Abstract
Introduction
Human rabies can be overlooked in places where this disease is now rare. Its diagnosis is further confused by a negative history of exposure (cryptogenic rabies), by a Guillain-Barré syndrome (GBS) type of presentation, or by symptoms indicating another diagnosis, eg, acute brachial neuritis (ABN).
Case presentation
A 19-year-old Mexican, with no past health problems, presented with a two-day history of left shoulder, arm, and chest pain. He arrived in Louisiana from Mexico five days prior to admission. Of particular importance is the absence of a history of rabies exposure and immunization. On admission, the patient had quadriparesis, areflexia, and elevated protein in the cerebrospinal fluid, prompting a diagnosis of GBS. However, emerging neurological deficits pointed towards acute encephalitis. Rabies was suspected on hospital day 11 after common causes of encephalitis (eg, arboviruses) have been excluded. The patient tested positive for rabies IgM and IgG. He died 17 days after admission. Negri bodies were detected in the patient’s brain and rabies virus antigen typing identified the vampire bat as the source of infection.
Conclusion
Rabies should be suspected in every patient with a rapidly evolving GBS-like illness—even if there is no history of exposure and no evidence of encephalitis on presentation. The patient’s ABN-like symptoms may be equivalent to the pain experienced by rabies victims near the inoculation site.
Keywords: rabies, cryptogenic, Guillain-Barré syndrome, acute brachial neuritis, vampire bat
Introduction
The control of canine rabies has greatly reduced the incidence of human rabies infection in various parts of the world, including the United States.1 In the State of Louisiana alone, the last report of human rabies was in 1953.2 The low index of suspicion for rabies in places where this disease is now rare increases the likelihood of a failure or delay in diagnosing rabies and implementing public health safety measures.
Rabies infection is easily overlooked if the infected individual has no history of animal bite or other forms of exposure to rabies (cryptogenic rabies). Nearly all cases of cryptogenic human rabies have been attributed to bat bites and most cases of human rabies acquired from a bat bite are cryptogenic. The absence of a history of bite is most likely due to the patient not recognizing, minimizing, or forgetting the bite incident.3
Most patients with rabies manifest a characteristic pattern of symptoms.4 A flu-like prodrome and pain at the inoculation site herald the onset of illness. Hydrophobia and anxiety signal the early encephalitic stage. Fever, tachycardia, salivation, perspiration and other signs of autonomic instability appear along with delirium. Bulbar dysfunction, facial palsy, ophthalmoplegia, and other cranial nerve deficits become evident as the encephalitis deepens and as the patient gradually slips into a coma. Occasionally, rabies presents as a paralytic disease5 and can be mistaken for Guillain-Barré syndrome (GBS), an acute autoimmune disorder of the peripheral nervous system.6 Moreover, the initial presentation of rabies may include symptoms that indicate another disease; eg, shoulder and arm pain and weakness may raise the suspicion of acute brachial neuritis (ABN), an inflammatory disorder affecting the brachial plexus and/or nerves in the upper extremity.7
We report a case of cryptogenic human rabies with initial manifestations that mimic GBS and ABN. The delay in suspicion of rabies in our patient resulted in the exposure of a large number of health-care workers to the virus.
Case Presentation
The patient, a 19-year-old male migrant farm worker from Michoacán, Mexico, arrived in Louisiana five days prior to admission. Two days later, after his first day of work in the sugar plantation, he felt pain on his left chest and shoulder. Thinking he was dehydrated, he drank a few glasses of water before going to bed. The next day, the pain was worse and he was taken to a hospital in Pointe Coupee, Louisiana. A cardiac cause of the pain was ruled out and the patient was transferred to University Hospital in New Orleans, Louisiana.
On admission, history was first obtained through an interpreter and later by one of the authors (JSM), a Spanish-speaking physician. The patient’s chief complaint was left shoulder pain radiating to his chest and left arm. He had no history of animal bite and he was never vaccinated against rabies. His father and brother, who arrived five days later, gave the same history. The patient was alert but anxious and distressed by pain; his left arm was tucked to his chest to avoid exacerbation of pain by movement. He had no fever and no signs of meningeal irritation. The initial examination revealed moderate weakness of the left biceps, triceps and deltoids, and a left Horner’s syndrome raising the suspicion of acute brachial neuritis (ABN). Then, in a matter of hours, he developed mild weakness of the left wrist flexors and extensors, the right biceps and triceps, and the left and right hip flexors and adductors, and he was found to be areflexic. Magnetic resonance imaging (MRI) of the brain was normal but cerebrospinal fluid (CSF) analysis showed mild lymphocytic pleocytosis (8 cells/mm3, 67% lymphocytes, 12% neutrophils), elevated protein (118 mg/dL), normal glucose, and no organisms. The patient was diagnosed with Guillain-Barré syndrome (GBS) and transferred to the intensive care unit where he received a course of intravenous immunoglobulin (IVIG).
