Abstract
We propose a new classification of severe early childhood caries (S-ECC): hypoplasia-associated severe early childhood caries (HAS-ECC). This form of caries affects mostly young children living at or below poverty, characterized by structurally damaged primary teeth that are particularly vulnerable to dental caries. These predisposing developmental dental defects are mainly permutations of enamel hypoplasia (EHP). Anthropologists and dental researchers consider EHP an indicator for infant and maternal stresses including malnutrition, a variety of illnesses, and adverse birthing conditions. Differentiation of HAS-ECC from other forms of early childhood caries is warranted because of its distinct etiology, clinical presentation, and eventual management. Defining HAS-ECC has important clinical implications: Therapies that control or prevent other types of caries are likely to be less effective with HAS-ECC because the structural integrity of the teeth is compromised prior to their emergence into the oral cavity. By the time these children present to the dentist, the treatment options often become limited to surgical management under general anesthesia. To prevent HAS-ECC, dentists must partner with other health providers to develop interventions that begin with pregnant mothers, with the aim of eliminating or ameliorating the covariates accompanying poverty, including better pre- and post-natal care and nutrition.
Keywords: caries, tooth development, odontogenesis, pediatric dentistry, Streptococcus mutans, access to care
Caries Affects Mainly the Poor
As the numbers of children living at or below the poverty level increase in the US and globally, the incidence of early childhood caries rises, despite falling caries prevalence within the general child population (US Department of Health and Human Services, 2000; Oliveira et al., 2006; Psoter et al., 2006; Vargas and Ronzio, 2006). This rising “epidemic” of caries correlates roughly with the rising number of children living in poverty and poor health. It has been asserted that 80% of caries can be found within 20% of the population—this 20% being the nation’s poorest. The most recent US Census reports one in five American children living at or below poverty, a proportion that continues to grow (http://www.census.gov/prod/2011pubs/acsbr10-05.pdf). Although being poor per se does not result in caries, a substandard diet consisting mainly of processed food high in sugar and low in protein is the necessary co-condition for most forms of caries, along with certain risk factors enumerated below. Communities that lack access to traditional food sources because of their unavailability and unaffordability, such as the Native American Indian population, are an example. Equally troublesome is that the inner-city and rural poor fill their caloric needs with these low-value foods, leading to another ever-increasing form of malnutrition, obesity. Although obesity is often mistaken as evidence of adequate nutrition, it is yet another form of malnutrition, and its prevalence is increasing, particularly among the nation’s poor (http://www.cdc.gov/obesity/childhood/data.html). Obesity is a risk factor for unfavorable birth outcomes and developmental enamel defects (Needleman et al., 1992) that can lead to increased susceptibility to caries. Maternal obesity is also associated with “nursing bottle caries” (Johnsen, 1982).
A yet-to-be-defined but growing cohort of children suffers from a severe and rampant form of dental caries, so destructive that often by age 3 yrs, most primary teeth are so damaged that their restoration involves outpatient hospitalization. In the US, these children are mainly members of a minority group, including recent immigrants, farm workers, Native American Indians, and Eskimos, who share the common attributes of being poor and/or detached from their traditional lifestyle and diet. Worldwide, this same form of aggressive childhood caries can be found among minority and indigenous groups, where poverty intersects with inadequacies in perinatal health care and poor nutrition. This form of caries has also been called “rampant caries”, “nursing bottle caries”, “baby bottle tooth decay”, and, more recently, severe early childhood caries (S-ECC) (Davies, 1998; Drury et al., 1999; Ismail and Sohn, 1999; Vadiakas, 2008) and is concentrated mainly among the poorest of children (Oliveira et al., 2006).
We propose and define a subgroup of S-ECC having specific antecedent conditions common to children living in poverty: one or multiple perinatal stresses resulting in enamel hypoplasia (EHP) coupled with a dependence on low-cost, processed food high in sugars and carbohydrates. A third, but less-well-supported, antecedent is the higher levels and/or early colonization of the dentition by cariogenic bacteria such as the mutans streptococci. We name this specific form of severe-ECC as Hypoplasia-associated Severe Early Childhood Caries (HAS-ECC). Other terms used to describe this condition likely include or overlap with HAS-ECC, as will be discussed further.
Here we reviewed a select number of studies supporting the argument that HAS-ECC constitutes a distinct and biologically plausible subgroup of S-ECC with clearly defined etiological or causative antecedents from which risk assessment and preventive/management strategies can be proposed and tested. It is not the intent of this article to review exhaustively all aspects of early childhood caries, since comprehensive and informative reviews already exist (Davies, 1998; Seow, 1998; Drury et al., 1999; Ismail and Sohn, 1999; Vadiakas, 2008). Our intention is to connect the existing studies that specifically define HAS-ECC and attempt to assemble a group of risk factors that may be used by healthcare workers to attenuate or anticipate this debilitating disease before extensive damage occurs.
