SUMMARY
Axons damaged by acute injury, toxic insults or during neurodegenerative diseases undergo Wallerian or Wallerian-like degeneration, which is an active and orderly cellular process but the underlying mechanisms are poorly understood [1–3]. Drosophila has been proven a successful system for modeling human neurodegenerative diseases [4,5]. In this study, we established a novel in vivo model of axon injury using the adult fly wing. The wing nerve highlighted by fluorescent protein markers can be directly visualized in living animals and be precisely severed by a simple wing cut, making it highly suitable for large-scale screening. Using this model, we confirmed an axonal protective function of Wlds and Nmnat [3]. We further revealed that knockdown of endogenous Nmnat triggered spontaneous, dying-back axon degeneration in vivo. Intriguingly, axonal mitochondria were rapidly depleted upon axotomy or downregulation of Nmnat. The injury-induced mitochondrial loss was dramatically suppressed by upregulation of Nmnat, which also protected severed axons from degeneration. However, when mitochondria were genetically eliminated from axons, upregulation of Nmnat was no longer effective to suppress axon degeneration. Together, these findings demonstrate an essential role of endogenous Nmnat in maintaining axonal integrity that may rely on and function by stabilizing mitochondria.
RESULTS AND DISCUSSION
A new model of axon injury using the Drosophila wing
To define a feasible nerve tract for injury studies, we used the GAL4/UAS system to express mCD8-GFP (mGFP) to illuminate the nervous system in different parts of the adult fly. This approach revealed the wing as an intriguing and flexible system for such study (Figure 1A): (1) the fly wing is semitransparent and the wing nerve highlighted by mGFP can be directly visualized on live animals; (2) the wing nerve forms a stereotypical axon track, allowing precise and reproducible axotomy; and (3) wings are dispensable for survival, thus manipulation of genes that would otherwise be lethal can be assessed and the injury response can be followed for weeks.
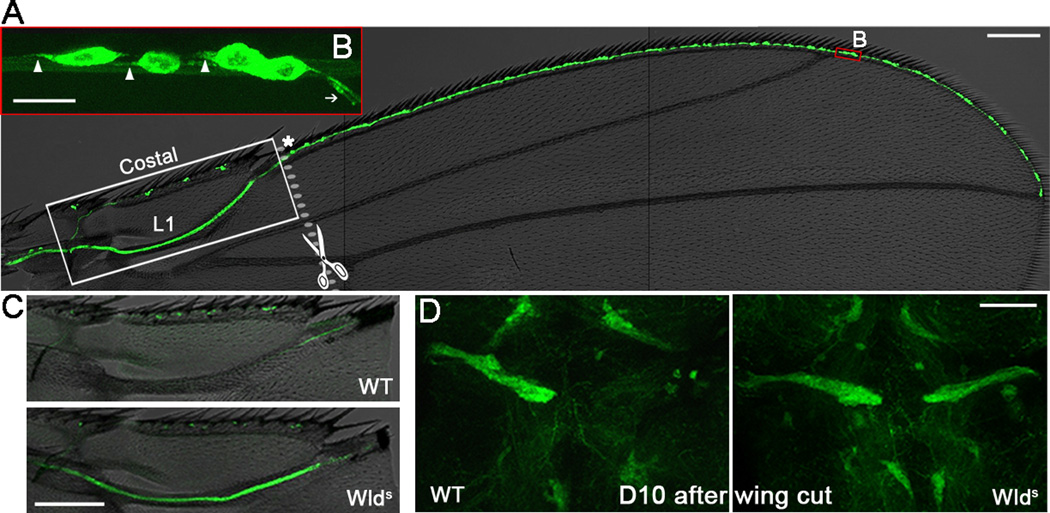
Figure 1. The Drosophila wing model of axon injury and degeneration.
(A) The nerve tract along the wing margin (L1 and costal veins) is highlighted by mGFP using a dpr-GAL4 driver. A higher magnification of the red box area is shown in (B). Arrowheads, axons; arrow, dendrites; asterisk, the last cluster of L1 neurons. (C–D) On D10 after wing cut, axonal mGFP was undetectable in the wing arch (C) and the thoracic ganglion (D) of wildtype (WT) flies but still robust in Wlds flies. Scale bar: 100 µm in (A) and (C), 10 µm in (B), and 50 µm in (D).
In order to find a wing nerve -specific GAL4 driver, we examined a large number of GAL4 driver lines and eventually found dprpGaw (dpr-GAL4). dpr, defective proboscis extension response, is expressed in a subset of chemosensory neurons of the gustatory system in the wing, leg and labellum, and is involved in the inhibitory salt response [6]. In the wing, the cell bodies of dpr+ neurons sit at the base of chemosensory bristles along the anterior wing margin (Figure 1A). They send out long axons merging into the wing nerve (Figure 1B), which projects into the thoracic ganglion of the central nervous system (CNS) (Figure 1D).
To sever the wing axons, we used handheld scissors to cut the wing (Figure S1A) at the junction site of the L1 vein and the costal vein, which is right after the last cluster of L1 neurons (Figure 1A, asterisk). A cut at this site transected all the axons of the L1 vein but left the costal vein intact as an internal control. This simple method allowed fast, precise and reproducible axotomy, making it suitable for large-scale screens. Further, no increased animal death was noted following the wing cut. Similar to removing the fly sensory organs [7], cutting the wing nerve caused axon degeneration. By 10 days after cut (D10), mGFP almost completely disappeared from the L1 wing vein (Figure 1C) and the projection in the thoracic ganglion (Figure 1D). In contrast, the uninjured costal vein retained mGFP signal, indicating that loss in the injured L1 axons was cell-autonomous and not due to a decline of mGFP expression for other reasons, such as aging.
Wallerian degeneration slow, Wlds, is a chimeric protein containing the N-terminus of an E4 ubiquitin ligase, Ube4U, and the entire protein sequence of nicotinamide mononucleotide adenylyltransferase 1 (Nmnat1) [8]. Wlds and Nmnat are known to suppress axon degeneration [7–12]. Wlds expression in the wing dramatically suppressed the injury-induced mGFP loss (Figure 1C–D, Figure S1B–C). This result confirmed that the transected wing axons indeed underwent Wallerian degeneration and that loss of mGFP was a direct consequence of axon destruction.
Characterization of injury-induced axon degeneration
We confirmed the injury-induced axonal response with additional fluorescent markers including mCD8-CherryRFP (mChRFP, Figure S2A) and a tubulin-tagged CherryRFP (data not shown). To carefully characterize the degenerative progress, we developed an evaluation system to assess the severity of degeneration (Figure S2B). Normally, the uninjured wing axons displayed a smooth, fiber-like mChRFP appearance. Upon axotomy, the injured wing nerve became massively fragmented and gradually lost the axonal mChRFP signal along the axons with time (Figure 2A). It is worth noting that, while the injured wing nerve appeared to degenerate progressively, severed olfactory axons were noted to degenerate simultaneously in the fly brain [7]. This discrepancy is likely because the transected wing nerve is sufficiently long (~500 µm) to resolve the rapid progression of axon degeneration, compared to the olfactory axons in the brain (~50 µm).