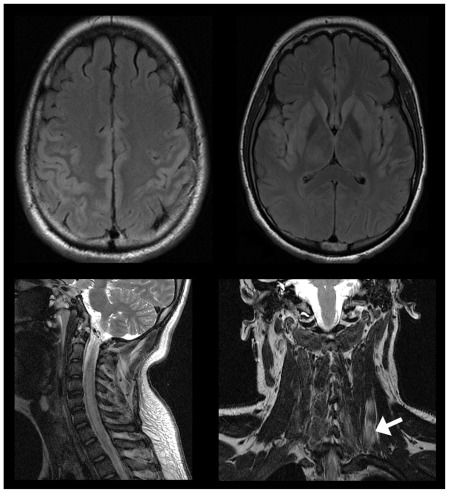
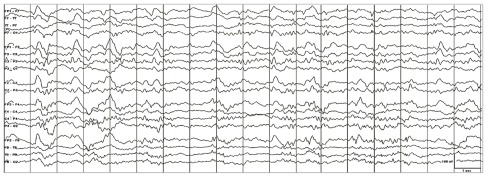
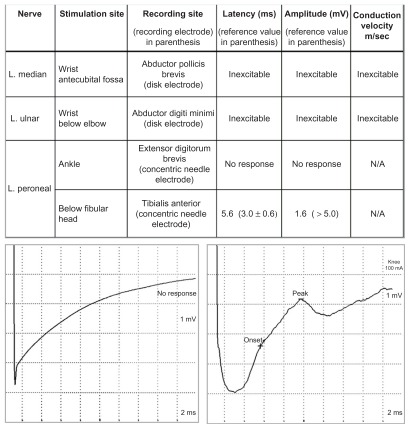
On the second hospital day, he manifested fever (temperature 38.4 C), delirium, and respiratory distress prompting intubation and initiation of acyclovir and antibiotics to cover for possible CNS infection. In less than a week, he developed dysautonomia, dysarthria and dysphagia, respiratory failure, bifacial paralysis, bilateral ophthalmoplegia, and quadriplegia. From delirium, he slipped into stupor, then from stupor into coma. Repeat MRI on the fifth hospital day showed increased signal in the brain, cervical cord, and neck muscles (Fig. 1). Electroencephalography (EEG) showed diffuse slowing and periodic discharges (Fig. 2). A second lumbar puncture revealed increasing pleocytosis (87 cells/mm3, 97% lymphocytes), increasing CSF protein (233 mg/dL), and decreasing CSF glucose (37 mg/dL). Nerve conduction studies (NCS) showed inexcitable left median and ulnar nerves: maximal stimulation of these nerves did not produce any compound muscle action potential (CMAP) or visible muscle twitch. Left peroneal nerve stimulation below the fibular head produced low- amplitude CMAPs with normal latency (Fig. 3).
Figure 1.
Magnetic resonance imaging (MRI) showing an abnormal increase in signal intensity in parts of the cerebral cortex (upper left), subcortical gray matter (upper right), and cervical cord (lower left). There is also increase in signal in the left neck muscles (lower right; arrow).
Figure 2.
Scalp electroencephalogram (EEG) showing continuous generalized slowing and periodic discharges. This abnormal pattern is seen in the EEG of patients with widespread cerebral dysfunction, often due to an underlying encephalopathic or encephalitic process.
Figure 3.
Nerve conduction studies (NCS) showing inexcitable left median and ulnar nerves with stimulation at the wrist and elbow, absence of response of the left peroneal nerve with stimulation at the ankle, and low-amplitude compound muscle action potential (CMAP) with stimulation of the left peroneal nerve below the fibular head (see table above). Only two tracings are shown: left median nerve stimulation at the wrist (left tracing) and left peroneal nerve stimulation below the fibular head (right tracing).