Current Definitions of ECC are Inadequate
The commonly used term “early childhood caries” or ECC is presently defined mainly by the number of teeth affected, absent either a pattern affiliation or an etiologically based definition. (Johnsen, 1984; Johnsen et al., 1987; Davies, 1998; Drury et al., 1999; Ismail and Sohn, 1999). The current definition of ECC remains remarkably non-informative, e.g., one or more instances of caries anywhere in the primary dentition in a child under 6 yrs of age (American Academy of Pediatric Dentistry; AAPD, 2011). A more severe form of caries affecting children under the age of 3 yrs with decay to the smooth surfaces is referred to as Severe Early Childhood Caries (S-ECC) (Drury et al., 1999; Ismail and Sohn, 1999). Two challenges exist with these definitions: (1) They describe numbers of teeth or surfaces affected (greater than 1) but fail to distinguish differences in patterns or etiologies (Johnsen, 1984; Johnsen et al., 1987; Psoter et al., 2006); and (2) they imply that, by nature of its numerical definition, caries in early childhood might be a continuum, with S-ECC as just a more severe form of ECC. Moreover, the distinction between S-ECC and ECC is not universally acknowledged (Davies, 1998; Seow et al., 2009). Challenges further hampering this effort to define various forms of ECC, particularly S-ECC, include the fact that: (1) no national registry exists for S-ECC treated in an outpatient hospital setting; (2) those most likely knowledgeable to diagnose and/or manage S-ECC – dental professionals – generally see children only after teeth are badly damaged by caries, thus obscuring contributing preconditions such as EHP or other developmental defects of teeth; and (3) children living in poverty or in rural communities lack access to health care in general, so early signs of S-ECC, even if evident, continue to progress rapidly to the point of limited treatment options. We contend that some, perhaps most, of the cases of S-ECC associated with nursing bottles (or rampant caries) are consistent with what we will define as HAS-ECC. We propose that a subgroup of S-ECC (formerly referred to as rampant caries or “nursing bottle caries”) can be more precisely defined based on relevant criteria including age of onset, pattern consistent with EHP, severity of destruction, rapidity of progression, and bacteriological profiles.
Enamel Hypoplasia (EHP)
Enamel hypoplasia (EHP) is one of several forms of developmental defects of enamel (DDE) identified by the Federation Dentaire Internationale (FDI) Commission on Oral Health (1992) and defined as a quantitative disturbance of mineralized tissue formation during tooth development. For the primary dentition, the disturbance occurs either pre-natally or in early childhood (Seow, 1991; Li et al., 1995; Seow et al., 2005). These defects generally correspond to the developmental stage and degree of insult as the primary teeth form and are most commonly present in the readily visible primary incisors, canines, and first molars. Both pre- and post-natal stresses adversely affect ameloblasts and odontoblasts during tooth formation and can result in both hypoplastic and hypomineralized enamel (Suckling, 1989; Sabel et al., 2008). The most common evidence of perinatal stress affecting tooth formation is found in virtually every child and presents subclinically as the neonatal line (NNL) (Sabel et al., 2008). The NNL appears in both enamel and dentin of primary teeth, denoted by an accentuated stria, similar to the incremental line called the striae of Retzius (lines of von Ebner in dentin), the result of injury or stress to ameloblasts and odontoblasts at the time of birth. Prematurity or low birthweight leads to an increase in both the extent (width) and vertical position of the NNL (Sabel et al., 2008). Interestingly, children delivered by cesarean section have less-pronounced neonatal lines than infants who are products of prolonged or difficult labor (Sabel et al., 2008).
Similarly, ameloblasts and odontoblasts can be adversely affected in both the pre- and post-natal period of tooth development by other antecedents, including malnutrition in mother or child, illness such as measles, and maternal risk factors like obesity, smoking, liver disease, drug and alcohol use, and other risk factors leading to prematurity (Table). Key studies definitively linking the adverse effects of malnutrition and EHP were conducted by Sweeney and others (Sweeney and Guzman, 1966; Sweeney et al., 1969, 1971), resulting in a most pronounced form of EHP termed ‘linear hypoplasia’. Crucial to the line of evidence, Needleman and co-workers (Needleman et al., 1991, 1992) collected and examined exfoliated teeth from an urban Boston population, thus connecting adverse antecedent conditions to enamel defects. The subsequent work of Seow and colleagues demonstrated prematurity and low birthweight to be major contributors to EHP (Seow et al., 1987, 2009; Seow, 1991, 1997a,b, 1998; Pascoe and Seow, 1994; Lai et al., 1997).
Table.
Pre- and Post-natal Antecedents Linked to Developmental Enamel Defects and Enamel Hypoplasia
| Risk Factors Associated with EHP | Reference |
|---|---|
| Pre-natal (maternal) malnutrition | Alvarez et al., 1993; Infante and Gillespie, 1976; Needleman et al., 1991; Sweeney and Guzman, 1966; Sweeney et al., 1971 |
| Premature or low-birthweight (< 2500 g) infant | Lai et al., 1997 |
| Low socio-economic status | Milgrom et al., 2000; Needleman et al., 1992; Oliveira et al., 2006; Vargas and Ronzio, 2006 |
| Mineral deficiency | Seow et al., 1989 |
| Infant measles | Needleman et al., 1992 |
| Parental smoking | Needleman et al., 1992 |
| Delayed pre-natal care, low Apgar score, greater maternal weight at time of delivery | Needleman et al., 1992 |
| Maternal liver disease | Seow et al., 1991 |
| Respiratory distress, maternal and post-natal infections, GI tract infections, anemia, failure to thrive | Pascoe and Seow, 1994 |
The duration, severity, and combinations of antecedent risk factors likely influence the extent to which the dental tissue is damaged. Indeed, most of the antecedent factors listed in the Table are common covariates of poverty, e.g., inadequate and poor-quality nutrition and other high-risk behaviors. EHP has been linked to respiratory distress, post-natal infections, gastrointestinal tract infections, anemia, failure to thrive (Pascoe and Seow, 1994), and liver disease (Seow et al., 1991). It is important to note that HAS-ECC is not confined solely to children living in poverty; many of these disturbances just named can also occur in non-malnourished individuals with the risk factors named above.
The timing and extent of the various insult(s) are dutifully recorded within the forming enamel and dentin, reflecting the stage of tooth development. As a chronometer for an infant’s life events, these insults can leave their mark more than once on the developing tooth, and the defects can extend to every tooth surface of the primary dentition (see Fig. 1). Typically, EHP manifests on those teeth that are at critical stages of secretion of organic matrices and mineralization around the time of insult (i.e., the central incisors, laterals, and canines, and less frequently on first and second molars that are mineralized post-natally). When such perinatal injuries manifest as EHP and are visible on the tooth surfaces, the hypoplasic bands or defects progress toward the incisal tip of the teeth, from centrals to molars, producing what we call the characteristic and bilateral symmetrical “frown” pattern (Fig. 1). It is often possible to predict the time of insult based on the position and extent of EHP from visual examination, and more precisely from microscopic cross-sections (Fig. 2).
Figure 1.
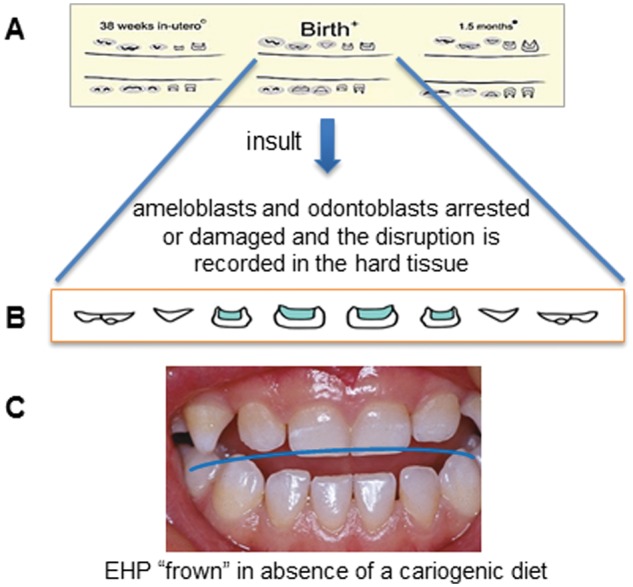
Pre- and post-natal developmental formation of the primary dentition. (A) Drawings from the recent histological study by AlQahtani and co-workers (AlQahtani et al., 2010). (B) The average developmental stage of the primary maxillary anteriors at birth (redrawn by permission of the authors). (C) Clinical representation of EHP that occurred around birth. Note the downward curve or “frown” coincidental to the stage of individual tooth development at time of insult. The child was malnourished but did not have access to refined sugar.
Figure 2.
Macroscopic and microscopic views of an incisor with HAS-ECC. (A) An extracted central incisor from a four-year-old child. A faint band of EHP including the mesial surface presents a large caries lesion. (B) Cross-section of the same incisor and imaged with polarized light microscopy. Note the pronounced neonatal line in both enamel and dentin with overlying evidence of insult occurring close to the birth of the infant. Also present is perturbed enamel structure in the region of the opaque band appearing clinically in (A). (C) Backscattered electron imaging in the scanning electron microscope (BSE-SEM) over the region of hypoplastic enamel reveals significant demineralization (color-coded orange, yellow, green, and blue, with decreasing density in enamel at left). (D) SEM images of the surface of enamel overlying the area of opacity and clear areas of disruption to surface integrity promoting retentive niches for cariogenic bacteria. (E) SEM images of other sites on the surface of the tooth display irregularities as well.
EHP and other developmental defects are not new to the human condition. For decades, anthropologists used EHP to estimate the health and living conditions of prehistoric populations. EHP records not only evidence of chronic malnutrition but also life-threatening forms of metabolic disturbances, including kidney and heart disease, diabetes, infections with both viral and bacterial agents, and fetal/neonatal conditions such as prematurity, Rh incompatibility, and allergies (Cook and Buikstra, 1979). Investigators have also suggested that some EHP may have a genetic predisposition, the degree of which so far appears to be less important than environmental factors (Taji et al., 2011).
Differentiating pre-eruptively-formed EHP from the pre-carious “white-spot” lesions associated with plaque accumulation and cariogenic diet can be difficult (Needleman et al., 1992; Seow, 1997a; Oliveira et al., 2006). Clinicians often attribute the appearance of chalky, opaque enamel along the gingival margin where plaque accumulates to poor diet and oral hygiene rather than to EHP. Since tooth eruption and development are correlated events, the location of demineralization due to caries often coincides with that due to EHP. Indeed, caries and a damaged enamel surface often occur together, but importantly, the critical distinction is that the defects caused by EHP precede (and promote) the overlying caries. Moreover, dental professionals typically do not see infants when teeth first emerge, so EHP goes undetected. Unlike the demineralization and subsequent caries thought to be the result of prolonged or inappropriate nursing bottle practices (i.e., nursing bottle caries or baby bottle syndrome), EHP is already present prior to tooth eruption into the oral cavity. In other words, primary teeth with EHP, particularly those in the anterior deciduous dentition, are structurally damaged before emerging into the oral cavity. As we will discuss later, defects in the surface become readily colonized niches for cariogenic bacteria such as Streptococcus mutans.
To this point, it seems plausible that the clinical entity called “nursing bottle caries” can co-exist with or exacerbate pre-existing EHP or be a separate and distinct form of S-ECC. The case for nursing bottle caries as a distinct subgroup of S-ECC comes from the observation that caries manifests mostly in the primary maxillary anterior teeth that are exposed to bottle contents at nighttime and follows the gingival margin, where plaque accumulates. That the lower incisors are usually not affected, presumably due to the protective effect of saliva, is compelling. We would argue that HAS-ECC and nursing bottle caries are not mutually exclusive events, often correlated, but clinical examinations of very young children as teeth emerge along with the pattern of caries would facilitate a differential diagnosis. As noted by Reisine and Douglass (1998), nursing bottle excesses may or may not lead to caries. Perhaps those who do develop “nursing bottle caries” have EHP as an antecedent. Further delineation between nursing bottle caries and HAS-ECC awaits properly designed studies with clearly defined variables.
Histological findings in primary teeth affected by EHP reveal the effects of various insults to developing teeth, serving as precise chronometers of events both pre- and post-natally. The NNL serves as a point of reference as to when the insult occurred. Periods of food scarcity, complications of birth, and exposure to infectious diseases leave their signature on the developing tooth and skeletal structures. Fig. 2A shows an extracted central incisor from a four-year-old child with HAS-ECC examined at different depths, revealing a faint band of EHP (arrow) including the mesial surface presenting a large caries lesion that extended into the pulp. A cross-section of the same incisor imaged by polarized light microscopy illustrates the pronounced neonatal line in both enamel and dentin with overlying evidence of insult represented as mild hypoplasia soon after birth. Polarizing microscopy also reveals a change in below-surface enamel structure in the vicinity of the opaque band appearing clinically (Fig. 2A). Since this was likely the result of demineralization, we performed density-dependent backscattered electron imaging in the scanning electron microscope (BSE-SEM) imaging of the block of enamel adjacent to the section prepared for Fig. 2B. The color-coded BSE grey level image is shown in Fig. 2C (inset indicates that mineralization density decreases from warm to cool colors). The enamel mineralization density of affected enamel decreased under the area of EHP; blue and green represent the least dense enamel (a density similar to that of dentin, at right), while yellow and orange represent increasing density, and unaffected enamel is pink. SEM images of the surface of enamel in the vicinity of the opacity and hypoplastic enamel (Fig. 2D) reveal clear areas of disruption to surface integrity, promoting retentive niches for the colonization of cariogenic bacteria such as mutans streptococci. SEM imaging of other sites on the surface of the tooth display irregularities as well, such as chipping and palling of enamel (Fig. 2E). Clinical presentations depicted in Fig. 3 and Appendix Fig. 1 represent examples of what we propose as HAS-ECC (see Fig. 3 and Appendix Fig. 1).
Figure 3.
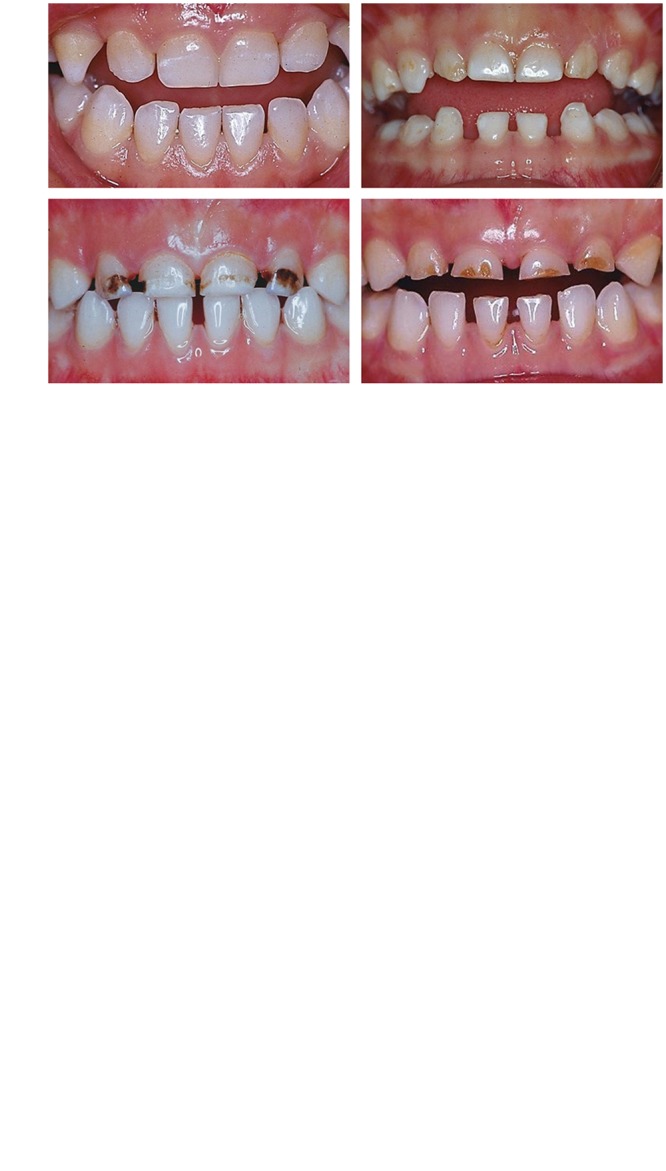
The clinical presentations from preschool children with various degrees of caries and/or EHP of HAS-ECC. Panel A shows EHP on multiple primary teeth without active caries that we propose to define as HAS-ECC, stage 0. Panel B shows primary teeth with EHP and a minor to moderate degree of caries that we propose to define as HAS-ECC, stage 1. Panel C shows primary teeth with EHP and severe caries which we propose to define as HAS-ECC, stage 2. These preschool children were from impoverished families, mainly from China, with a documented history of malnutrition (Li et al., 1995). Almost all of them were colonized with moderate to high levels of mutans streptococci (Li et al., 1994).
In some cases, the primary teeth are so undermined by EHP defects that entire sheets of enamel appear to fracture away. This pattern of apparent fracture parallel to the labial surface (and lines of Retzius) supports the case for EHP undermining the integrity of the enamel and differs from cavitation that occurs from the surface due to caries alone. Such en masse loss of enamel is evident from clinical images shown in Fig. 3 and Appendix Figs. 1 and 2.
Histological findings reveal irregular crystalline formation along the lines of Retzius, sometimes accumulating on the surface perikymata as pits or lines (Seow et al., 2005; Sabel et al., 2008) (Fig. 2). Teeth from pre-term children, many of whom have EHP, exhibit hypomineralized enamel and dentin with indications of porosity (Rythen et al., 2008, 2010). Seow et al. (2005) reported 20% less mineralized enamel overlying EHP as well as aberrant surface defects similar to those shown in Fig. 2. It is noteworthy that Seow and co-workers found surface defects in 52% of teeth in which no EHP was visible, indicating that EHP may not be clinically apparent in many cases.
That EHP is a major risk factor for subsequent caries requires longitudinal observations. Three such studies exist (Lai et al., 1997; Oliveira et al., 2006; Targino et al., 2011). Oliveira and co-workers showed that primary teeth with EHP are 15 times more likely to develop caries compared with non-EHP teeth. These investigators further made the critical distinction between white-spot lesions and EHP based on pattern differences and low SES. A recent study by Milgrom et al. (2000) showed EHP as a covariant to caries risk as well.
The Connection Between EHP and the Mutans Streptococci
While it seems logical that surface irregularities on the smooth surfaces of the teeth from EHP would promote the colonization of mutans streptococci (MS), the pivotal study linking EHP and elevated levels of MS was first reported by Li and co-investigators in 1994 (Li et al., 1994). Examining over 480 three- to four-year-old undernourished children in Miyun, China, they showed that children with EHP had elevated levels of MS compared with non-EHP children, attributed to the presence of a retentive niche provided by EHP defects, which, in turn, promoted higher colonization levels. Subsequent studies by Wan et al. (2003) confirmed that low-birthweight children with EHP were 4.4 times more likely to be infected by MS than non-EHP children. Although these authors concluded that early infection among low-birthweight children was contrary to the concept of a “window of infectivity” derived from an urban American population (Caufield et al., 1993), their findings do support the underlying premise of the window concept, i.e., presence of colonization-favorable ecological sites dictates the window’s margins. Instead of fissures in molars, the presence of smooth-surface retentive areas would be expected to open the window of infectivity sooner, as Wan et al. reported (Wan et al., 2003).
Earlier colonization by MS in young children generally translates into increased caries rates (Kohler et al., 1988). The association between MS and rampant or nursing bottle caries has convincing support in the literature. For example, van Houte and co-workers (1982) reported that some children with nursing caries had levels of MS as high as 60% of the total cultivable microbiota. Lesser MS counts, but still greater than 10% of the cultivable biota, were reported by Berkowitz et al. (1984). In our population of HAS-ECC Hispanic children from Bellevue Hospital in New York City, we observed elevated levels of MS in saliva, but averaging less than 1% of the total cultivable biota (Li et al., 2007). More recent studies, based on cultivation (Tanner et al., 2011) and microarray together with a16S rRNA survey (Becker et al., 2002), confirmed a clear presence of S. mutans in most, but not all, cases of S-ECC. MS in levels over 1% of the total biota are considered a risk indicator for caries (Loesche, 1986). Microbes other than, or in addition to, MS, which preferentially colonize retentive sites include the lactobacilli (Li et al., 1994; Marchant et al., 2001; Becker et al., 2002; Gross et al., 2010; Yang et al., 2010) and possibly several novel species of microbes (Li et al., 2007; Tanner et al., 2011). It is also noteworthy that the overall microbial diversity in plaque of children with S-ECC (HAS-ECC) is significantly less diverse than that of caries-free controls (Li et al., 2007).
Collectively, these studies suggest that children with HAS-ECC are prone to early colonization by MS, due to retentive surface defects associated with EHP. While most of the literature points to MS as the major pathogen associated with HAS-ECC, presumably due to its predilection for colonizing retentive sites, other cariogenic bacteria with similar ecological requirements are likely to be found.
Putting It All Together – Defining HAS-ECC
Thus, we present the following model for the pathogenesis of HAS-ECC (Fig. 4). Primary teeth forming in utero are adversely affected by various insults to embryonic cells responsible for dentin and enamel formation. These insults are comprised mainly of covariates associated with low SES and poverty, including malnutrition and possibly other dietary deficiencies, low birthweight and prematurity, pre- and post-natal infectious diseases, and a host of other risk factors affecting both mother and newborn. The collective impact results in various manifestations of tooth damage, clinically presenting in most cases as EHP. EHP is most often seen in the maxillary anterior teeth of the primary dentition but can extend to other teeth, including molars. Teeth with EHP are vulnerable to early and elevated colonization by cariogenic bacteria, notably MS and lactobacilli, promoting early caries at the ecological sites of enamel defects, leading to what we call HAS-ECC. However, early or elevated colonization of MS and other cariogenic bacteria is necessary, but not sufficient. Without a caries-promoting diet high in fermentable carbohydrates, EHP probably would not progress to HAS-ECC. It is the duality of EHP and poor diet that defines HAS-ECC. We further suggest that the present definition of ECC encompasses not just one disease, but several, each distinct with different natural histories, antecedent contributors, anatomical sites, bacterial etiology, severity or aggressiveness, chronic vs. acute status, and distribution within different racial/ethnic groups. The divisions become blurred in these groups because of shared common etiologies, i.e., cariogenic bacteria and a caries-promoting diet. Some have attributed S-ECC to nursing bottle and even prolonged breast-feeding habits, but this association is mainly a correlation, not causation. We define HAS-ECC as a subgroup of what is already termed S-ECC but is etiologically distinct from other possible causes of S-ECC such as nursing bottle caries, although we would argue that some or most of the cases attributed to nursing bottle caries have underlying EHP and would be more appropriately called HAS-ECC.
Figure 4.

Model of the sequence of events leading to HAS-ECC (see text). MS, mutans streptococci.
Acknowledgments
The impetus for writing this article was to a large extent the result of a series of symposia championed by Dr. Dee Robertson and sponsored by the American Dental Association. We thank Catherine Schon, Howard Needleman, and Dee Robertson for their valued insight and critical comments. Shiyong Liu, Peking University School of Stomatology. provided most of the HAS-ECC photos, and Bin Hu prepared the histological sections.
Footnotes
Portions of the research reported here were supported by grants DE13937 and DE019455 from NIDCR. Research support was also provided by the 2010 Max Planck Research Award to TGB, administered by the Max Planck Society and the Alexander von Humboldt Foundation in respect of the Hard Tissue Research Program in Human Paleobiomics.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- AlQahtani SJ, Hector MP, Liversidge HM. (2010). Brief communication: the London atlas of human tooth development and eruption. Am J Phys Anthropol 142:481-490 [DOI] [PubMed] [Google Scholar]
- Alvarez JO, Caceda J, Woolley TW, Carley KW, Baiocchi N, Caravedo L, et al. (1993). A longitudinal study of dental caries in the primary teeth of children who suffered from infant malnutrition. J Dent Res 72:1573-1576 [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatric Dentistry (2011). Policy on early childhood caries (ECC): classifications, consequences, and preventive strategies. http://www.aapd.org/media/policies_Guidelines/P_ECCClassifications.pdf [PubMed]
- Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, et al. (2002). Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40:1001-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz RJ, Turner J, Hughes C. (1984). Microbial characteristics of the human dental caries associated with prolonged bottle-feeding. Arch Oral Biol 29:949-951 [DOI] [PubMed] [Google Scholar]
- Caufield PW, Cutter GR, Dasanayake AP. (1993). Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res 72:37-45 [DOI] [PubMed] [Google Scholar]
- Cook DC, Buikstra JE. (1979). Health and differential survival in prehistoric populations: prenatal dental defects. Am J Phys Anthropol 51:649-664 [DOI] [PubMed] [Google Scholar]
- Davies GN. (1998). Early childhood caries—a synopsis. Community Dent Oral Epidemiol 26(1 Suppl):106S-116S [DOI] [PubMed] [Google Scholar]
- Drury TF, Horowitz AM, Ismail AI, Maertens MP, Rozier RG, Selwitz RH. (1999). Diagnosing and reporting early childhood caries for research purposes. A report of a workshop sponsored by the National Institute of Dental and Craniofacial Research, the Health Resources and Services Administration, and the Health Care Financing Administration. J Public Health Dent 59:192-197 [DOI] [PubMed] [Google Scholar]
- FDI (1992). A review of developmental defects of enamel (DDE) index. Commission on Oral Health, Research & Epidemiology. Report of a Federation Dentaire Internationale Working Group. Int Dent J 42:411-426 [PubMed] [Google Scholar]
- Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, et al. (2010). Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol 48:4121-4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante PF, Gillespie GM. (1976). Dental caries experience in the deciduous dentition of rural Guatemalan children ages 6 months to 7 years. J Dent Res 55:951-957 [DOI] [PubMed] [Google Scholar]
- Ismail AI, Sohn W. (1999). A systematic review of clinical diagnostic criteria of early childhood caries. J Public Health Dent 59:171-191 [DOI] [PubMed] [Google Scholar]
- Johnsen DC. (1982). Characteristics and backgrounds of children with “nursing caries”. Pediatr Dent 4:218-224 [PubMed] [Google Scholar]
- Johnsen DC. (1984). Dental caries patterns in preschool children. Dent Clin North Am 28:3-20 [PubMed] [Google Scholar]
- Johnsen DC, Bhat M, Kim MT, Hagman FT, Allee LM, Creedon RL, et al. (1987). Caries in primary dentition. Ohio Dent J 61:47-52 [PubMed] [Google Scholar]
- Kohler B, Andreen I, Jonsson B. (1988). The earlier the colonization by mutans streptococci, the higher the caries prevalence at 4 years of age. Oral Microbiol Immunol 3:14-17 [DOI] [PubMed] [Google Scholar]
- Lai PY, Seow WK, Tudehope DI, Rogers Y. (1997). Enamel hypoplasia and dental caries in very-low birthweight children: a case-controlled, longitudinal study. Pediatr Dent 19:42-49 [PubMed] [Google Scholar]
- Li Y, Navia JM, Caufield PW. (1994). Colonization by mutans streptococci in the mouths of 3- and 4-year-old Chinese children with or without enamel hypoplasia. Arch Oral Biol 39:1057-1062 [DOI] [PubMed] [Google Scholar]
- Li Y, Navia JM, Bian JY. (1995). Prevalence and distribution of developmental enamel defects in primary dentition of Chinese children 3-5 years old. Community Dent Oral Epidemiol 23:72-79 [DOI] [PubMed] [Google Scholar]
- Li Y, Ge Y, Saxena D, Caufield PW. (2007). Genetic profiling of the oral microbiota associated with severe early-childhood caries. J Clin Microbiol 45:81-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ. (1986). Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant S, Brailsford SR, Twomey AC, Roberts GJ, Beighton D. (2001). The predominant microflora of nursing caries lesions. Caries Res 35:397-406 [DOI] [PubMed] [Google Scholar]
- Milgrom P, Riedy CA, Weinstein P, Tanner AC, Manibusan L, Bruss J. (2000). Dental caries and its relationship to bacterial infection, hypoplasia, diet, and oral hygiene in 6- to 36-month-old children. Community Dent Oral Epidemiol 28:295-306 [DOI] [PubMed] [Google Scholar]
- Needleman HL, Leviton A, Allred E. (1991). Macroscopic enamel defects of primary anterior teeth–types, prevalence, and distribution. Pediatr Dent 13:208-216 [PubMed] [Google Scholar]
- Needleman HL, Allred E, Bellinger D, Leviton A, Rabinowitz M, Iverson K. (1992). Antecedents and correlates of hypoplastic enamel defects of primary incisors. Pediatr Dent 14:158-166 [PubMed] [Google Scholar]
- Oliveira AF, Chaves AM, Rosenblatt A. (2006). The influence of enamel defects on the development of early childhood caries in a population with low socioeconomic status: a longitudinal study. Caries Res 40:296-302 [DOI] [PubMed] [Google Scholar]
- Pascoe L, Seow WK. (1994). Enamel hypoplasia and dental caries in Australian aboriginal children: prevalence and correlation between the two diseases. Pediatr Dent 16:193-199 [PubMed] [Google Scholar]
- Psoter WJ, Pendrys DG, Morse DE, Zhang H, Mayne ST. (2006). Associations of ethnicity/race and socioeconomic status with early childhood caries patterns. J Public Health Dent 66:23-29 [DOI] [PubMed] [Google Scholar]
- Reisine S, Douglass JM. (1998). Psychosocial and behavioral issues in early childhood caries. Community Dent Oral Epidemiol 26(1 Suppl):32S-44S. [DOI] [PubMed] [Google Scholar]
- Rythen M, Noren JG, Sabel N, Steiniger F, Niklasson A, Hellstrom A, et al. (2008). Morphological aspects of dental hard tissues in primary teeth from preterm infants. Int J Paediatr Dent 18:397-406 [DOI] [PubMed] [Google Scholar]
- Rythen M, Sabel N, Dietz W, Robertson A, Noren JG. (2010). Chemical aspects on dental hard tissues in primary teeth from preterm infants. Eur J Oral Sci 118:389-395 [DOI] [PubMed] [Google Scholar]
- Sabel N, Johansson C, Kuhnisch J, Robertson A, Steiniger F, Noren JG, et al. (2008). Neonatal lines in the enamel of primary teeth—a morphological and scanning electron microscopic investigation. Arch Oral Biol 53:954-963 [DOI] [PubMed] [Google Scholar]
- Seow WK. (1991). Enamel hypoplasia in the primary dentition: a review. ASDC J Dent Child 58:441-452 [PubMed] [Google Scholar]
- Seow WK. (1997a). Clinical diagnosis of enamel defects: pitfalls and practical guidelines. Int Dent J 47:173-182 [DOI] [PubMed] [Google Scholar]
- Seow WK. (1997b). Effects of preterm birth on oral growth and development. Aust Dent J 42:85-91 [DOI] [PubMed] [Google Scholar]
- