate hypofractionated radiotherapy has been hypothesized to enjoy a radiobiological advantage over conventional fractionation (8, 39), and because it is the fractionation regimen used both by Cleveland Clinic’s well tolerated and effective study of prostate only radiotherapy in 770 patients (39) and by the experimental arm of ongoing RTOG trial 0415.
Conclusion
Pelvic nodal IMRT dose escalation to 56 Gy delivered concurrently with 70 Gy of hypofractionated prostate radiotherapy in 28 fractions over 5–1/2 weeks is a resource-efficient, convenient and well-tolerated regimen that produces acceptable preliminary biochemical control rates. No in-field pelvic nodal failures have been observed to date despite 2 peri-aortic failures outside the treatment volume. While the benefits of pelvic nodal irradiation using conventional 45–50.4 Gy doses remain unproven, pelvic nodal dose escalation combined with better tools for patient selection could provide a new avenue for exploring its potential benefits.
Footnotes
Conflicts of Interest Notification: The authors have no conflicts of interest to disclose.
This work was presented at the 2010 American Radium Society Annual Meeting. Grant Support by NCI–R01CA106835
References
- 1.Roach M, 3rd, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21:1904–1911. doi: 10.1200/JCO.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Lawton CA, DeSilvio M, Roach M, 3rd, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94–13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asbell SO, Krall JM, Pilepich MV, et al. Elective pelvic irradiation in stage A2, B carcinoma of the prostate: analysis of RTOG 77–06. Int J Radiat Oncol Biol Phys. 1988;15:1307–1316. doi: 10.1016/0360-3016(88)90225-8. [DOI] [PubMed] [Google Scholar]
- 4.Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25:5366–5373. doi: 10.1200/JCO.2006.10.5171. [DOI] [PubMed] [Google Scholar]
- 5.King CR, Kapp DS. Radiotherapy after prostatectomy: is the evidence for dose escalation out there? Int J Radiat Oncol Biol Phys. 2008;71:346–350. doi: 10.1016/j.ijrobp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 6.King CR, Spiotto MT. Improved outcomes with higher doses for salvage radiotherapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2008;71:23–27. doi: 10.1016/j.ijrobp.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 7.Shanahan TG, Mehta MP, Bertelrud KL, et al. Minimization of small bowel volume within treatment fields utilizing customized “belly boards”. Int J Radiat Oncol Biol Phys. 1990;19:469–476. doi: 10.1016/0360-3016(90)90559-3. [DOI] [PubMed] [Google Scholar]
- 8.Ritter M, Forman J, Kupelian P, et al. Hypofractionation for prostate cancer. Cancer J. 2009;15:1–6. doi: 10.1097/PPO.0b013e3181976614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–848. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 10.Hong TS, Tome WA, Jaradat H, et al. Pelvic nodal dose escalation with prostate hypofractionation using conformal avoidance defined (H-CAD) intensity modulated radiation therapy. Acta Oncol. 2006;45:717–727. doi: 10.1080/02841860600781781. [DOI] [PubMed] [Google Scholar]
- 11.Lawton CA, Michalski J, El-Naqa I, et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–387. doi: 10.1016/j.ijrobp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Roach M, 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 16.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 17.Cagiannos I, Karakiewicz P, Eastham JA, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–1803. doi: 10.1097/01.ju.0000091805.98960.13. [DOI] [PubMed] [Google Scholar]
- 18.Bluestein DL, Bostwick DG, Bergstralh EJ, et al. Eliminating the need for bilateral pelvic lymphadenectomy in select patients with prostate cancer. J Urol. 1994;151:1315–1320. doi: 10.1016/s0022-5347(17)35239-4. [DOI] [PubMed] [Google Scholar]
- 19.Briganti A, Karakiewicz PI, Chun FK, et al. Percentage of positive biopsy cores can improve the ability to predict lymph node invasion in patients undergoing radical prostatectomy and extended pelvic lymph node dissection. Eur Urol. 2007;51:1573–1581. doi: 10.1016/j.eururo.2007.01.108. [DOI] [PubMed] [Google Scholar]
- 20.Briganti A, Chun FK, Salonia A, et al. Validation of a nomogram predicting the probability of lymph node invasion among patients undergoing radical prostatectomy and an extended pelvic lymphadenectomy. Eur Urol. 2006;49:1019–1026. doi: 10.1016/j.eururo.2006.01.043. discussion 1026–1017. [DOI] [PubMed] [Google Scholar]
- 21.Conrad S, Graefen M, Pichlmeier U, et al. Prospective validation of an algorithm with systematic sextant biopsy to predict pelvic lymph node metastasis in patients with clinically localized prostatic carcinoma. J Urol. 2002;167:521–525. doi: 10.1016/S0022-5347(01)69077-3. [DOI] [PubMed] [Google Scholar]
- 22.Bader P, Burkhard FC, Markwalder R, et al. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J Urol. 2002;168:514–518. doi: 10.1016/s0022-5347(05)64670-8. discussion 518. [DOI] [PubMed] [Google Scholar]
- 23.Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol. 2002;167:1681–1686. [PubMed] [Google Scholar]
- 24.Briganti A, Chun FK, Salonia A, et al. Critical assessment of ideal nodal yield at pelvic lymphadenectomy to accurately diagnose prostate cancer nodal metastasis in patients undergoing radical retropubic prostatectomy. Urology. 2007;69:147–151. doi: 10.1016/j.urology.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Golimbu M, Morales P, Al-Askari S, et al. Extended pelvic lymphadenectomy for prostatic cancer. J Urol. 1979;121:617–620. doi: 10.1016/s0022-5347(17)56906-2. [DOI] [PubMed] [Google Scholar]
- 26.Burkhard FC, Bader P, Schneider E, et al. Reliability of preoperative values to determine the need for lymphadenectomy in patients with prostate cancer and meticulous lymph node dissection. Eur Urol. 2002;42:84–90. doi: 10.1016/s0302-2838(02)00243-9. discussion 90–82. [DOI] [PubMed] [Google Scholar]
- 27.Clark T, Parekh DJ, Cookson MS, et al. Randomized prospective evaluation of extended versus limited lymph node dissection in patients with clinically localized prostate cancer. J Urol. 2003;169:145–147. doi: 10.1016/S0022-5347(05)64055-4. discussion 147–148. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen PL, Chen MH, Hoffman KE, et al. Predicting the risk of pelvic node involvement among men with prostate cancer in the contemporary era. Int J Radiat Oncol Biol Phys. 2009;74:104–109. doi: 10.1016/j.ijrobp.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 29.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 30.Heesakkers RA, Jager GJ, Hovels AM, et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology. 2009;251:408–414. doi: 10.1148/radiol.2512071018. [DOI] [PubMed] [Google Scholar]
- 31.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 32.Da Pozzo LF, Cozzarini C, Briganti A, et al. Long-term follow-up of patients with prostate cancer and nodal metastases treated by pelvic lymphadenectomy and radical prostatectomy: the positive impact of adjuvant radiotherapy. Eur Urol. 2009;55:1003–1011. doi: 10.1016/j.eururo.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 33.McCammon R, Rusthoven KE, Kavanagh B, et al. Toxicity assessment of pelvic intensity-modulated radiotherapy with hypofractionated simultaneous integrated boost to prostate for intermediate- and high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:413–420. doi: 10.1016/j.ijrobp.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 34.Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 76:S101–107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 35.Joon DL, Hasegawa M, Sikes C, et al. Supraadditive apoptotic response of R3327-G rat prostate tumors to androgen ablation and radiation. Int J Radiat Oncol Biol Phys. 1997;38:1071–1077. doi: 10.1016/s0360-3016(97)00303-9. [DOI] [PubMed] [Google Scholar]
- 36.Pollack A, Ashoori F, Sikes C, et al. The early supra-additive apoptotic response of R3327-G prostate tumors to androgen ablation and radiation is not sustained with multiple fractions. Int J Radiat Oncol Biol Phys. 2000;46:153–158. doi: 10.1016/s0360-3016(99)00387-9. [DOI] [PubMed] [Google Scholar]
- 37.Pollack A, Salem N, Ashoori F, et al. Lack of prostate cancer radiosensitization by androgen deprivation. Int J Radiat Oncol Biol Phys. 2001;51:1002–1007. doi: 10.1016/s0360-3016(01)01750-3. [DOI] [PubMed] [Google Scholar]
- 38.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92–02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 39.Kupelian PA, Willoughby TR, Reddy CA, et al. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2. 5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2007;68:1424–1430. doi: 10.1016/j.ijrobp.2007.01.067. [DOI] [PubMed] [Google Scholar]