CSF and blood samples were sent to the laboratory to find the cause of the patient’s encephalitis. Almost all common causes of encephalitis were eliminated by the battery of tests, including arboviruses, herpes simplex virus, human immunodeficiency virus, syphilis, and Lyme disease. As the diagnosis became more and more elusive, the possibility of rabies was considered—even in the face of a negative history of animal bite. On the eleventh hospital day, the Louisiana Office of Public Health was informed of the possibility of a rabies case and infection precautions were instituted. CSF and serum specimens were sent to the Centers for Disease Control and Prevention (CDC) for testing and the patient was immunized against rabies. Both CSF and serum tested positive for rabies virus immunoglobulin G and M (IgG and IgM). The patient died seventeen days after admission.
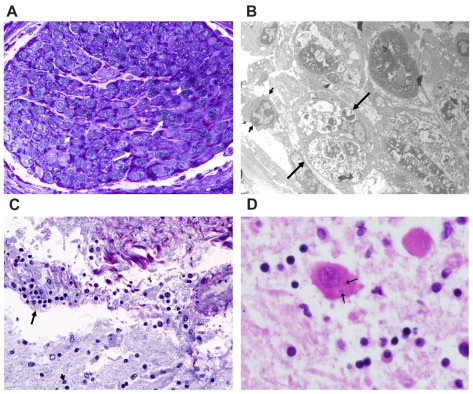
Postmortem histological examination of the left brachial plexus demonstrated loss of axons, degeneration of myelin, and an accompanying monocytic infiltrate (Fig. 4A). Electron microscopy of the nerve revealed a mixture of lymphocytes and monocytes within the nerve with some monocytes containing partially degraded myelin. There was destruction of several myelin sheaths and occasional preservation of a remnant axon. No viral particles were identified (Fig. 4B). Histological examination of the brain showed moderate lymphocytic infiltrate within the meninges with extension to the underlying cortex, indicating meningoencephalitis (Fig. 4C). Negri bodies were also detected in the cerebral cortex (Fig. 4D). Postmortem brain tissue was also analyzed by CDC. Rabies virus antigen was detected in the tissue sample and antigenic typing with monoclonal antibodies showed that the rabies virus which infected the patient is in fact a variant associated with vampire bats. This finding was further confirmed by subsequent nucleic acid amplification and sequencing.
Figure 4.
Neuropathological findings: (A) Section of the left brachial plexus demonstrating focal destruction of myelin (long arrows) and occasional monocytes (short arrows) [toluidine blue and basic fuschin, 400X]. (B) Electron micrograph of a left brachial plexus nerve showing histiocytes (monocytes) filled with phagocytised and degraded myelin (long arrows); lymphocytes are occasionally present (short arrow) [uranyl acetate and lead citrate, 3520X]. (C) Cortical meninges with moderate lymphocytic infiltrate (arrows) extending into the underlying cortex (arrowhead) [toluidine blue, basic fuschin, 400X]. (D) Neuron containing sharply demarcated round to oval eosinophilic cytoplasmic inclusions known as Negri bodies (arrows). Note scant inflammation in the background [hematoxylin and eosin, 400X].
Discussion
Three factors played a role in delaying the diagnosis of rabies in our patient: (1) a low index of suspicion for rabies in a place where the disease is now rare, (2) a negative history of animal bite or exposure to rabies, and (3) an atypical clinical presentation of the disease. Symptomatic human rabies is almost always fatal1 and delaying its diagnosis is unlikely to impact prognosis; however, a delayed diagnosis of rabies can have adverse public health consequences.2
As in most cases of cryptogenic rabies,3 our patient most likely forgot or ignored the animal bite that infected him with rabies. Using rabies virus antigen typing and nucleic acid amplification and sequencing, CDC was able to trace the source of the patient’s rabies to the vampire bat (Fig. 5).2 Vampire bats are endogenous only in Latin America, indicating that the patient acquired rabies from a vampire bat bite while he was still in Mexico.8 Although vampire bats are currently the leading cause of human rabies in Latin America, our case represents the first human rabies case in the United States with a vampire bat source.2
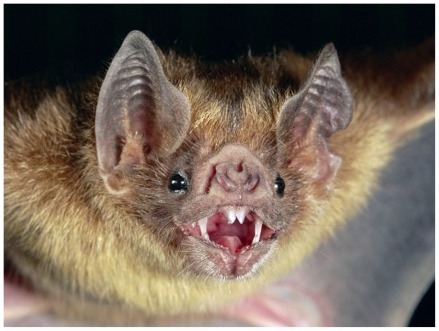
Figure 5.
The common vampire bat (Desmodus rotundus) is one of three species of hematophagous (blood-feeding) bats that are native to Mexico, Brazil, Chile, Argentina, and other places in Central and South America. The vampire bat transmits rabies to both humans and domestic livestock. It is now the leading cause of human rabies in Latin America. However, no human rabies case (before our case) has been linked to the vampire bat In the United States. Photograph by Michael and Patricia Fogden/Corbis; with permission from the National Geographic.
Acute brachial neuritis (ABN) or neuralgic amyotrophy is an idiopathic disorder affecting the brachial plexus and/or individual nerves of the upper extremity; it typically presents as acute unilateral shoulder pain followed, several days later, by flaccid paralysis of shoulder and arm muscles.7 We believe that the patient’s ABN-like symptoms are equivalent to the pain and weakness manifested by rabies victims at the inoculation site and that the patient acquired rabies infection from a bat bite on the left shoulder or neck. This hypothesis is supported by the MRI finding of signal abnormalities in the patient’s left neck muscles (Fig. 1). However, the patient’s mother told public health authorities that the patient was bitten by a bat on the left heel 15 days prior to the onset of illness.2 Based on our current understanding of rabies pathophysiology,9 the 15-day incubation period is not long enough to account for virus spread from the foot to the brain. Therefore, we maintain the hypothesis that the patient acquired rabies from a vampire bat bite on the left shoulder.
Guillain-Barré syndrome (GBS) is an acute autoimmune disorder of the peripheral nervous system. The classic form of GBS is a demyelinating polyradiculoneuritis with an ascending pattern of weakness, areflexia, and CSF protein elevation in the absence of pleocytosis.6 Rabies can have a GBS-like presentation (paralytic rabies), often with weakness beginning in the bitten extremity, but ultimately progressing to quadriparesis and bifacial weakness.5,10 Interestingly, patients who receive the rabies vaccine after a bite, but develop the disease anyway, are more likely to have a GBS-like presentation.11 GBS has also been associated with older formulations of rabies vaccine cultured in mammalian brain tissue, but not with newer formulations derived from chick embryo cells.12 A negative history of vaccination against rabies or other infections prior to the onset of illness automatically rules out vaccine-induced GBS in our patient.
The patient’s weakness, areflexia, and CSF protein elevation were initially attributed to GBS. However, instead of showing the characteristic “demyelinating” features of classic GBS, the patient’s nerve conduction studies demonstrated the presence of inexcitable motor nerves—an abnormality that indicates severe axonopathy or neuronopathy (Fig. 3). While etiologically non-specific, this finding is most consistent with paralytic rabies, in which axonal or neuronal degeneration appear to be the most plausible mechanism of peripheral nerve injury.10,13,14 However, some investigators consider demyelination15,16 or the combination of axonopathy and demyelination13,14 as the mechanism of peripheral nerve injury in paralytic rabies.
Rabies was suspected in our patient on hospital day 11 and CDC confirmed this diagnosis the next day. The public health impact of this rabies case is staggering. In Louisiana, public health officials and hospital infection control staff conducted risk assessment of 204 patient contacts and administered post-exposure prophylaxis to 95 individuals, including 68 healthcare workers. In Mexico, public health authorities conducted a vaccination campaign of cats and dogs and implemented measures to reduce the local vampire bat population in the State of Michoacán.2
Conclusion
This is the only reported case of human rabies in the State of Louisiana in the last 56 years. It is also the first case report of death from vampire bat rabies virus in the United States. More importantly, this case is a glaring example of how a low index of suspicion, a negative history of exposure, and an atypical clinical presentation can delay the diagnosis of rabies to the detriment of the public—especially the health-care workers.
Rabies should be suspected immediately in a patient with a rapidly evolving GBS-like illness – even if there is no history of exposure and no evidence of encephalitis on initial evaluation. The emergence of encephalitic signs increases the probability of rabies. The patient’s ABN-like symptoms may be equivalent to the pain experienced by rabies victims near the inoculation site. Awareness of these diagnostic pitfalls can help clinicians recognize rabies early, thereby mitigating exposure and the need for post-exposure prophylaxis.
Acknowledgements
We are grateful to all individuals and parties whose combined effort and expertise resulted in a definitive diagnosis of rabies. Dr. Deepu Thoppil, Dr. Samuel Bairu, and the University Hospital Infectious Diseases and Critical Care staff excluded common etiologies and considered rabies a possibility. The University Hospital Section of Neuroradiology provided high-quality images of the brain, cervical cord, and neck muscles. The University Hospital Pathology Department performed postmortem studies for a definitive diagnosis of rabies. The Centers for Disease Control and Prevention (CDC) performed serologic tests which confirmed the diagnosis and rabies virus antigen typing which identified the vampire bat as the source of the infection. We are also grateful to the Louisiana Office of Public Health, to the Infection Control staff of University Hospital, and to all health officials in Mexico who addressed the public health issues surrounding this case in a timely manner.
Footnotes
Consent
An informed consent could not be obtained directly from the patient because he rapidly slipped into delirium and expired in 15 days. A verbal informed consent was provided by the patient’s father and cousin for publication of this case report and accompanying images. It is considered that the individual is not identifiable and this and also public interest considerations outweigh any possible harms arising from the absence of written consent.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
ECM, JSM, LSG, RDC conceived the idea and prepared the manuscript. ECM was the consulting neurologist and JSM was the neurology resident for this case. JSM, a native Spanish speaker, was constantly in communication with the patient and the family. REA, ESP, AJS performed the electrophysiological studies and provided invaluable inputs on how to present the case from a neurological perspective. RDC performed the neuropathological studies to confirm the diagnosis of rabies.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Wunner WH, Briggs DJ. Rabies in the 21 century. PLoS Negl Trop Dis. 2010 Mar 30;4(3):e591. doi: 10.1371/journal.pntd.0000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Human rabies from exposure to a vampire bat in Mexico—Louisiana, 2010. MMWR Morb Mortal Wkly Rep. 2011 Aug 12;60(31):1050–2. [PubMed] [Google Scholar]
- 3.Gibbons RV. Cryptogenic rabies, bats, and the question of aerosol transmission. Ann Emerg Med. 2002;39(5):528–36. doi: 10.1067/mem.2002.121521. [DOI] [PubMed] [Google Scholar]
- 4.Jackson AC. Rabies. Neurol Clin. 2008;26:717–26. doi: 10.1016/j.ncl.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Chopra JS, Banerjee AK, Murthy JM, Pal SR. Paralytic rabies: a clinico-pathological study. Brain. 1980;103(4):789–802. doi: 10.1093/brain/103.4.789. [DOI] [PubMed] [Google Scholar]
- 6.Sumner AJ. The physiological basis for symptoms in Guillain-Barré syndrome. Ann Neurol. 1981;(Suppl 9):28–30. doi: 10.1002/ana.410090706. [DOI] [PubMed] [Google Scholar]
- 7.England JD, Sumner AJ. Neuralgic amyotrophy: an increasingly diverse entity. Muscle Nerve. 1987;10:60–8. doi: 10.1002/mus.880100112. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz M, Chávez CB. Rabies in Latin America. Neurol Res. 2010;32(3):272–7. doi: 10.1179/016164110X12645013284257. [DOI] [PubMed] [Google Scholar]
- 9.Rupprecht CE, Hemachudha T. Rabies. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. 3rd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004. pp. 243–59. [Google Scholar]
- 10.Hemachudha T, Wacharapluesadee S, Mitrabhakdi E, Wilde H, Morimoto K, Lewis RA. Pathophysiology of human paralytic rabies. J Neurovirol. 2005;11(1):93–100. doi: 10.1080/13550280590900409. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh JB, Roy M, Lahiri K, Bala AK, Roy M. Acute flaccid paralysis due to rabies. J Pediatr Neurosci. 2009;4(1):33–5. doi: 10.4103/1817-1745.49106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf. 2009;32:309–23. doi: 10.2165/00002018-200932040-00005. [DOI] [PubMed] [Google Scholar]
- 13.Sheikh KA, Ramos-Alvarez M, Jackson AC, Li CY, Asbury AK, Griffin JW. Overlap of pathology in paralytic rabies and axonal Guillain-Barre syndrome. Ann Neurol. 2005;57(5):768–72. doi: 10.1002/ana.20482. [DOI] [PubMed] [Google Scholar]
- 14.Hemachudha T, Laothamatas J, Rupprecht CE. Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol. 2002;1(2):101–9. doi: 10.1016/s1474-4422(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 15.Mitrabhakdi E, Shuangshoti S, Wannakrairot P, et al. Difference in neuropathogenetic mechanisms in human furious and paralytic rabies. J Neurol Sci. 2005;238(1–2):3–10. doi: 10.1016/j.jns.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Gadre G, Satishchandra P, Mahadevan A, et al. Rabies viral encephalitis: clinical determinants in diagnosis with special reference to paralytic form. J Neurol Neurosurg Psychiatry. 2010;81(7):812–20. doi: 10.1136/jnnp.2009.185504. [DOI] [PubMed] [Google Scholar]