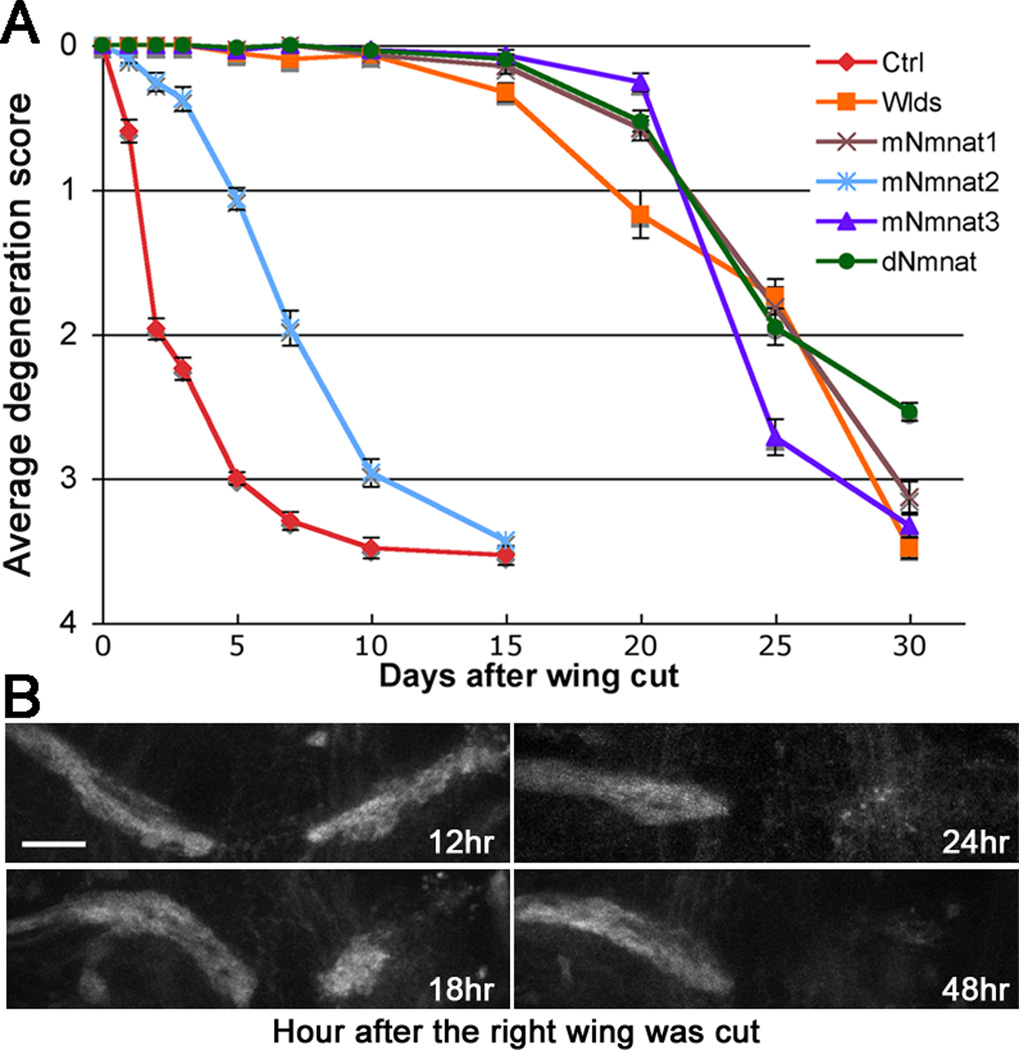
Figure 2. Time course of injury-induced axon degeneration in the wing cut model.
(A) Axon degeneration scores (see Figure S2B) of flies expressing UAS-Luciferase (Ctrl) or Wlds, mNmnats, or dNmnat at indicated time after injury. Mean ± SEM is shown, n = 22~35. (B) The thoracic projection was examined at indicated time after the right wing was cut. The left wing was uncut and its projection was used as an internal control. Scale bar: 20 µm.
We then examined the effect of Wlds, three mouse Nmnat (mNmnat) and Drosophila Nmnat (dNmnat) on axon degeneration. Consistent with previous studies [7–13], expression of Wlds, mNmnat1, mNmnat3, and Drosophila Nmnat (dNmnat) all dramatically delayed axon degeneration (p < 0.0001, Figure 2A). To our surprise, even mNmnat2, which fails to protect severed olfactory axons in flies [13] and is unprotective unless highly overexpressed (eg. 50 times more than Wlds) in mammalian cultures [14,15], significantly suppressed degeneration of the injured wing nerve (p < 0.0001, D1~D10, Figure 2A). This result demonstrates that the wing model of axon injury is not only reliably consistent, but also sufficiently sensitive such that both strong players like Wlds and less potent modifiers such as mNmnat2 can be identified.
Of significance, degeneration of the injured wing nerve not only displayed the characteristics of Wallerian degeneration such as progressive axonal fragmentation, but also exhibited a clear retrograde directionality: axonal fragmentation appeared earlier and was more prominent in the distal axon region than in the region that is proximal to the injury site (Figure S2A). In the thoracic ganglion, significant loss of the distal axon projection was seen as early as 18 hr and completed within 24~48 hr after the wing cut (Figure 2B). At D1, however, degeneration of injured axons within the wing appeared to have just started (Figure 2A, Figure S2B).
Endogenous dNmnat is required for axonal integrity
A similar retrograde pattern of axon degeneration – “dying-back” – has been noted in other neurodegenerative situations such as peripheral neuropathy [1,2]. This prompted us to consider whether, in addition to injury-induced axon degeneration, this model may be applicable to define mechanisms of axon degeneration associated with toxic and genetic disorders [1,2]. Toward this end, we first determined if it was possible to trigger spontaneous axon degeneration in uninjured wing nerve by modulating the activity of endogenous genes. Since dNmnat overexpression potently suppressed axon degeneration (Figure 2A and [7,13,16,17]) and depletion of mNmnat2 led to Wallerian-like degeneration in neurite culture [14], we hypothesized that endogenous dNmnat may be required for normal axonal maintenance.
To test this hypothesis, we generated a fly line that expressed mChRFP to highlight the axons and RNAi against endogenous dNmnat using the dpr-GAL4 driver (see Methods; for simplicity, we refer to this line as “dpr>RNAi-dNmnat” fly). The wing of dpr>RNAi-dNmnat flies had normal gross morphology (Figure 3A). The wing neurons and axons developed normally and appeared intact in young adult flies on the day of eclosion (D0, Figure 3B). Within 24 hr, however, the distal axons in the wing arch started to degenerate, evidenced by robust fragmentation of axonal mChRFP; the more proximal axons in the middle and the tip of the wing showed only minor or no axon fragmentation (D1, Figure 3B, and FigureS3A–C). During the next 24~48 hr, the wing nerve underwent rapid degeneration that proceeded retrogradely toward the proximal axons and the cell bodies (Figure 3D). By D3, massive fragmentation of mChRFP was seen throughout the entire wing nerve (Figure 3B, 3D). An inspection of the wing projection in the thoracic ganglion revealed that the distal axon ends were already undetectable in young adult flies as early as 10 hr (Figure S3D–E).
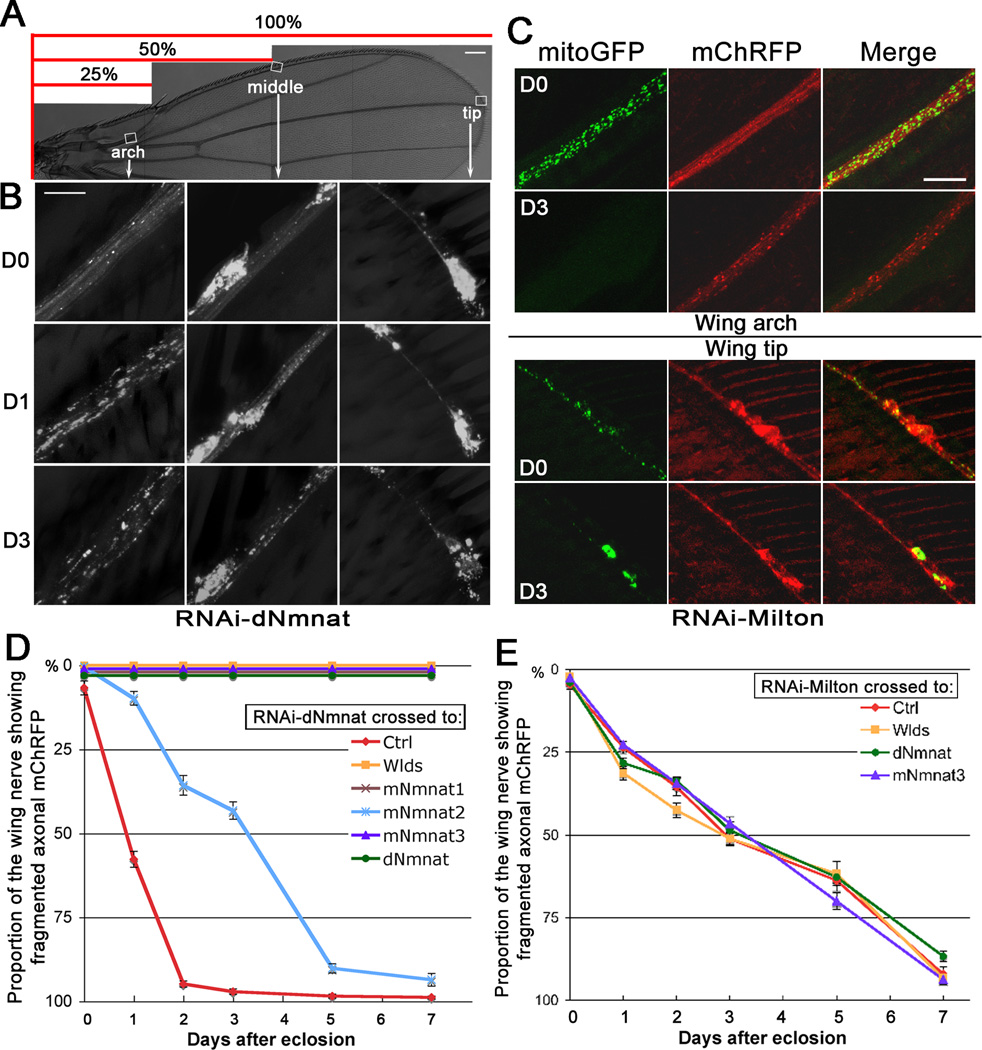
Figure 3. Knockdown of Nmnat or Milton in the wing nerve induces spontaneous axon degeneration.
(A–B) dpr>RNAi-dNmnat flies exhibited retrograde, spontaneous axon degeneration. Fragmentation of axonal mChRFP was seen earlier and more prominent in the wing arch (distal axons) than in the wing tip (proximal axons). (C) dpr>RNAi-Milton flies had normally distributed mitoGFP and smooth axonal mChRFP on D0. On D3, mitoGFP was depleted from the axons (wing arch) and retained in the cell bodies (wing tip); axonal mChRFP was massively fragmented in the wing arch but remained continuous in the wing tip. (D–E) Degeneration curves of dpr>RNAi-dNmnat (D) and dpr>RNAi-Milton (E) flies, plotted as proportion of fragmented wing nerve (see the scale in (A)). Ctrl, UAS-Luciferase. Mean ± SEM is shown, n = 14~42. Scale bar: 100 µm in (A), 10 µm in (B), and 20 µm in (C).
We confirmed that the spontaneous degeneration was indeed due to loss of dNmnat by rescuing the degeneration with upregulation of dNmnat (p < 0.0001) (Figure 3D). The rescue was not because of a titration of the GAL4 driver, as no rescue was seen when dpr>RNAi-dNmnat was crossed to a non-related UAS-Luciferase line (Ctrl, Figure 3D). Next, we determined whether Wlds and mNmnats could also rescue dpr>RNAi-dNmnat flies. As shown in Figure 3D, Wlds, mNmnat1 and mNmnat3 completely blocked this degeneration (p < 0.0001, D1~D7), while mNmnat2, although less potent, also significantly slowed the degeneration progress (p < 0.0001, D1~D5, and p < 0.05 on D7). Moreover, the extent of protection provided by the three mNmnats was similar to that of their relative potency in protecting injury-induced axon degeneration (Figure 2A). Thus, downregulation of dNmnat triggered spontaneous degeneration that was remarkably similar to axon degeneration induced by an acute injury, further suggesting a mechanistic link between the endogenous Nmnat activity and axon degeneration.
Although dNmnat mutant flies have been reported to exhibit a severe loss of the photoreceptor cell structural integrity [16], the spontaneous axon degeneration in dpr>RNAi-dNmnat flies did not appear to be simply an event following the neuronal cell death. Instead, axon degeneration occurred prior to any noticeable change in the cell bodies (Figure 3B and Figure S3). Moreover, we examined a collection of transgenic flies expressing various neurotoxic proteins in the wing model, but none of them caused spontaneous axon degeneration (data not shown).
Depletion of axonal mitochondria leads to axon degeneration that cannot be suppressed by Nmnat
To further confirm that the dying-back degeneration seen in dpr>RNAi-dNmnat flies represents an axonal mechanism but not merely a result of unhealthy neurons, we determined whether an insult limited to the axons, such as depleting axonal mitochondria by downregulating Milton, would cause spontaneous axon degeneration. Milton is a mitochondria-associated protein required for kinesin-dependent anterograde transport of mitochondria; in milton mutant flies, mitochondria were absent from axons, but were normally distributed and appeared to be functional in cell bodies [18, 19]. Hence, we generated a fly line with downregulated Milton levels in the dpr+ neurons (referred as dpr>RNAi-Milton), and used UAS-mitoGFP [20] to highlight mitochondria in the wing nerve (Movie S1).
Like dpr>RNAi-dNmnat flies, dpr>RNAi-Milton flies developed normally with fully extended wings (Figure 3C). In young adults (D0), mitoGFP was abundant in both the axons and the cell bodies. On D3, however, the mitochondria appeared to be gradually withdrawn from the axons, ending up with mitoGFP highly condensed in the neuronal cell bodies. Knockdown of Milton indeed led to spontaneous, progressive axon degeneration. This was first seen in the distal axon ends of the wing nerve projection in the thorax in adults as young as 10 hr (Figure S3F). This was followed by fragmentation of axonal mChRFP in the distal axon region (D3, wing arch, Figure 3C), which proceeded retrogradely to span the entire wing nerve (Figure 3E).
Impaired axonal transport of proteins and organelles such as mitochondria is known to be an early and perhaps causative event in many human neurodegenerative diseases [21], thus it was not entirely unexpected to see that interruption of mitochondrial transport led to axon degeneration. To our surprise, however, Wlds, mNmant3 (the mitochondrial Nmnat isoform) and dNmnat all failed to mitigate axon degeneration induced by downregulation of Milton (Figure 3E), despite that they are potent axon protectors in numerous models of neural injury and neurodegenerative diseases [1–3]. Thus, although Nmnat could delay axon degeneration caused by mitochondrial or oxidative stress [10], complete depletion of axonal mitochondria abolished axon protection of Wlds and Nmnat.
Axonal mitochondria are rapidly depleted upon nerve injury and loss of dNmnat
We noticed that axon degeneration in dpr>RNAi-Milton flies did not progress as fast as that in dpr>RNAi-dNmnat flies, possibly because the effect of loss of Milton on mitochondria and axon maintenance is indirect and thus slower. This relatively slower progression may also be the reason why axon degeneration is not noted in Milton mutant larvae, where the time window of examination is limited [18,19]. Also, molecular mechanisms controlling axon degeneration during developmental stages and in adults may be distinct [3].
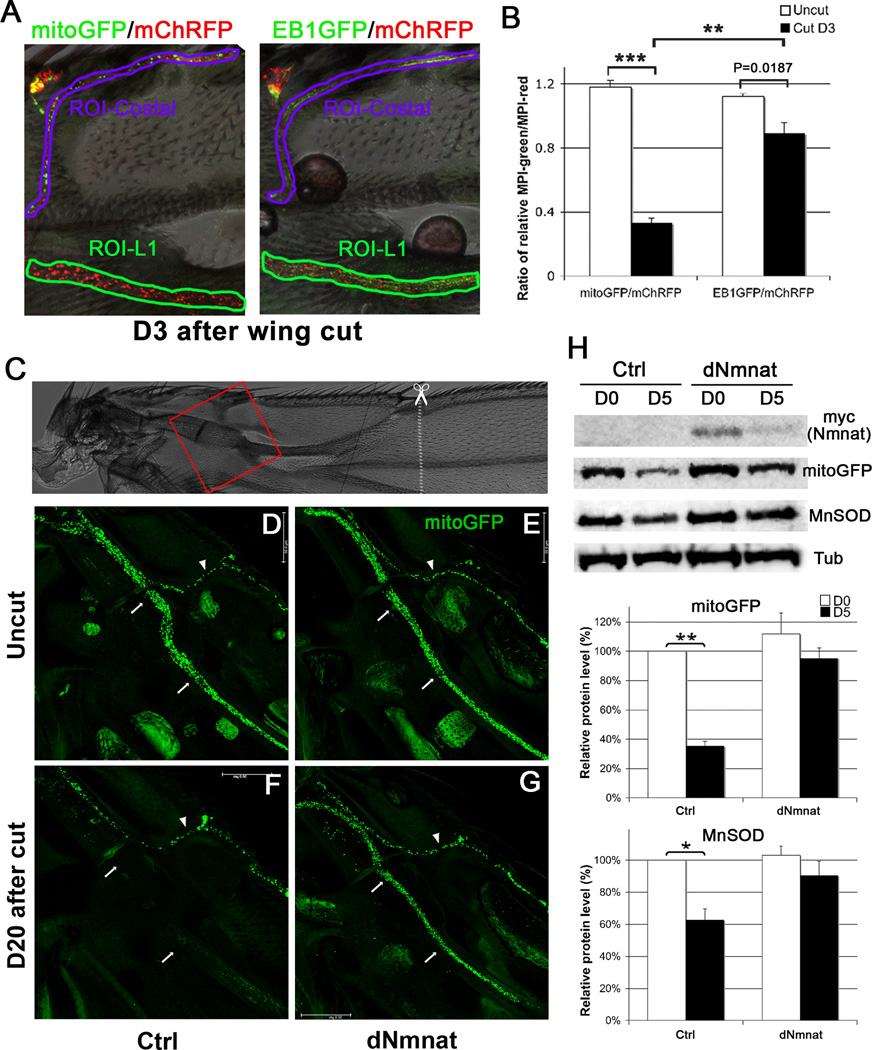
Since both wing cut and knockdown of dNmnat caused a rapid axonal response, we speculated that these insults might have a direct effect on axonal mitochondria. Intriguingly, axotomy not only disrupts axonal transport of mitochondria [22], but also induced rapid loss of mitoGFP (~77%) along with massive fragmentation of axonal mChRFP (Figure 4A, 4C). Knockdown of dNmnat also elicited a robust mitoGFP loss (~60%, Figure 4B, 4D). The rapid loss of mitoGFP was unlikely due to a simple dissociation of mitochondria from disassembled microtubules, as another microtubule-associated protein marker, EB1GFP [23], did not disappear as rapidly as mitoGFP upon axotomy (Figure S4B). Further, we confirmed axotomy-induced mitochondrial loss by immunoblots of injured wings, which showed a significant reduction of the mitochondrial specific form of superoxide dismutase (SOD), manganese SOD (MnSOD) [24]. And, upregulation of dNmnat dramatically preserved the mitoGFP signal and MnSOD in the injured wing nerve (Figure 4A, 4C and Figure S4C–H). Thus, mitochondrial loss may be an early event in response to axon injury, which can be suppressed by Nmnat overexpression
Figure 4. Mitochondria are rapidly depleted upon nerve injury or knockdown of dNmnat.
(A) Injury induced rapid mitoGFP loss and mChRFP fragmentation in the severed wing nerve, which was dramatically preserved by upregulation of dNmnat. (B) dpr>RNAi-dNmnat flies had normal mitoGFP and mChRFP appearance on D0. On D3, mitoGFP became undetectable and axonal mChRFP was massively fragmented. (C–D) Quantification of mitoGFP and mChRFP intensity in (A) and (B), respectively (also see Figure S4A). Mean ± SEM is shown, n = 6~9. * p < 0.01, *** p < 0.0001. Scale bar: 20 µm.
Nmnat is a key enzyme in NAD+ biosynthesis, involved in many metabolic redox reactions and essential for cell survival [25,26]. Although there has been controversy about the requirement of its enzymatic activity [16,17], recent studies indicate that the NAD+-synthesizing activity is crucial for protecting injured axons [12,13,15,27]. Given that mitochondria purified from Nmnat-overexpressing animals are capable of increased ATP production [11] and that Nmnat can delay axon degeneration caused by mitochondrial or oxidative stress in neurite culture [10], it is likely that Nmnat maintains normal axonal integrity and confers protection to injured axons by stabilizing mitochondrial function and/or integrity.
Several reports have pointed to a mitochondrial location of Nmnat’s axon protective function. Transgenic mice and flies expressing mNmnat3 achieve axonal protection as potent as Wlds [11,13]. The axon protection is correlated with localization of Nmnat enzymatic activity to the mitochondrial matrix [11]. The axon-targeted mNmnat1 protein is transported in coordination with the movement of mitochondria [28]. Further, activation of the mitochondrial permeability transition pore abolishes the axon protection conferred by Wlds [29]. Here we reveal that degeneration of the wing nerve caused by downregulating Milton, which prevents mitochondrial transport down the axons, was not protected by concomitant expression of Wlds or Nmnat. Since blockage of Nmnat axonal entry also abolishes its protective effect [28], it will be interesting to determine whether the inability of Nmnat to prevent axon degeneration in dpr>RNAi-Milton flies is because the arrest of mitochondria in the cell bodies also prevents axonal localization of the overexpressed Wlds and dNmnat proteins, or whether these proteins are delivered to axons but are ineffective in the absence of axonal mitochondria.
To conclude, we present a novel in vivo model for axon injury and degeneration based on the adult fly wing. Using this model, we uncovered: (1) endogenous dNmnat is required for axonal integrity; (2) axonal mitochondria are depleted rapidly upon axotomy or downregulation of dNmnat; (3) upregulation of dNmnat preserves mitochondria in injured axons and delays Wallerian degeneration; and (4) removal of mitochondria from axons abolishes the protective effect of Wlds and Nmnat. The levels of mNmnat2 rapidly decline in mammalian neurite culture upon injury [14]. Drosophila has only one gene encoding Nmnat and axon degeneration was observed as early as 18 hr after axotomy (Figure 2B), suggesting a rapid turnover of dNmnat (also see Figure S4H). Hence, reduction of Nmnat levels, either by rapid turnover of Nmnat upon axotomy or genetic knockdown of dNmnat, may render instability and/or dysfunction of mitochondria, and thus triggering axon degeneration. The self-destructive mechanisms of axon degeneration in injury and loss of endogenous Nmnat appear to converge at axonal mitochondria. This may underlie the morphological similarity between Wallerian degeneration and spontaneous axon degeneration in dying-back diseases. As such, endogenous Nmnat and axonal mitochondria may be key to identifying additional downstream events, and therefore providing exciting new targets for therapeutic interventions of both acute neural injury and chronic axonal disorders.
Supplementary Material
ACKNOWLEDGEMENTS
We thank H.J. Bellen, M. Freeman, M. Rolls, the BDSC, and the TRiP for providing fly strains, and L. Hao, S. Shieh, A. Berson, L. Yang, A. Li, Z. Chen, and N. Liu for helpful discussion and critical reading of the manuscript. N.M.B. is an investigator of the Howard Hughes Medical Institute. This work was also supported by a Fidelity Foundation grant and an NIH EUREKA award (grant 1R01NS066312) to N.M.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes four figures, one movie, and the Experimental Procedures.
REFERENCES
- 1.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 2005;11:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 2.Hilliard MA. Axonal degeneration and regeneration: a mechanistic tug-of-war. J. Neurochem. 2009;1:23–32. doi: 10.1111/j.1471-4159.2008.05754.x. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu. Rev. Neurosci. 2010:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilen J, Bonini NM. Drosophila as a model for human neurodegenerative disease. Annu. Rev. Genet. 2005:153–171. doi: 10.1146/annurev.genet.39.110304.095804. [DOI] [PubMed] [Google Scholar]
- 5.Lessing D, Bonini NM. Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nat. Rev. Genet. 2009;6:359–370. doi: 10.1038/nrg2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura M, Baldwin D, Hannaford S, Palka J, Montell C. Defective proboscis extension response (DPR), a member of the Ig superfamily required for the gustatory response to salt. J. Neurosci. 2002;9:3463–3472. doi: 10.1523/JNEUROSCI.22-09-03463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;6:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, Wagner D, Perry VH, Coleman MP. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc. Natl. Acad. Sci. U.S.A. 2000;21:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur. J. Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 10.Press C, Milbrandt J. Nmnat delays axonal degeneration caused by mitochondrial and oxidative stress. J. Neurosci. 2008;19:4861–4871. doi: 10.1523/JNEUROSCI.0525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yahata N, Yuasa S, Araki T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J. Neurosci. 2009;19:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki Y, Vohra BP, Baloh RH, Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J. Neurosci. 2009;20:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avery MA, Sheehan AE, Kerr KS, Wang J, Freeman MR. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J. Cell Biol. 2009;4:501–513. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8(1):e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan T, Feng Y, Zheng J, Ge X, Zhang Y, Wu D, Zhao J, Zhai Q. Nmnat2 delays axon degeneration in superior cervical ganglia dependent on its NAD synthesis activity. Neurochem. Int. 2010;1:101–106. doi: 10.1016/j.neuint.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhai RG, Cao Y, Hiesinger PR, Zhou Y, Mehta SQ, Schulze KL, Verstreken P, Bellen HJ. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 2006;12:e416. doi: 10.1371/journal.pbio.0040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai RG, Zhang F, Hiesinger PR, Cao Y, Haueter CM, Bellen HJ. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature. 2008;7189:887–891. doi: 10.1038/nature06721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;6:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- 19.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006;4:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell. 2006;4:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 2008:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 22.Misgeld T, Kerschensteiner M, Bareyre FM, Burgess RW, Lichtman JW. Imaging axonal transport of mitochondria in vivo Nat. Methods. 2007;7:559–561. doi: 10.1038/nmeth1055. [DOI] [PubMed] [Google Scholar]
- 23.Stone MC, Roegiers F, Rolls MM. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol. Biol. Cell. 2008;10:4122–4129. doi: 10.1091/mbc.E07-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee S, Forde R, Belton A, Duttaroy A. SOD2, the principal scavenger of mitochondrial superoxide, is dispensable for embryogenesis and imaginal tissue development but essential for adult survival. Fly. 2011;1:39–46. doi: 10.4161/fly.5.1.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau C, Niere M, Ziegler M. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front. Biosci. 2009:410–431. doi: 10.2741/3252. [DOI] [PubMed] [Google Scholar]
- 26.Jayaram HN, Kusumanchi P, Yalowitz JA. NMNAT expression and its relation to NAD metabolism. Curr. Med. Chem. 2011;13:1962–1972. doi: 10.2174/092986711795590138. [DOI] [PubMed] [Google Scholar]
- 27.Conforti L, Wilbrey A, Morreale G, Janeckova L, Beirowski B, Adalbert R, Mazzola F, Di Stefano M, Hartley R, Babetto E, et al. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J. Cell Biol. 2009;4:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babetto E, Beirowski B, Janeckova L, Brown R, Gilley J, Thomson D, Ribchester RR, Coleman MP. Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo. J. Neurosci. 2010;40:13291–13304. doi: 10.1523/JNEUROSCI.1189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrientos SA, Martinez NW, Yoo S, Jara JS, Zamorano S, Hetz C, Twiss JL, Alvarez J, Court FA. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J. Neurosci. 2011;3:966–978. doi: 10.1523/JNEUROSCI.4065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.