Abstract
Respiratory viruses (RVs) are known to be major causes of morbidity and mortality in recipients of hematopoietic stem cell transplants (HSCTs), but prospective long-term studies are lacking. We prospectively screened all adult HSCT recipients (172 allogeneic [alloHSCT] and 240 autologous [autoHSCT]) who underwent transplantation during a 4-year period (1999 to 2003) for the development of a first episode of symptomatic upper respiratory tract infections and/or lower respiratory tract infections (LRTI) by an RV. RVs studied were influenza A and B viruses (n = 39), human respiratory syncytial virus (n = 19), human adenoviruses (n = 11), human parainfluenza viruses 1 to 3 (n = 8), human enteroviruses (n = 5), human rhinoviruses (n = 3), and the recently discovered human metapneumoviruses (n = 19). During the study, 51 and 32 cases of RV symptomatic infections were identified of alloHSCT and autoHSCT recipients (2-year incidence, 29% and 14%, respectively). Risk factors for progression of upper respiratory tract infection to LRTI included severe (<0.2 × 109/L) and moderate (<0.2 × 109/L) lymphocytopenia in alloHSCT (P = .02) and autoHSCT (P = .03). Death from LRTI was attributed to an RV in 8 alloHSCT recipients. Symptomatic RV had no effect on 2-year outcomes, with the possible exception of influenza A and B virus infections in autoHSCT: these were associated with nonrelapse mortality (P = .02). In conclusion, this prospective trial allows an estimation of the minimum incidence of a first RV infection in adult HSCT recipients and identifies risk factors for acquisition of an RV infection and progression to LRTI; this should aid in the design of future studies. In addition, human metapneumovirus should be added to the potentially serious causes of RV infections in HSCT.
Keywords: Respiratory viruses, Hematopoietic stem cell transplantation, Respiratory tract infection, Prospective study
INTRODUCTION
Common respiratory viruses (RVs) have been recognized as a potential cause of severe pneumonia in recipients of hematopoietic stem cell transplants (HSCTs) [1–16]. In this patient population, these viruses cause symptomatic upper respiratory tract infections (URTI) as in the general population, but they seem to have a higher tendency to progress to severe pneumonia or lower respiratory tract infections (LRTIs), with a mortality as high as 50% to 70% [2,3,11,17–19]. Single-center and multicenter studies have analyzed the characteristics and outcome of symptomatic RV infections by human respiratory syncytial virus (HRSV), influenza A and B viruses (FLUAV and FLUBV), human parainfluenza viruses (HPIVs) 1 to 3, human rhinovirus (HRV) [20,21], human enteroviruses (HEVs) [20–23], and human adenoviruses (HAdVs). However, most studies are retrospective case series of hospitalized patients only or are small and short prospective studies in hospitalized patients [5] or outpatients [13,24]. The epidemiology of each of these viruses is unique and varies from one region to another, as does the relative proportion of each type of virus and the risk of developing pneumonia and death. The most common RVs in all series, however, are HRSV, FLUAV/FLUBV, and HPIV 1 to 3. Because all of these viruses are easily transmitted between individuals, RV infections can be rapidly spread in an HSCT inpatient unit or acquired in the community by outpatients during epidemics [3,19, 25,26]. Thus, a thorough analysis of incidence and risk factors for symptomatic RV infections requires a prospective study of all HSCT recipients during hospitalization and after discharge.
In an effort to establish the incidence, risk factors, and outcome of symptomatic RV infections in adult HSCT recipients in our region (Barcelona, Spain), we performed a prospective 4-year study of all patients with symptoms of URTI, LRTI, or both during their inpatient and outpatient follow-up. In addition to the prospective testing for FLUAV/FLUBV, HRSV, HPIV 1 to 3, HAdV, HEV, and HRV, we also retrospectively analyzed patient samples for the presence of human metapneumovirus (HMPV), a newly described member of the Paramyxoviridae family belonging to the Metapneumovirus genus. Since its initial description in 2001 [27], HMPV has been shown to be a common cause of URTI and LRTI in children [28], but their have been no prior studies in HSCT recipients.
PATIENTS AND METHODS
Patient Population and Study Design
This 4-year prospective study included all adult patients who underwent an HSCT between September 1999 and October 2003 at the Division of Hematology of the Hospital de la Santa Creu i Sant Pau, Barcelona. All such patients who had signs and symptoms of a URTI or LRTI in the inpatient and outpatient settings underwent a detailed clinical evaluation, and samples from the upper and/or lower respiratory tract were screened for RVs. After informed consent was obtained, patients with symptoms of URTI underwent a nasopharyngeal aspiration (NPA), whereas patients with LRTI (ie, pneumonia) underwent a bronchoalveolar lavage (BAL) when clinically possible. Patients with pneumonia but no signs of URTI did not undergo an NPA. A brief questionnaire, clinical examination, chest radiographs, or computed tomographic (CT) scans during the episode allowed us to classify them as URTI or LRTI. At our institution, since 1995 thoracic CT scans are always quickly performed in HSCT recipients with persistent fever or respiratory symptoms with an abnormal or normal plain chest radiograph and no identified pathogen in the sputum. All patients with LRTIs then undergo BAL, unless the patient is in respiratory failure that could be aggravated by the BAL. Patients with a positive NPA for an RV (both outpatients and inpatients) were asked to return to the clinic (or an inpatient resampling) at least weekly for further clinical and radiologic assessment and microbiological analysis until all symptoms resolved and the virus was cleared from the NPA.
Microbiological Methods
All clinical specimens were kept on ice and processed within 2 hours of sampling. Samples were processed for RVs through antigen detection by immunofluorescence (IF), viral culture, or both. For IF (direct IF for HRSV and indirect IF for the other viruses), the specimens were spotted onto glass slides and processed by standard techniques [29]. The presence of viral antigen in respiratory cells was indicated by the appearance of characteristic intracellular apple-green fluorescence in 1 or more cells. Labeled antibodies against FLUAV and FLUBV, HPIV 1 to 3, HRSV, and HAdV were used. Samples without respiratory epithelial cells were considered inconclusive. For viral culture, specimens were inoculated into each of 4 cell lines: human fibroblasts (MRC5), human epithelial cells (Hep-2 and A-549), and Madin-Darby canine kidney cells. Viral cultures were incubated for 2 weeks for RVs and 4 weeks for cytomegalovirus (CMV) on a roller drum at 35°C (A-549 and Hep-2) or in static position (MRC5 and Madin-Darby canine kidney cells). Viruses were identified on the basis of cytopathic effects in cell cultures and confirmed by staining with fluorescein-conjugated monoclonal antibodies [29,30]. BAL samples were also processed for routine aerobic, anaerobic, mycobacterial, parasitic, and fungal identification techniques. Both viral cultures and IF were applied for FLUAV/FLUBV, HRSV, HAdV, and HPIV 1 to 3 in all samples. The picornaviruses (HRV and HEV) were studied by viral culture, and the type of HEV was later identified, as previously described [31]. All these techniques were performed in our virology laboratory on a daily basis.
In addition, duplicate specimen aliquots (approximately 100 µL) from all BAL and NPA samples were collected in a NucliSens Extractor (BioMérieux, Barcelona, Spain) to preserve RNA and stored at −70°C. Specimen aliquots were later shipped to a metapneumovirus (MPV) reference laboratory (Vanderbilt University, Nashville, TN) and analyzed by reverse-transcription polymerase chain reaction (RT-PCR) and gene sequencing for MPV, as previously described in detail [28]. In brief, RT-PCRs were performed in duplicate by using the OneStep RT-PCR kit (Qiagen, Valencia, CA). Primers amplified a 170–base pair fragment of the L (polymerase) gene that is highly conserved among HMPV isolates [27,28]. Products were gel-purified and cloned into a commercial plasmid vector (Promega, Madison, WI). The nucleotide sequences of both complementary DNA strands of the insert were determined on an ABI 377 Prism instrument in the Vanderbilt DNA Sequencing Core. Samples were considered positive if they had a unique sequence or were positive in both PCR reactions. Sequences were aligned to each other and to published HMPV sequences by using ClustalW alignment in MacVector (Accelrys, San Diego, CA).
Definitions
There are no widely agreed and consistently used definitions of the signs and symptoms required for defining the presence of a URTI or LRTI in this patient population. Thus, a variety of definitions have been used in clinical studies, but none of these definitions has been assessed in detail for its reliability and validity. Our definitions were adapted from definitions for symptomatic respiratory tract infections in chronic-care facilities [32] and from our previous experience with a clinical-based definition [1,33].
To precisely classify all episodes of symptomatic respiratory tract infections, all patients who developed respiratory symptoms (as defined below) and who consented to enter the study filled out daily symptom diaries with the help of experienced nursing or medical staff. To exclude allergic reactions, respiratory symptoms had to be present for at least 2 days for patients to enter the study.
A URTI was defined as the presence of 2 or more of the following symptoms: rhinorrhea and sneezing, nasal/sinus congestion, otitis media, pharyngitis, or dry cough (similar to the common cold or pharyngolaryngitis syndrome [32]), with a normal chest examination and chest roentgenogram. Criteria for LRTI (or pneumonia) included (1) new-onset coughing, with or without widespread fine crepitation, rhonchi, coarse rales, or wheezes on lung auscultation; (2) a new pulmonary infiltrate (defined by a plain chest radiograph, high-resolution CT, or both); and (3) isolation of the RV(s) involved in a BAL or lung biopsy. The presence of a copathogen was defined as the isolation of the RV in addition to pathogenic bacterial species, fungal species (such as Aspergillus species), or other opportunistic viruses, especially CMV.
A first episode of infection was defined as the period during which the patient had symptoms of URTI, LRTI, or both, whether or not an RV was isolated. A further episode of infection required the presence of a symptom-free period and negativization of the NPA for any RVs isolated during the previous episode. This study, however, focuses only on a first episode of RV infection per patient (unless otherwise specified), as described later in detail. A mixed viral infection was defined by the isolation of more than 1 RV during the same episode and samples. An inpatient (nosocomial) RV infection was considered when a patient had been in the hospital for at least 3 days and symptoms of the infection developed during hospitalization. Death from pneumonia was defined as death as a result of respiratory failure during the episode of LRTI. Progression of a URTI to an LRTI was defined as the onset of pneumonia in patients with a prior or concurrent URTI, whereas patients with pneumonia without a URTI were considered as having an isolated LRTI. Lymphocyte counts of <0.5 × 109/L and 0.2 × 109/L were defined as moderate and severe lymphocytopenia, respectively.
Infection-Control Measures and Therapy of RV
Recommended infection-control measures were routinely used to avoid transmission of RVs from patient to patient and patient to hospital staff and vice versa [34]. At our unit, all rooms are equipped with positive-pressure ventilation in sealed rooms, with an anteroom for handwashing and change of protective clothing. High-efficiency particulate air filtration is incorporated. Airflow is strictly monitored to ensure that the pressure differentials are maintained, and at least 12 air changes per hour are performed. Microbiological sampling is regularly performed to detect relevant pathogens in the air.
All health care workers, patients, and visitors with symptoms of URTI were required to avoid access to inpatient wards or the outpatient clinics. Symptomatic inpatients and outpatients underwent virologic testing of NPA, as discussed previously. Inpatients with an RV infection were placed in reverse-isolation individual rooms until they were both asymptomatic and negative for RVs. Isolation rooms were equipped with dedicated stethoscopes and other equipment, and visitors and health care workers were required to wear gowns and gloves and to wash their hands before and after all routine patient contact. However, no systematic measures were taken to avoid outpatient RV infections during community outbreaks. Yearly influenza vaccination of patients, hospital staff, and family members were strongly recommended, according to established criteria [11].
All patients with LRTI by HRSV and HPIV 1 to 3 received inhaled ribavirin (6 g/d for 5 to 7 days) with a small particle aerosol generator. For URTI and LRTI by FLUAV and no risk factors for resistance (defined by prior contact with a patient with FLUAV infection treated with amantadine or recent use of amantadine by the patient), oral amantadine (100 mg twice daily for 7 to 14 days) was an accepted therapy. From late 1999, oseltamivir (75 mg twice daily for 5 days) or zanamivir (one 10-mg inhalation every 12 hours for 5–10 days) was recommended for therapy of URTI or LRTI, and these drugs were used if the patient had risk factors for amantadine-resistant FLUAV and in all symptomatic FLUBV infections [35]. However, therapy of URTI by any RV to avoid progression to LRTI (preemptive therapy) was mandatory only if the patient had recently undergone transplantation (before day +60) or if the patient had active graft-versus-host disease (GVHD) under treatment. However, in patients without these risk factors, treating physicians were free to decide whether to treat patients who were ambulatory with an isolated URTI according to their own clinical judgment, because there is no formal proof of the clinical benefit of these preemptive therapies [3,11]. Patients were also treated with concomitant antibiotics, antifungals, or antivirals directed at isolated copathogens, as indicated.
Statistical Analysis
All patients were followed up until April 2004 and were censored before this date in case of death from any cause, relapse of the underlying disease that required salvage chemotherapy, or the start of conditioning for a second HSCT performed within tandem protocols. All analyses were performed for a first episode of RV infection in all patients to prevent bias introduction by entering patients more than once in the outcome analyses. Thus, patients were enrolled in this study only once, but later episodes were studied and managed in the same manner, although they are not analyzed in detail herein. The incidence of LRTI and URTI by RV infection was calculated by using cumulative incidence estimates and taking into account the competing risks (nonrelapse mortality [NRM] and disease relapse), and all other usual transplantation outcomes were analyzed with standard methods [36,37]. Univariate analyses of risk factors for symptomatic RV infections were performed with univariate Cox regression models, whereas multivariate analyses were performed with Cox proportional hazards regression. Variables with a P value of <.10 were included in the prior univariate testing. For categorical variables, the χ2 statistic or Fisher exact tests were used to establish differences in their distribution, whereas the Mann-Whitney U test was used to compare continuous variables. Tests of significance were 2 sided, with a significance level of P ≤ .05. Development of an RV infection, acute (aGVHD) and chronic GVHD (cGVHD), neutropenia, or lymphocytopenia was entered into univariate and multivariate models as a time-dependent covariate. Because risk factors for complications from RV infections probably differ between allogeneic and autologous HSCT recipients (alloHSCT and autoHSCT, respectively), separate risk factor analyses were performed for each transplant group. Quantitative variables were reentered as qualitative or binary variables only if at least a trend for statistical significance (P < .08) was found.
The only variables analyzed for their effect on symptomatic RV infections and outcomes were age, sex, disease group (leukemia/myelodysplasia versus lymphoid malignancies), disease status (early versus advanced; see Table 1 for details), prior autoHSCT, donor type (genoidentical sibling versus alternative donors; only for alloHSCT), stem cell source (bone marrow [BM] versus peripheral blood stem cells [PBSCs]), T-cell depletion by CD34+ cell selection (only for alloHSCT), type of conditioning (myeloablative versus reduced intensity; only for alloHSCT), time after transplantation of the RV infection (early [before day +30], intermediate [day +31 to +90], late [after day +90 to 1 year], and very late (beyond 1 year), close household contact with children younger than 12 years, presence of moderate and severe lymphocytopenia at the time of RV infection, neutropenia (<0.5 × 109/L), type of RV isolated, donor/recipient CMV serostatus, nosocomial or outpatient infection, use of steroids before RV infection (>0.5 mg/kg daily for >2 weeks in the previous 2 months), and development of aGVHD or cGVHD (only for alloHSCT). Unless otherwise specified, no further variables were explored, to reduce the chances of identifying possible risk factors by chance.
Table 1.
Patient Characteristics and Overall Transplantation Outcomes
| Variable | Allogeneic HSCT | Autologous HSCT |
|---|---|---|
| Number | 172 | 240* |
| Median age, y (range) | 44 (19–71) | 49 (19–71) |
| Sex (M/F) | 105/67 | 143/97 |
| Patient CMV seropositive (immunoglobulin G) | 135 (78.6) | 192 (80) |
| Nosocomial infections | 74 (43) | 91 (38) |
| Underlying disease | ||
| Chronic myelogenous leukemia | 21 (12.2) | 4 (1.7) |
| Acute myeloid leukemia/myelodysplasia | 61 (35.5) | 35 (14.5) |
| Acute lymphoblastic leukemia | 11 (6.4) | 5 (2.1) |
| Non-Hodgkin lymphoma/CLL | 33 (19.2) | 66 (27.5) |
| Hodgkin disease | 15 (8.7) | 34 (14.2) |
| Multiple myeloma | 20 (11.6) | 88 (36.7) |
| Other | 11 (6.4) | 8 (3.3) |
| Status at transplantation† | ||
| Early | 58 (33.7) | 148 (61.7) |
| Advanced | 114 (66.3) | 92 (38.3) |
| Second HSCT | 57 (33.1) | 12 (5) |
| Donor type | ||
| HLA-identical sibling | 145 (84.3) | |
| HLA-matched unrelated | 12 (7) | |
| Mismatched related or unrelated | 15 (8.7) | |
| Close household contact with children < 12 y | 95 (55) | 115 (48) |
| Stem cell source | ||
| Peripheral blood | 134 (77.9) | 222 (92.5) |
| Bone marrow | 38 (21.9) | 18 (7.5)‡ |
| CD34+-selected PBSC | 19 (11) | |
| Conditioning regimen | ||
| TBI-based ablative | 38 (22) | 40 (16.7) |
| Chemotherapy-only ablative | 22 (12.9) | 200 (82.3) |
| Reduced intensity | 112 (65.1) | |
| Follow-up median, d (range) | 445 (8–1606) | 297 (14–1582) |
| For survivors | 698 (31–1606) (n = 107) | 356 (96–1582) (n = 188) |
| Developed aGVHD (% CumInc. 95% CI) | 97 (51.7, 44.2–59.2) | NA |
| Grades II–IV | 75 (39.4, 32.2–47.6) | |
| Grades III–IV | 30 (15.4, 9.4–21.4) | |
| Developed cGVHD (% CumInc, 95% CI)§ | 86 (49.5, 41.5–57.5) | NA |
| 1-y extensive cGVHD | 40 (23.1, 10.6–35.6) | |
| Relapse incidence (95% CI) | ||
| 1 y | 16.6 (9.7–23.5) | 26.7 (21.9–32.5) |
| 2 y | 23.6 (15.5–31.7) | 42.9 (34.3–51.5) |
| Overall survival (95% CI) | ||
| 1 y | 70 (63–77) | 80.6 (74.8–86.4) |
| 2 y | 59.5 (67.3–51.7) | 74.2 (67.2–81.2) |
Data are n (%) unless otherwise noted.
CMV indicates cytomegalovirus; CLL, chronic lymphocytic leukemia; CumInc, cumulative incidence; CI, confidence interval; aGVHD, acute graft-versus-host disease; cGVHD, chronic GVHD; HSCT, hematopoietic stem cell transplantation; TBI, total body irradiation; NA, not applicable; CML, chronic myelogenous leukemia.
Includes 2 syngeneic HSCTs.
Disease phase at transplantation was categorized as early (acute leukemia or poor-risk myelodysplasia in first complete remission, untreated good-risk myelodysplasia, first chronic phase chronic myelogenous leukemia, lymphoid malignancy in first remission, multiple myeloma in first complete, or partial, response after chemotherapy) and advanced (acute leukemia or myelodysplasia in second or higher complete remission, relapsed acute leukemia or myelodysplasia, accelerated and blastic phase CML, lymphoid malignancy in second or higher remission, refractory or relapsed lymphoid malignancy, and any indication for a second transplantation).
Source included both bone marrow and peripheral blood in 13 cases.
A total of 29 to 172 were not evaluable for cGVHD because they were not alive and progression free beyond day + 100.
RESULTS
A total of 412 HSCTs were performed during the 4-year study period, and the minimum follow-up was 6 months for all cases. Thirty-eight patients received 2 HSCTs during the study period as part of protocols that incorporate tandem transplantations, and thus the total number of patients included was 386. Unless otherwise specified, the term patient refers to each individual HSCT recipient. One hundred seventy-two patients received an alloHSCT, whereas 240 received an autoHSCT. Patient characteristics in both transplant groups are shown in detail in Table 1.
Microbiological Results
During the study period, 83 alloHSCT (48%) and 94 autoHSCT (39%) recipients were sampled at least once by NPA, BAL, or both because they met the criteria for URTI, LRTI, or both and gave their informed consent. The initial sample analyzed was an NPA in 137 cases, a BAL sample in 23 cases, and both NPA and BAL in 17 cases. Table 2 details the results of the initial sample(s) analyzed.
Table 2.
Number and Cumulative Incidence of the Most Common Symptomatic RV Infections*
| Variable | Allogeneic HSCT (n = 172) |
Autologous HSCT (n = 240) |
|---|---|---|
| No. patients sampled at least once | 83 (48%) | 94 (39%) |
| No. patients with a documented RV infection* | 51 | 32 |
| 2-y incidence of any documented RV infection | 29.1 (19.1–36.7)† | 14.1 (6.9–19.7)† |
| No. of patients with a URTI by an RV | 41 | 32 |
| 2-y incidence of URTI by an RV | 20.1 (11.7–28.5)† | 14.1 (6.9–19.7)† |
| No. of patients with an LRTI by an RV | 29 | 8 |
| 2-y incidence of LRTI by an RV | 12.5 (5.1–17.9)† | 2.5 (0.1–5.1)† |
| No. of patients with an infection by FLUAV/FLUBV | 24 | 15 |
| 2-y incidence of infection by FLUAV/FLUBV | 13.1 (7.1–19.1)‡ | 6.1 (2.6–9.6)‡ |
| No. of patients with an infection by HRSV | 12 | 7 |
| 2-y incidence of infection by HRSV | 7.5 (2.1–12.6)§ | 2.9 (0.9–4.9)§ |
| No. of patients with an infection by HMPV | 9 | 7 |
| 2-y incidence of infection by HMPV | 5.2 (1.7–9.7) | 2.9 (0.9–5.2) |
| No. of patients with a monomicrobial RV infection | 42 | 25 |
| FLUAV/FLUBV | 13/4 | 10/1 |
| HRSV | 7 | 4 |
| HAdV | 6 | 1 |
| HPIV 1–3 | 3 | 2 |
| HEV | 2 | 1 |
| Human rhinovirus | 2 | — |
| Human metapneumovirus | 5 | 6 |
| No. of patients with a mixed RV infection‖ | 9 | 7 |
| FLUAV/FLUBV + HRSV | 4 | 3 |
| FLUAV/FLUBV + HAdV | 1 | 1 |
| HPIV + HAdV/HEV/FLUAV | —/—/1 | 1/1/— |
| HEV + HAdV | 1 | — |
| HMPV + HAdV/HRSV/HAdV FLUAV | —/1/1 | 1/—/— |
Data in parentheses are 95% confidence intervals unless otherwise specified.
HSCT indicates hematopoietic stem cell transplantation; RV, conventional respiratory virus; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; HRSV, human respiratory syncytial virus; HMPV, human metapneumovirus; HAdV, human adenovirus; HEV, human enteovirus.
All data refer to a first episode of RV infection(s). Twelve patients (8 allogeneic and 4 autologous transplant recipients) developed 2 or more episodes of RV infection during the study period (see text for details).
P < .001.
P = .02.
P = .09.
Mixed RV infections refer to the identification of more than 1 type of RV during the same clinical episode.
Initial microbiological results
Overall, an RV was identified in 83 episodes (47%) in the initial samples, and more than 1 RV was found during the same episode in 16 patients (19%). Mixed viral infections by RVs were considered coinfections if both RVs were present in the initial samples or were considered superinfections if the new RV appeared in follow-up NPA during the episode of URTI. Details on the initial RVs isolated are shown in detail in Tables 2 and 3. For FLUAV/FLUBV, HRSV, HPIV 1 to 3, and HAdV, the initial samples were positive by both IF and cell culture in 54% of the samples, whereas only IF or culture was positive in 29% and 17% of cases, respectively. As previously described, the presence of HMPV was detected retrospectively by RT-PCR, although the samples and clinical data had been collected prospectively.
Table 3.
Clinical Presentation and Outcome of 83 Episodes of Clinically Symptomatic Infections by Respiratory Viruses in Posttransplantation Patients
| FLUAV (n = 33) or FLUBV (n = 6) |
HRSV (n = 19) |
HAdV (n = 11) |
HPIV 1–3 (n = 8) |
HEV (n = 5) |
HRV (n = 3) |
HMPV (n = 16) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | (Allo Only) | Allo | Auto |
| Total related to type of HSCT, n* | 24 | 15 | 12 | 7 | 7 | 4 | 4 | 4 | 3 | 2 | 3 | 9 | 7 |
| Nosocomial infection | 8 (67) | 6 (40) | 5 (42) | 3 (43) | 2 (29) | 2 (50) | 3 (75) | — | — | 2 | 5 (56) | 3 (43) | |
| Seasonal occurrence in autumn or winter | 3 (14) | 1 (7) | 2 (17) | — | 1 (14) | 1 (25) | — | 2 (50) | 1 (34) | 1 | 2 | 2 (22) | 1 (14) |
| 14 (54) | 8 (53) | 9 (75) | 6 (86) | 4 (57) | 2 (50) | 1 (25) | 1 (25) | 2 (66) | 1 | 3 (33) | 3 (43) | ||
| Period after HSCT | |||||||||||||
| Early (< 30 d) | 9 (37) | 5 (33) | 3 (25) | 3 (43) | 1 (14) | 1 (25) | 2 (50) | 1 (25) | 2 | 4 (44) | 4 (57) | ||
| Intermediate (31–90 d) | 4 (17) | 5 (33) | 4 (33) | 3 (43) | 1 (14) | 1 | 2 (50) | 1 (25) | 1 (34) | — | 1 | 1 (11) | 2 (29) |
| Late (91 d to 1 y) and very late (> 1 y) | 11 (46) | 5 (33) | 5 (42) | 1 (14) | 5 (71) | 2 (50) | — | 2 (50) | 2 (66) | 2 (100) | 4 (44) | 1 (14) | |
| Documented sites of infection | |||||||||||||
| URTI only, n = 50 (25/25) | 11 (45) | 11 (74) | 4 (33) | 4 (57) | 3 (43) | 4 (100) | 1 (25) | 3 (75) | 2 (66) | 2 (100) | 3 (100) | 5 (56) | 6 (86) |
| URTI - LRTI, n = 23 (16/7) | |||||||||||||
| Initial URTI - LRTI, n = 8 (5/3) | 2 (8) | 2 (13) | 2 (16) | 3 (43) | 1 (14) | — | — | — | 1 (34) | — | — | — | |
| URTI followed by LRTI, n = 15 (11/4) | 3 (13) | 2 (13) | 3 (25) | 1 (14) | 2 (28) | — | 3 (75) | — | 2 (22) | 1 (14) | |||
| LRTI only, n = 14 (13/1) | 8 (34) | — | 3 (25) | — | 1 (14) | — | — | 1 (25) | 2 (22) | — | |||
| Symptoms in patients with URTI† | n = 30 | n = 13 | n = 10 | n = 7 | n = 5 | n = 2 | n = 10 | ||||||
| Rhinorrhea | 22 (73) | 9 (69) | 9 (90) | 7 (100) | 5 (100) | 2 (100) | 9 (90) | ||||||
| Nasal congestion and/or sneezing | 19 (63) | 9 (69) | 10 (100) | 6 (86) | 5 (100) | 2 (100) | 10 (100) | ||||||
| Sinusitis | 2 (7) | — | — | — | — | — | 2 (20) | ||||||
| Pharyngitis-laryngitis | 6 (20) | — | 2 (20) | 2 (29) | — | 2 (100) | 6 (60) | ||||||
| Frontal headache | 2 (7) | 5 (38) | 3 (30) | — | 2 (40) | — | 6 (60) | ||||||
| Conjunctivitis | — | 3 (23) | 2 (20) | — | 4 (67) | 2 (100) | 2 (20) | ||||||
| Tracheobronchial cough (no pneumonia) | 14 (47) | 4 (40) | 3 (30) | 6 (36) | 4 (80) | — | 4 (40) | ||||||
| Fever (> 37.5°C) | 13 (43) | 2 (15) | 5 (50) | 4 (57) | 5 (100) | 2 (100) | 6 (60) | ||||||
| Number of weekly follow-up NPA performed‡ | |||||||||||||
| 1 wk | 12/18 | 7/14 | 6/9 | 4/5 | 1/4 | 1/3 | 2/4 | 2/2 | NA | NA | NA | ||
| 2 wk | 7/11 | 2/4 | 4/5 | 2/2 | 1/1 | 0/3 | 0/3 | 0/2 | |||||
| ≥3 wk | 5/10 | 1/2 | 2/7 | 1/2 | 1/1 | 0/1 | — | ||||||
| Specific antiviral therapy: total treated n (%) | 16 (67) | 7 (47) | 10 (83) | 4 (57) | 4 (57) | — | 2 (50) | 1 (25) | NA | NA | NA | ||
| Death attributed to RV (n, % with LRTI) | 3 (23) | 0/4 | 2 (25) | 0/3 | 2 (50) | — | 0/3 | 6/1 | 0/1 | — | 2 (50) | 0/1 | |
| No other copathogens found | 1 | — | 1 | — | 1 | — | — | — | |||||
| Other copathogens involved | 2 | — | 1 | — | 2 (50) | — | |||||||
| Invasive aspergillosis | 2 | — | 2 | — | |||||||||
| Grant-negative bacilli | 1 | — | — | — | |||||||||
| Cytomegalovirus | 1 | — | 1 | — | 1 | — | 1 | — | |||||
Data are n (%) unless otherwise indicated.
There were 51 alloHSCTs and 32 auto HSCTs.
HSCT indicates hematopoietic stem cell transplantation; RV, respiratory virus; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; NPA, nasopharyngeal aspirate; NA, not applicable.
The number of respiratory viruses was 101 in 83 episodes, because mixed viral episodes (> 1 respiratory virus isolated) occurred in 9 alloHSCT and 7 autoHSCT recipients (detailed in Table 2).
Specific URTI symptoms for each virus include both alloHSCT and autoHSCT because there were no differences in any symptom between the 2 transplant groups.
Numbers refer to still-positive NPAs in follow-up samples/number of patients who were resampled at each time point.
Follow-up microbiological results and duration of viral shedding
Other viral and nonviral pathogens were isolated from the lower respiratory tract or a follow-up NPA in many patients, as shown in detail in Table 3. A new RV was isolated during the same clinical episode but at a follow-up (not initial) sample in 5 alloHSCT and 4 autoHSCT recipients (RV superinfections). Thus, the total numbers of infections by the different RVs during the 83 episodes studied (including initial coinfections and, later, superinfections) were 39 FLUAV/FLUBV, 19 HRSV, 16 HMPV, 11 HAdV, 8 HPIV 1 to 3, 5 HEV, and 3 HRV.
Follow-up NPAs were systematically requested at weekly intervals only for symptomatic RV infections with rapid diagnostic tests (IF and rapid shell vial viral cultures for FLUAV/FLUBV, HRSV, HAdV, and HPIV 1–3). The initial RVs were isolated again in follow-up NPA in 36 (60%) of 60 at the 1-week follow-up visit, 20 (40%) of 49 at 2 weeks, and 8 (42%) of 19 at 3 weeks: 2 (22%) of 9 patients tested positive beyond 3 weeks, with no significant differences between transplant groups and type of RV. The median duration of viral shedding in patients who came to follow-up sampling was 2 weeks (range, 0–12 weeks).
Forty-nine (80%) of 61 patients with an initial URTI were screened at least weekly for ≥2 weeks to document viral clearance. In these patients, we assessed factors that influenced prolonged viral shedding in NPA, defined as a persistently positive NPA for the initial RV for ≥2 weeks. In all 49 evaluable cases, multivariate analysis found only 2 risk factors: (1) mixed viral URTI (hazard ratio [HR], 15; 95% confidence interval [CI], 2.5–91; P = .003); and (2) nonearly disease status at transplantation (HR, 6; 95% CI, 1.47–23; P = .02). In autoHSCT (n = 19 evaluable cases), the only possible risk factor identified was mixed viral URTI (HR, 11; 95% CI, 0.99–130; P = .053), whereas in alloHSCT (n = 30 evaluable cases), 3 possible risk factors were identified in multivariate analysis: (1) mixed viral URTI (HR, 33; 95% CI, 1.4–4765; P = .03), (2) nonearly disease status at transplantation (HR, 18; 95% CI, 1.3–245; P = .03), and (3) prior aGVHD grades II to IV (HR, 8; 95% CI, 1.02–64; P = .05). Of note, use of oseltamivir/zanamivir, amantadine, or inhaled ribavirin (see section on therapy) showed a trend toward reducing prolonged viral shedding in univariate testing (5/14 [36%] patients treated versus 11/16 [69%] in untreated patients; P = .07).
Incidence of RV Infections
Allogeneic HSCT
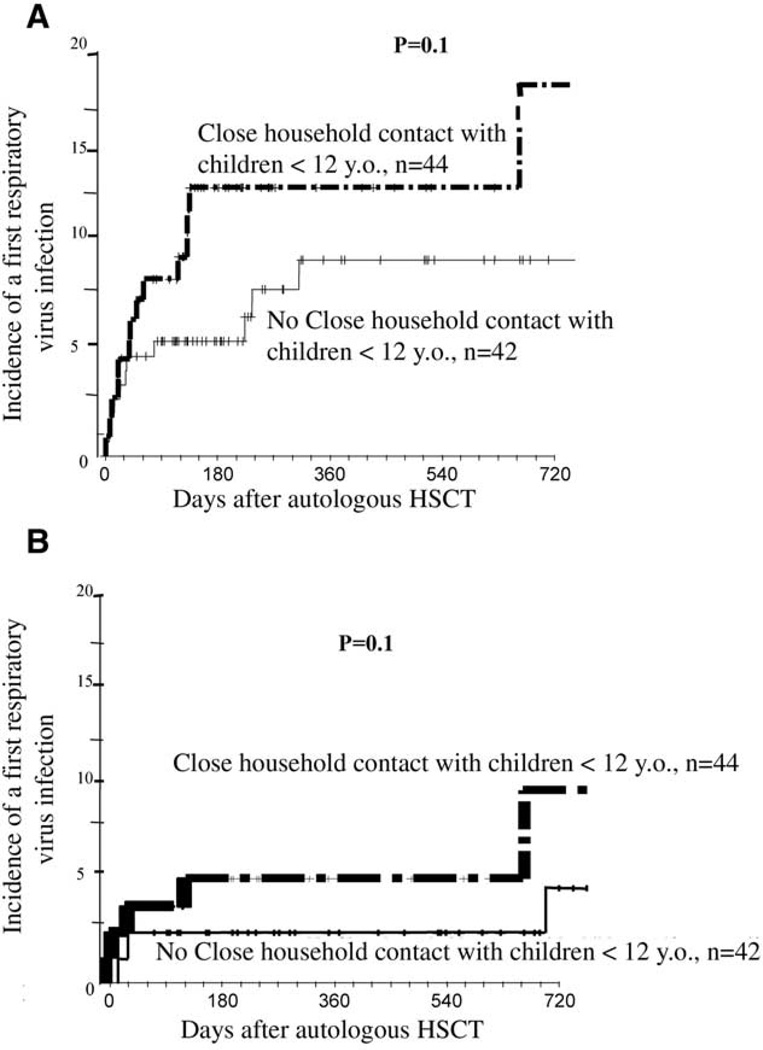
Fifty-one alloHSCT recipients tested positive for an RV virus during the study period, and more than 1 RV was identified during the same episode in 14 (9 coinfections and 5 superinfections) of these patients, as detailed in Table 2. The 2-year cumulative incidence of a first episode of RV infection was 29.1% (95% CI, 19.1%–36.7%), and 29 patients developed confirmed LRTI by RVs (incidence, 12.5%; 95% CI, 5.1%–17.9%). The median onset of symptomatic RV infections was 169 days (range, 3–963 days). In univariate analysis, risk factors for a higher risk of developing any RV infection were (1) close household contact with children younger than 12 years (41% versus 16%; P = .007), (2) alternative donor (nongenoidentical sibling) HSCT (58% versus 28%; P = .04), and (3) cGVHD (33% versus 19%; P = .03). Trends were found for prior autoHSCT (P = .08), CD34+ cell selection (P = .07), and reduced-intensity conditioning (P = .08). In multivariate analysis, only close household contact with children younger than 12 years (HR, 2.5; 95% CI, 1.3–5; P = .009) and cGVHD (HR, 1.5; 95% CI, 1.03–2.04; P = .03) were associated with the risk of developing an RV infection (Figure 1). When the final univariate and multivariate models were applied to nosocomial and outpatient respiratory tract infections, close household contact with children remained an independent risk factor in both subgroups. In addition to a first episode of RV infection, 8 alloHSCT recipients developed 2 or more episodes of RV infection (all initially URTIs) during the study period. Seven episodes occurred during a different winter season, and 1 occurred during the same winter. The RVs involved were FLUAV/FLUBV in 4 cases and HRSV, HPIV 3, HAdV, HEV, and HRV in 1 case each. Two of these 8 URTIs progressed to LRTI, and 1 patient died from HAdV and FLUAV pneumonia.
Figure 1.
Cumulative incidence of development of a first documented episode of respiratory virus infection in allogeneic transplant recipients. A, Incidence in 86 patients who developed cGVHD according to whether they had (heavy line) or did not have (light line) close household contact with children (<12 years): this incidence was 45% and 19%, respectively, on day +720. B, Incidence in 57 evaluable patients who did not develop cGVHD according to whether they had (heavy line) or did not have (light line) close household contact with children (< 12 years): this incidence was 29% and 11%, respectively, on day +720.
Autologous HSCT
Thirty-two autoHSCT recipients tested positive for an RV virus, and more than 1 RV was identified during the same episode in 11 (7 coinfections and 4 superinfections) of these patients. The 2-year cumulative incidence of symptomatic RV infections was 14.1% (95% CI, 6.9%–19.7%), and only 8 patients developed confirmed LRTI by an RV (incidence, 2.5%; 95% CI, 0.1%–5.1%). The median time to onset of symptomatic RV infections was 164 days (range, 1–1222 days). Thus, although the incidence of both URTI and LRTI was much higher in alloHSCT recipients (Table 2), the median time to a first episode was the same in both groups. In univariate analysis, risk factors for a higher risk of developing any RV infection in autoHSCT were (1) close household contact with children younger than 12 years (20% versus 9%; P = .05), (2) advanced disease status at transplantation (18% versus 6%; P = .03), (3) BM as the stem cell source (24% versus 11%; P = .06), and (3) patient CMV seropositivity (17% versus 4%; P = .05). In multivariate analysis, close household contact with children younger than 12 years (HR, 2.2; 95% CI, 1.1–6.9; P = .05) and advanced disease status (HR, 2.9; 95% CI, 1.1–7.7; P = .03) were the only risk factors identified (Figure 2). In addition to a first episode of RV infection, 4 autoHSCT recipients developed 2 or more episodes of RV infection during the study period, but all of these episodes occurred during a different winter season. The RVs involved were FLUAV in 2 cases and HRSV, HPIV 3, and HRV in 1 case each. One case of FLUAV URTI progressed to LRTI, from which the patient fully recovered.
Figure 2.
Cumulative incidence of development of a first documented episode of respiratory virus infection in autoHSCT recipients. A, Incidence in 92 patients with advanced underlying disease status at transplantation according to whether they had (heavy line) or did not have (light line) close household contact with children (<12 years): this incidence was 18% and 9%, respectively, on day +720. B, Incidence in 148 patients with early underlying disease status at transplantation according to whether they had (heavy line) or did not have (light line) close household contact with children (<12 years): this incidence was 10% and 4%, respectively, on day +720.
The most commonly isolated RVs in both transplant groups were FLUAV/FLUBV (n = 39), HRSV (n = 19), and MPV (n = 16). The proportion of nosocomial and community-acquired RV infections did not differ significantly between transplant groups (nosocomial infections: 24 [47%] alloHSCTs versus 12 [38%] autoHSCTs). Additionally, no clustering of nosocomial infections occurred during the study period, and both these and community-acquired symptomatic RV infections occurred during community outbreaks by each specific RV analyzed (Figure 1A). The proportion of episodes that occurred before day +90 was somewhat higher in autoHSCT: early (days 0 to +30) plus intermediate (days +31 to +90) infections, 56% versus 76% in alloHSCT and autoHSCT, respectively, and late (day +91 to 1 year) plus very late (>1 year after transplantation) infections, 44% versus 24%, respectively (P = .08).
LRTI (Pneumonia) by an RV: Number, Risk Factors, and Outcome
Of the 83 patients with a first episode of RV infection, 37 developed a first LRTI during the study episode (29 alloHSCTs and 8 autoHSCTs). Eleven patients had only an LRTI, without a concomitant or prior URTI (11 and 0 cases, respectively). Eight patients had both a URTI and an LRTI at initial diagnosis (6 and 3 cases, respectively), whereas 15 patients had an initial URTI that progressed to an LRTI during the study episode (12 and 5 cases, respectively). In 9 patients with an LRTI, more than 1 RV was isolated from lower respiratory tract samples (5 alloHSCTs and 4 autoHSCTs). BAL confirmed the presence of an RV in the lower respiratory tract in 24 (65%) cases of LRTIs (all 14 cases of initial LRTI, 4/8 cases of initial URTI and LRTI, and 6/15 secondary LRTIs from a prior URTI), whereas 13 additional cases (all alloHSCTs) were documented in lung tissue.
Allogeneic HSCT
In univariate analysis, risk factors for developing an LRTI were (1) advanced disease status at transplantation (17% versus 5%; P = .01), (2) alternative donor (nongenoidentical sibling) HSCT (28% versus 5%; P = .05), (3) prior autoHSCT (28% versus 6%; P = .04), and (4) cGVHD (16% versus 5%; P = .01). Trends were found for grades II to IV aGVHD (18% versus 8%; P = .08) and stem cell source (40% for cord blood versus 9% for PBSC versus 11% for BM; P = .10). In multivariate analysis, only alternative donors (HR, 1.7; 95% CI, 1.05–2.9; P = .05) and cGVHD (HR, 1.6; 95% CI, 1.1–2.6; P = .05) were associated with the risk of developing an LRTI.
When all 172 allografts were analyzed, a strong risk factor for developing an LRTI by an RV was having documented a recent (< 6 weeks) URTI by the same virus or viruses. Eighteen (44%) of 41 patients with a URTI developed an LRTI by the same RV, whereas 11 (8%) of 131 with no documented URTI developed an LRTI (2-year cumulative incidence of LRTI: 30% [95% CI, 2%–34%] versus 8% [95% CI, 3%–13%], respectively; P < .01). This variable, however, was not introduced in multivariate Cox models even though it was highly significant in univariate testing, because variables that predispose to the development of LRTI may also increase the risk of URTI and/or the progression of URTI to LRTI (mainly alternative [nongenoidentical sibling] donors, cGVHD, and aGVHD).
Death was attributed to an LRTI by an RV in 8 (28%) of the 29 alloHSCT recipients. The RVs involved in fatal cases were FLUAV/FLUBV (3/13 [23%] pneumonias), HRSV (2/8 [25%] pneumonias), HAdV (1/4 [25%] pneumonias), and MPV (2/4 [50%] pneumonias). However, significant copathogens were found in 5 of the 8 deaths from LRTI (see Table 3 for details).
Autologous HSCT
In univariate analysis, risk factors for developing an LRTI were stem cell source (3% for BM versus 0% for PBSCs; P = .01) and male sex (5% versus 0%; P = .05). Because of the small number of events and risk factors in univariate testing, a multivariate analysis could not be performed. None of the 8 autoHSCT recipients with pneumonia died.
Other pathogens found in LRTI
A copathogen known to be definitively involved in opportunistic LRTIs was identified in BAL or lung samples in 5 alloHSCT recipients (2 with gram-negative bacilli, 2 with invasive pulmonary aspergillosis, 1 with CMV, and 1 with both CMV and invasive pulmonary aspergillosis). In autoHSCT, only 1 case of invasive pulmonary aspergillosis coexisted with an RV (Table 3).
Risk for Progression of URTI to LRTI
As described previously, 23 (33%) of the 70 patients who presented with symptoms of a URTI either had pneumonia (n = 8) or progressed to pneumonia (n = 15) during the follow-up period (18/41 [44%] alloHSCT and 5/29 [17%] autoHSCT; P < .05).
Allogeneic HSCT
The median interval between URTI and the first signs of LRTI was 7 days (range, 2–32 days). In univariate analysis, risk factors for progression of a URTI to LRTI included (1) severe lymphocytopenia (<0.2 × 109/L) at the onset of the URTI (50% versus 20%; P = .04), (2) alternative donor (nongenoidentical sibling) HSCT (39% versus 22%; P = .05), (3) disease group (12% in leukemia/myelodysplasia versus 50% in lymphoid malignancies; P = .05), (4) prior grade II to IV aGVHD (44% versus 22%; P = .04), and (5) prior extensive cGVHD (40% versus 20%; P = .04). Trends were found for stem cell source (30% for PBSCs versus 11% for BM; P = .10) and type of RV isolated (3/4 HPIV 1–3, 3/7 HRSV, 2/5 HAdV, 3/14 FLUAV/FLUBV, and 2/7 MPV; P = .10). In multivariate analysis, only severe lymphocytopenia (HR, 7.3; 95% CI, 1.3–40.9; P = .02) and grade II to IV aGVHD (HR, 3.8; 95% CI, 1.1–15.5; P = .05) were associated with the risk of developing an LRTI from a prior URTI.
Autologous HSCT
Because only 5 autoHSCT recipients had a URTI that progressed to an LRTI, risk factors could not be reliably determined. However, the presence of moderate lymphocytopenia (<0.5 × 109/L) at the onset of the URTI seemed to increase the risk (31% versus 9%; P = .03), and a trend was observed for the presence of neutropenia (<0.5 × 109/L) at the onset of infection (33% versus 0%; P = .10). Additionally, the type of RV involved also seemed to increase this risk (5/25 with FLUAV/FLUBV, HRSV, or MPV versus 0/7 for other RVs). As in alloHSCT, a strong association was found between developing an LRTI by an RV and a recent URTI by the same virus or viruses. Eight (25%) of 32 patients with a URTI developed an LRTI by the same RV, whereas 0 of 208 with no documented URTI developed an LRTI (2-year cumulative incidence of LRTI: 31% [95% CI, 6%–56%] versus 0%, respectively; P < .0001).
Effect of Symptomatic RV Infections on NRM and Survival
Development of an LRTI by any RV (introduced as a time-dependent variable) was associated with the 100-day and 2-year NRM estimates in all 412 patients in univariate analysis. Nine of 37 patients with and 26 of 375 without an LRTI by an RV experienced NRM. The 100-day and 2-year cumulative incidences of NRM were 9.2% (95% CI, 1.2%–17.2%) and 22% (95% CI, 11%–33%), respectively, in patients with LRTI by an RV and 3.7% (95% CI, 0.1%–7.9%) and 14% (95% CI, 10%–18%), respectively, in patients without this complication (P < .01). However, when alloHSCT and autoHSCT recipients were analyzed separately, LRTI by an RV had no effect on early or late NRM (data not shown). However, in all 412 patients, the 1- and 2-year overall survival (OS) for patients who developed an RV (all infections or LRTIs) did not differ from the OS in those who did not develop any episode of RV infection. Nevertheless, in multivariate analysis of OS, symptomatic RV infections were kept in all models and analyzed as time-dependent variables, as indicated in “Statistical Analysis.”
In alloHSCT recipients, variables that decreased 2-year OS (final multivariate model) were (1) development of invasive aspergillosis (HR, 5.6; 95% CI, 3.7–8.6; P = .01), (2) advanced disease phase (HR, 1.9; 95% CI, 1.01–4.4; P = .05), (3) CD34+ cell–selected allogeneic PBSC transplant (HR, 3.3; 95% CI, 1.7–6.6; P = .01), (4) development of grade III/IV aGVHD (HR, 1.4; 95% CI, 1.03–1.9; P = .05), and (5) not developing cGVHD (HR, 3.5; 95% CI, 2.5–4.9; P = .001). Variables that increased the 2-year NRM (final multivariate model) were (1) development of invasive aspergillosis (HR, 3.3; 95% CI, 1.2–9.3; P = .04), (2) CD34+ cell–selected allogeneic PBSC transplant (HR, 2.1; 95% CI, 1.12–2.86; P = .04), (3) developing aGVHD grade III/IV (HR, 6.6; 95% CI, 2.5–11.6; P = .01), and (4) use of conventional myeloablative conditioning (ie, not receiving a reduced-intensity conditioning regimen; HR, 3.2; 95% CI, 1.1–9.06; P = .05).
In autoHSCT recipients, the only variables analyzed that decreased the 2-year OS (final multivariate model) were (1) advanced disease phase (HR, 2.1; 95% CI, 1.1–5.4; P = .04) and (2) development of FLUAV/FLUBV infection (HR, 3.2; 95% CI, 1.3–8.1; P = .02). Variables found to increase the 2-year NRM (final multivariate model) were (1) development of FLUAV/FLUBV infection (HR, 2.2; 95% CI, 0.99–7.2; P = .06) and (2) age >60 years (HR, 3.8; 95% CI, 1.1–9.1; P = .03).
MPV infections
Details on the incidence and clinical characteristics of all the first episodes of each RV infection are shown in detail in Tables 2 and 4 and are thus not described in detail separately in the text. However, a brief description of MPV infections would be useful because this is, to our knowledge, the first description of these infections in HSCT recipients. MPV was isolated in 16 (9%) of 177 samples tested (5/40 [13%] BALs and 11/154 [7%] NPAs), and coinfections with other RVs were seen in 3 (19%) cases. MPV infections occurred at all time periods after transplantation in both alloHSCT (n = 9) and autoHSCT (n = 7) recipients, with a 2-year incidence of 5.2% (95% CI, 1.7%–9.75%) and 3% (95% CI, 0.9%–5.2%), respectively. Fifty percent of the infections were community acquired, and MPV pneumonia was documented in 4 (44%) of 9 and 1 (14%) of 7 alloHSCT and autoHSCT recipients, respectively. As with the other viruses studied, LRTIs were preceded by a URTI in 3 (60%) of 5 cases of pneumonia. Because these infections were diagnosed retrospectively, follow-up NPAs were not obtained to assess the duration of viral shedding.
Table 4.
Interaction in Each Transplant Group between Lymphocytopenia, Acute Grades II to IV GVHD (Only in AlloHSCT), Type of Respiratory Virus, and Having Received (or Not) Specific Antiviral Therapy with the Risk of Progression to LRTI in 61 Patients with Initial Isolated URTI by FLUAV/FLUBV, HRSV, HPIV 1 to 3, and Adenovirus (n/N, %)
| Progression of initial URTI to LRTI* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Received Specific Antiviral Therapy for the URTI |
Lymphocytopenia | Acute GVHD Grades II to IV |
Type of Respiratory Virus Isolated† | |||||||
| Transplant Type | Overall | Yes (n = 7) | No (n = 25) | Yes (n = 13) | No (n = 19) | FLUA/FLUBV (n = 27) | HRSV (n = 13) | HAdV (n = 9) | HPIV 1–3 (n = 7) | |
| Allogeneic HSCT | ||||||||||
| (n = 32) | 9/32 (28) | 4/7 (57)‡ | 5/25 (20)‡ | 6/13 (46)§ | 3/19 (16)§ | 3/14 (21) | 3/7 (43) | 2/5 (40) | 1/4 (25) | |
| Yes (n = 13)‖ | 3/13 (23) | 2/4 (50) | 1/9 (11) | 2/4 (50) | 1/9 (11) | 1/5 (20) | 2/6 (33) | 1/1 | 0/1 | |
| No (n = 23) | 6/19 (33) | 2/3 (67) | 4/16 (25) | 4/9 (44) | 2/10 (20) | 3/9 (33) | 1/1 | 1/4 | 1/3 | |
| Autologous HSCT |
||||||||||
| (n = 29) | 4/29 (14) | 3/11 (27)§ | 1/18 (6)§ | NA | NA | 2/13 (15) | 1/5 (20) | 1/4 (25) | 0/3 | |
| Yes (n = 6)‖ | 3/6 (50)¶ | 2/2 (100) | 1/4 (25) | NA | NA | 2/3 (66) | 1/2 (50) | 0/0 | 0/1 | |
| No (n = 23) | 1/23 (4)¶ | 1/9 (11) | 0/14 (0) | NA | NA | 0/10 | 0/3 | 1/4 | 0/2 | |
HSCT indicates hematopoietic stem cell transplantation; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; NA, not applicable; FLUA/FLUBV = human influenza A/influenza B; HRSV, human respiratory syncytial virus; HAdV, human adenovirus; HPIV, human parainfluenza virus.
Progression to LRTI for each RV was (alloHSCT and autoHSCT combined) (1) FLUA/BV, 5/27 (19%); (2) HRSV, 4/12 (33%); (3) HAdV, 2/9 (22%); (4) HPIV 1–3, 3/7 (43%); not included in this table: MPV, 3/14 (21%).
Four autoHSCT recipients had > 2 different RVs isolated from the same nasopharyngeal aspirates and are not included in these columns.
P = .07.
P = .10.
See text and Table 3 for details on antiviral therapy.
P = .02.
DISCUSSION
Our study provides several relevant observations about the incidence, clinical course, and complications of symptomatic RV infections after HSCT, with the important fact that all patients who underwent transplantation over the 4-year study period with respiratory tract infections were systematically screened for the presence of the major RVs and followed a standardized clinical protocol. More importantly, the search for RVs was uniformly applied not only on NPA, but also on all BAL, biopsy, and autopsy specimens obtained throughout the study. Because 80% of our HSCT recipients who develop LRTI undergo BAL and/or postmortem lung examination, the observed results of LRTIs by RVs are probably an accurate measure of the true frequency of these infections. Additionally, detailed case report forms were filled in by the patients and by the treating medical and nursing staff. This study design may give more exact information than previous retrospective or brief prospective studies focused on adult and/or pediatric patients with a specific documented RV infection; these studies focused mainly on hospitalized patients or analyzed mostly symptomatic RV infections that occurred during the first 3 to 4 months after HSCT [1–16,38]. Transversal studies, which do not allow determining risk factors for acquiring RV infections, have also analyzed all symptomatic RV infections diagnosed over a given time period, ranging from a few months [13] to 1 to 7 years [6,12,14,15,39]. A few studies have estimated the incidence of and risk factors for acquiring URTI and/or LRTI by retrospectively analyzing patients with a documented RV infection during a given time period and comparing them with all transplant patients during the same time period [2,4,16,33,40–42]. Ljungman et al. [12] performed a 1-year prospective multicenter study of RV infections acquired early after HSCT within the European Group for Blood and Marrow Transplantation Infectious Diseases Working Party. Surprisingly, only 40 symptomatic RV infections were reported in 1863 transplant patients, and this did not allow appropriate risk factor analyses. This low frequency was probably a consequence of low sampling in cases of URTI and/or nonstandardized laboratory studies for RVs. These 40 cases, however, were combined with an additional 53 cases obtained transversally in an effort to at least define risk factors for LRTI and death among infected patients.
Even with such a prospective intensive monitoring of all symptomatic patients, surely many RV infections, especially URTIs, were not diagnosed. Several reasons may lead to underestimating the true incidence of both URTI and LRTI by any RV. An important reason is the fact that we studied only symptomatic infections, and patients with mild symptoms of URTI are unlikely to attend the emergency outpatient clinic. Additionally, patients who live far away from our hospital and were referred for transplantation would be more likely to seek attention at their local center for common cold or flulike symptoms. Finally, more sensitive diagnostic testing with PCR-based methods for all known RVs would have undoubtedly identified more cases of symptomatic RV infections, which may double with very sensitive PCR techniques [13,43–46]. However, PCR methods for each type of RV are not standardized, and thus we chose to use viral culture and IF for the current study, except, of course, for HMPV infections. Thus, the incidences we found surely represent only a fraction of the actual cases of RV infections, especially uncomplicated isolated URTIs, experienced by our transplant patients.
With these caveats in mind, symptomatic RV infections were common after HSCT in our patient cohorts and were associated with a relatively high incidence of progressing to LRTI. URTIs and LRTIs by all RVs were seen among both autoHSCT and alloHSCT recipients. Recipients of alloHSCT seemed to have a higher risk, but, again, this may be the result of several biases that lead to more common sampling of alloHSCT recipients, who are probably more likely to attend the outpatient clinic with symptoms of a URTI. An interesting finding was that an apparent risk factor for acquiring an RV infection after HSCT was having close household contact with children. To our knowledge, this observation has not been previously reported. Nevertheless, this is a logical finding, because children develop many RV infections and are very contagious because of their high viral excretion and intimate contact with their close relatives [47]. Unfortunately, this risk factor is not modifiable in real-life clinical practice.
URTIs per se are often not perceived as a potentially serious problem for patients and HSCT physicians, but pneumonia (LRTI) is appropriately perceived as a serious complication, because several studies have found that mortality from an LRTI with the most commonly identified RVs may be high. Most authors place great emphasis on avoiding patient infection with rigorous infection-control policies and, if a URTI is diagnosed, in avoiding the progression to an LRTI, although this may not be possible with current therapies. In our study, the incidence of a first LRTI by an RV was 13% in alloHSCT and 2.5% in autoHSCT recipients; in alloHSCT recipients, the development of pneumonia was due primarily to the presence of moderate to severe GVHD, alternative donor transplantation, and severe lymphocytopenia. No large differences between the main RVs were observed, but the number of LRTIs per each specific RV was small. With respect to currently available antiviral agents that may be active against certain RVs, inhaled ribavirin is by far the most commonly recommended antiviral agent for HRSV and HPIV 1 to 3, whereas FLUAV and FLUBV are both susceptible to the neuraminidase inhibitors oseltamivir and zanamivir. Amantadine is active against FLUAV, but resistant strains develop rapidly in vivo [48]. However, most retrospective studies have failed to show any significant benefit of inhaled ribavirin on the progression of an initial URTI to pneumonia by HRSV and HPIV 1 to 3 [3,4,6,11,26,42,49–51]. With respect to FLUAV/FLUBV, small patient series have also failed to show any clear benefit of preemptive therapy with amantadine/rimantadine or the neuraminidase inhibitors (oseltamivir/zanamivir), but the data available are very scant [2,39,52]. Because early therapy with oseltamivir has been shown to obtain clinically relevant benefits in large randomized trials in immunocompetent adults [48,53], we might see a benefit in our immunocompromised hosts if adequately designed prospective trials were performed. Our results on preemptive therapy of URTI were in line with previous studies. Because lymphocytopenia and grade II to IV aGVHD are strongly interrelated variables and were the main risk factors for progression to pneumonia in our study, we analyzed whether there were interactions between these variables, specific antiviral therapy, and risk for progression to LRTI in each transplant group and type of RV (detailed in Table 4). Although the subgroups were too small to allow definitive conclusions, patients with lymphocytopenia or aGVHD seemed to be equally likely to develop pneumonia whether they were treated with antivirals or not, thus raising doubts about the value of preemptive therapy in patients with URTIs. Preemptive therapy, however, may be useful for epidemiologic reasons, as described later in this discussion.
Few studies have analyzed risk factors for LRTI by these viruses in HSCT. Of note, the presence of lymphocytopenia was an independent risk factor for LRTI and/or progression of URTI to LRTI in the few studies that included a reliable multivariate analysis. In a multicenter 1-year prospective study performed by the Infectious Disease Working Party of the European Group for Blood and Marrow Transplantation [12], lymphocytopenia was the most significant variable predisposing to LRTI when all RVs are analyzed together, but especially in HRSV infections. Two large retrospective studies from Seattle also identified lymphocytopenia as an independent risk factor for LRTI in patients with HRSV [54] and FLUAV/FLUBV [2] infections. Recently, our group published another prospective study based on a cohort of patients with hematologic diseases (who did not receive transplants), and it concluded that lymphocytopenia predicted the presence of RV pneumonia at any moment and had a borderline significance for the progression to pneumonia from a prior URTI [1].
In our current study, mortality from LRTI was relatively low and usually occurred in the presence of aggressive copathogens (6/8 deaths). The outcome of patients with pneumonia (especially by HRSV, HPIV 1–3, and FLUAV/FLUBV) has been historically poor, with mortality rates ranging from 50% to 85% [5,6,10,11,24,33,55–58]. Whimbey et al. [5] reported that the risk of LRTI and death was closely related to the interval between transplantation and the appearance of symptoms: >70% of patients developed LRTI if the infection was acquired during the first month after transplantation. However, recent studies from the same institution and others have reported an attributable mortality in LRTIs by these RVs to be much lower (10%–30%), with little or no effect of the period after HSCT in which it develops. These findings are similar to ours [11–16,41,54,59–62]. Because there is no clear evidence that currently available antiviral therapy reduces the mortality from LRTI by any RV, this reduction in mortality may be due to improvements in supportive care and reductions in the rates of copathogens (mainly pulmonary aspergillosis and CMV pneumonia). In fact, in some previous studies, the absence of aggressive copathogens in LRTIs seemed to translate into lower mortality, rather than the use of antiviral therapy [11,42]. The coexistence of RV pneumonia and other aggressive pulmonary infections in HSCT recipients is not surprising, and the incidence may actually increase if survival of these patients increases; however, establishment of a good and long-lasting immune system does not improve in parallel with survival.
It should be highlighted, however, that most studies performed to date have focused only on early NRM in which an RV LRTI is implicated, but its effect on long-term outcomes has not been measured. A notable exception is the retrospective analysis of parainfluenza virus infections by the Seattle group [4], in which 1-month NRM after an LRTI was relatively low (35%), but the 6-month NRM was 75%. Thus, RV infections may have indirect local or systemic effects that predispose to later NRM. This may be partly due to an increased risk of LRTI by other copathogens, but it may also indicate that symptomatic RV infections are surrogate markers of something else that has not been analyzed, or it may be a combination of several variables.
Because most patients with an initial URTI by FLUAV/FLUBV, HRSV, HPIV 1 to 3, and HAdV (49/61; 80%) were screened at least weekly for ≥2 weeks to document viral clearance, we were able to assess the factors that influenced prolonged viral shedding. Few prior studies have reported detailed data on the duration of viral shedding. A recent retrospective study from Seattle that included 51 HSCT recipients with a URTI by FLUAV/FLUBV [2] identified 3 possible risk factors for prolonged shedding: alloHSCT versus autoHSCT, steroid therapy ≥1 mg/kg/d, and not receiving antiviral therapy, especially oseltamivir. Machado et al. [39] found prolonged shedding in 12 (48%) of 25 patients with HRSV, 2 (9%) of 23 with FLUAV/FLUBV, and 1 (13%) of 8 with HPIV 1 to 3 URTIs. Unfortunately, risk factors were not described, although all patients with FLUAV/FLUBV were treated with oseltamivir or amantadine, whereas only a few patients with HRSV received therapy [15]. In contrast, we found only a trend for a reduction of the duration of viral shedding in patients treated with specific antiviral therapy (prolonged shedding in 5/14 [36%] patients treated versus 11/16 [69%] in untreated patients; P = .07), but it should be stressed that this was a prospective observational study and not a controlled treatment study, and therapy was given (according to protocol and standard hospital guidelines) mostly to sicker or higher-risk patients, as previously specified. However, the trend toward reduced viral shedding is a positive finding that may decrease the spread of these viruses and the risk of generating drug-resistant strains [48,63].
The most novel observation in our study was the relatively high incidence of HMPV URTI and LRTI in our patients. Because this virus was discovered recently [27], there are currently no published data on the implications of HMPV in HSCT recipients, except for a single case report [64]. Among healthy children, and probably also healthy adults, a significant portion of hitherto unexplained viral respiratory tract illnesses can now be attributed to the human MPV [28,65,66]. Several surveys on the burden of disease of HMPV infection have been conducted in various immunocompetent study groups and have suggested that clinically it is indistinguishable from HRSV and FLUAV/FLUBV infections, with recurrent epidemics during the winter months. Although there are no published data on MPV infections in our specific geographic area (Barcelona, which lies in the western Mediterranean), data from the neighboring Basque country, as in other areas in Europe and worldwide, have confirmed its presence in URTI and LRTI [67,68]. Thus, despite the lack of prior data, it is reasonable to believe that HMPV will infect immunocompromised individuals at least as commonly as the general population. For obvious reasons, MPV infections in our patients were studied retrospectively in stored frozen aliquots and with a PCR technique, which was performed in an experienced reference laboratory [28]. Thus, the incidence of HMPV infection is not comparable to the other RVs studied on site prospectively by IF and viral cultures. However, the 16 cases identified suggest that its clinical relevance in adult HSCT recipients is as important as that of HRSV, FLUAV/FLUBV, and the other common RVs. Of the 16 HMPV infections, 5 were LRTIs, and 2 alloHSCT recipients died from this infection, as detailed in Table 3. Recently, researchers from Seattle reported an abstract of a retrospective study of 211 BALs from 162 HSCT recipients: MPV was identified in 5 patients (3%) and 8 BAL samples (3.8%). More importantly, 4 of these patients died with the clinical and autopsy diagnosis of idiopathic pneumonia syndrome [69]. Thus, MPV must surely be added to the list of the potentially lethal RVs in our patient population.
In conclusion, this prospective study allowed an estimation of the minimum incidence of a first RV infection in adult HSCT recipients. It identified risk factors for acquisition of an RV infection, prolonged viral shedding in URTI, and development of URTI and progression to LRTI, which should aid in the design of future studies. In addition, the recently discovered HMPV should be added to the potentially serious causes of symptomatic RV infections in HSCT worldwide.
ACKNOWLEDGMENTS
This study was supported by a grant from Fondo de Investigaciones Sanitarias (FIS 00/0296). We are grateful to all the nursing and medical staff from the Division of Clinical Hematology for their continuous contribution to this study.
REFERENCES
- 1.Martino R, Ramila E, Rabella N, et al. Respiratory virus infections in adults with hematologic malignancies: a prospective study. Clin Infect Dis. 2003;36:1–8. doi: 10.1086/344899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 3.Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant. 2001;7(suppl):11S–15S. doi: 10.1053/bbmt.2001.v7.pm11777098. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 5.Whimbey E, Champlin RE, Couch RB, et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;22:778–782. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 6.Champlin RE, Whimbey E. Community respiratory virus infections in bone marrow transplant recipients: the M.D. Anderson Cancer Center experience. Biol Blood Marrow Transplant. 2001;7(suppl):8S–10S. doi: 10.1053/bbmt.2001.v7.pm11777103. [DOI] [PubMed] [Google Scholar]
- 7.La Rosa AM, Champlin RE, Mirza N, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32:871–876. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 8.Lewis VA, Champlin R, Englund J, et al. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clin Infect Dis. 1996;23:1033–1037. doi: 10.1093/clinids/23.5.1033. [DOI] [PubMed] [Google Scholar]
- 9.Whimbey E, Elting LS, Couch RB, et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13:437–440. [PubMed] [Google Scholar]
- 10.Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27–30. doi: 10.1016/s0002-9343(97)00007-7. [DOI] [PubMed] [Google Scholar]
- 11.Ljungman P. Prevention and treatment of viral infections in stem cell transplant recipients. Br J Haematol. 2002;118:44–57. doi: 10.1046/j.1365-2141.2002.03515.x. [DOI] [PubMed] [Google Scholar]
- 12.Ljungman P, Ward KN, Crooks BN, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 13.Roghmann M, Ball K, Erdman D, et al. Active surveillance for respiratory virus infections in adults who have undergone bone marrow and peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;32:1085–1088. doi: 10.1038/sj.bmt.1704257. [DOI] [PubMed] [Google Scholar]
- 14.Raboni SM, Nogueira MB, Tsuchiya LR, et al. Respiratory tract viral infections in bone marrow transplant patients. Transplantation. 2003;76:142–146. doi: 10.1097/01.TP.0000072012.26176.58. [DOI] [PubMed] [Google Scholar]
- 15.Machado CM, Boas LS, Mendes AV, et al. Low mortality rates related to respiratory virus infections after bone marrow transplantation. Bone Marrow Transplant. 2003;31:695–700. doi: 10.1038/sj.bmt.1703900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small TN, Casson A, Malak SF, et al. Respiratory syncytial virus infection following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:321–327. doi: 10.1038/sj.bmt.1703365. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Champlin RE, Englund J, et al. Respiratory syncytial virus upper respiratory tract illnesses in adult blood and marrow transplant recipients: combination therapy with aerosolized ribavirin and intravenous immunoglobulin. Bone Marrow Transplant. 2000;25:751–755. doi: 10.1038/sj.bmt.1702228. [DOI] [PubMed] [Google Scholar]
- 18.Hohenthal U, Nikoskelainen J, Vainionpaa R, et al. Parainfluenza virus type 3 infections in a hematology unit. Bone Marrow Transplant. 2001;27:295–300. doi: 10.1038/sj.bmt.1702776. [DOI] [PubMed] [Google Scholar]
- 19.McCann S, Byrne JL, Rovira M, et al. Outbreaks of infectious diseases in stem cell transplant units: a silent cause of death for patients and transplant programmes. Bone Marrow Transplant. 2004;33:519–529. doi: 10.1038/sj.bmt.1704380. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, Champlin R, Couch R, et al. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29:528–532. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]
- 21.Ison MG, Hayden FG, Kaiser L, Corey L, Boeckh M. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis. 2003;36:1139–1143. doi: 10.1086/374340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakrabarti S, Osman H, Collingham KE, Fegan CD, Milligan DW. Enterovirus infections following T-cell depleted allogeneic transplants in adults. Bone Marrow Transplant. 2004;33:425–430. doi: 10.1038/sj.bmt.1704359. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez Y, Martino R, Badell I, et al. Pulmonary enterovirus infections in stem cell transplant recipients. Bone Marrow Transplant. 1999;23:511–513. doi: 10.1038/sj.bmt.1701605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ljungman P, Gleaves CA, Meyers JD. Respiratory virus infection in immunocompromised patients. Bone Marrow Transplant. 1989;4:35–40. [PubMed] [Google Scholar]
- 25.Cortez KJ, Erdman DD, Peret TC, et al. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis. 2001;184:1093–1097. doi: 10.1086/322041. [DOI] [PubMed] [Google Scholar]
- 26.Nichols WG, Erdman DD, Han A, et al. Prolonged outbreak of human parainfluenza virus 3 infection in a stem cell transplant outpatient department: insights from molecular epidemiologic analysis. Biol Blood Marrow Transplant. 2004;10:58–64. doi: 10.1016/j.bbmt.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 27.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabella N, Rodriguez P, Labeaga R, et al. Conventional respiratory viruses recovered from immunocompromised patients: clinical considerations. Clin Infect Dis. 1999;28:1043–1048. doi: 10.1086/514738. [DOI] [PubMed] [Google Scholar]
- 30.Gleaves CA, Rice DH, Meyers JD. Use of serum substitutes for the growth of four cell lines commonly used for virus isolation. J Virol Methods. 1990;28:171–178. doi: 10.1016/0166-0934(90)90032-b. [DOI] [PubMed] [Google Scholar]
- 31.Palacios G, Casas I, Tenorio A, Freire C. Molecular identification of enterovirus by analyzing a partial VP1 genomic region with different methods. J Clin Microbiol. 2002;40:182–192. doi: 10.1128/JCM.40.1.182-192.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19:1–7. doi: 10.1016/0196-6553(91)90154-5. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez Y, Martino R, Rabella N, et al. Community respiratory virus infections in patients with hematologic malignancies. Haematologica. 1999;84:820–823. [PubMed] [Google Scholar]
- 34.Dykewicz CA. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients: focus on community respiratory virus infections. Biol Blood Marrow Transplant. 2001;7(suppl):19S–22S. doi: 10.1053/bbmt.2001.v7.pm11777100. [DOI] [PubMed] [Google Scholar]
- 35.Englund JA, Champlin RE, Wyde PR, et al. Common emergence of amantadine- and rimantadine-resistant influenza A viruses in symptomatic immunocompromised adults. Clin Infect Dis. 1998;26:1418–1424. doi: 10.1086/516358. [DOI] [PubMed] [Google Scholar]
- 36.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: regression modeling. Bone Marrow Transplant. 2001;28:1001–1011. doi: 10.1038/sj.bmt.1703271. [DOI] [PubMed] [Google Scholar]
- 37.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant. 2001;28:909–915. doi: 10.1038/sj.bmt.1703260. [DOI] [PubMed] [Google Scholar]
- 38.Ison MG, Hayden FG. Viral infections in immunocompromised patients: what’s new with respiratory viruses? Curr Opin Infect Dis. 2002;15:355–367. doi: 10.1097/00001432-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Machado CM, Boas LS, Mendes AV, et al. Use of Oseltamivir to control influenza complications after bone marrow transplantation. Bone Marrow Transplant. 2004;34:111–114. doi: 10.1038/sj.bmt.1704534. [DOI] [PubMed] [Google Scholar]
- 40.Hassan IA, Chopra R, Swindell R, Mutton KJ. Respiratory viral infections after bone marrow/peripheral stem-cell transplantation: the Christie hospital experience. Bone Marrow Transplant. 2003;32:73–77. doi: 10.1038/sj.bmt.1704048. [DOI] [PubMed] [Google Scholar]
- 41.Chakrabarti S, Avivi I, Mackinnon S, et al. Respiratory virus infections in transplant recipients after reduced-intensity conditioning with Campath-1H: high incidence but low mortality. Br J Haematol. 2002;119:1125–1132. doi: 10.1046/j.1365-2141.2002.03992.x. [DOI] [PubMed] [Google Scholar]
- 42.Wingard JR, Nichols WG, McDonald GB. Supportive care. Hematology (Am Soc Hematol Educ Program) 2004:372–389. doi: 10.1182/asheducation-2004.1.372. [DOI] [PubMed] [Google Scholar]
- 43.Watzinger F, Suda M, Preuner S, et al. Real-time quantitative PCR assays for detection and monitoring of pathogenic human viruses in immunosuppressed pediatric patients. J Clin Microbiol. 2004;42:5189–5198. doi: 10.1128/JCM.42.11.5189-5198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Englund JA. Diagnosis and epidemiology of community-acquired respiratory virus infections in the immunocompromised host. Biol Blood Marrow Transplant. 2001;7(suppl):2S–4S. doi: 10.1053/bbmt.2001.v7.pm11777101. [DOI] [PubMed] [Google Scholar]
- 45.Van Elden LJ, van Loon AM, van der Beek A, et al. Applicability of a real-time quantitative PCR assay for diagnosis of respiratory syncytial virus infection in immunocompromised adults. J Clin Microbiol. 2003;41:4378–4381. doi: 10.1128/JCM.41.9.4378-4381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Elden LJ, van Kraaij MG, Nijhuis M, et al. Polymerase chain reaction is more sensitive than viral culture and antigen testing for the detection of respiratory viruses in adults with hematological cancer and pneumonia. Clin Infect Dis. 2002;34:177–183. doi: 10.1086/338238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musher DM. How contagious are common respiratory tract infections? N Engl J Med. 2003;348:1256–1266. doi: 10.1056/NEJMra021771. [DOI] [PubMed] [Google Scholar]
- 48.Couch RB. Prevention and treatment of influenza. N Engl J Med. 2000;343:1778–1787. doi: 10.1056/NEJM200012143432407. [DOI] [PubMed] [Google Scholar]
- 49.DeVincenzo J. Pre-emptive ribavirin for ‘RSV’ in BMT. Bone Marrow Transplant. 2000;26:113–114. doi: 10.1038/sj.bmt.1702436. [DOI] [PubMed] [Google Scholar]
- 50.Elizaga J, Olavarria E, Apperley J, Goldman J, Ward K. Parainfluenza virus 3 infection after stem cell transplant: relevance to outcome of rapid diagnosis and ribavirin treatment. Clin Infect Dis. 2001;32:413–418. doi: 10.1086/318498. [DOI] [PubMed] [Google Scholar]
- 51.Latchford T, Shelton BK. Respiratory syncytial virus in blood and marrow transplant recipients. Clin J Oncol Nurs. 2003;7:418–422. doi: 10.1188/03.CJON.418-422. [DOI] [PubMed] [Google Scholar]
- 52.Johny AA, Clark A, Price N, et al. The use of zanamivir to treat influenza A and B infection after allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29:113–115. doi: 10.1038/sj.bmt.1703343. [DOI] [PubMed] [Google Scholar]
- 53.Kaiser L, Wat C, Mills T, et al. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163:1667–1672. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- 54.Boeckh M, Gooley T, Englund J, et al. Respiratory syncytial virus (RSV) infection in HSCT recipients: risk factors for acquisition and lower respiratory tract disease [abstract] Blood. 2004;104:57a–58a. [Google Scholar]
- 55.Englund JA, Sullivan CJ, Jordan MC, et al. Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med. 1988;109:203–208. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- 56.Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Marrow Transplant. 2001;7(suppl):5S–7S. doi: 10.1053/bbmt.2001.v7.pm11777102. [DOI] [PubMed] [Google Scholar]
- 57.Ljungman P. Respiratory virus infections in bone marrow transplant recipients: the European perspective. Am J Med. 1997;102:44–47. doi: 10.1016/s0002-9343(97)00010-7. [DOI] [PubMed] [Google Scholar]
- 58.Whimbey E, Englund JA, Couch RB. Community respiratory virus infections in immunocompromised patients with cancer. Am J Med. 1997;102:10–18. doi: 10.1016/S0002-9343(97)80004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anaissie EJ, Mahfouz TH, Aslan T, et al. The natural history of respiratory syncytial virus infection in cancer and transplant patients: implications for management. Blood. 2004;103:1611–1617. doi: 10.1182/blood-2003-05-1425. [DOI] [PubMed] [Google Scholar]
- 60.Bredius RG, Templeton KE, Scheltinga SA, et al. Prospective study of respiratory viral infections in pediatric hemopoietic stem cell transplantation patients. Pediatr Infect Dis J. 2004;23:518–522. doi: 10.1097/01.inf.0000125161.33843.bb. [DOI] [PubMed] [Google Scholar]
- 61.Chakrabarti S, Collingham KE, Marshall T, et al. Respiratory virus infections in adult T cell-depleted transplant recipients: the role of cellular immunity. Transplantation. 2001;72:1460–1463. doi: 10.1097/00007890-200110270-00024. [DOI] [PubMed] [Google Scholar]
- 62.Hebart H, Einsele H. Specific infectious complications after stem cell transplantation. Support Care Cancer. 2004;12:80–85. doi: 10.1007/s00520-003-0511-3. [DOI] [PubMed] [Google Scholar]
- 63.Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug-resistant influenza A virus in an immunocompromised patient. N Engl J Med. 2003;348:867–868. doi: 10.1056/NEJM200302273480923. [DOI] [PubMed] [Google Scholar]
- 64.Cane PA, van den Hoogen BG, Chakrabarti S, Fegan CD, Osterhaus AD. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 2003;31:309–310. doi: 10.1038/sj.bmt.1703849. [DOI] [PubMed] [Google Scholar]
- 65.McIntosh K, McAdam AJ. Human metapneumovirus—an important new respiratory virus. N Engl J Med. 2004;350:431–433. doi: 10.1056/NEJMp038212. [DOI] [PubMed] [Google Scholar]
- 66.van den Hoogen BG, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23:S25–S32. doi: 10.1097/01.inf.0000108190.09824.e8. [DOI] [PubMed] [Google Scholar]
- 67.Vicente D, Montes M, Cilla G, Perez-Trallero E. Human metapneumovirus and chronic obstructive pulmonary disease. Emerg Infect Dis. 2004;10:1338–1339. doi: 10.3201/eid1007.030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vicente D, Cilla G, Montes M, Perez-Trallero E. Human metapneumovirus and community-acquired respiratory illness in children. Emerg Infect Dis. 2003;9:602–603. doi: 10.3201/eid0905.020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Englund J, Nichols WG, Kuypers J, et al. Fatal human metapneumovirus infections in stem cell transplant recipients [abstract] Blood. 2004;104:58a. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]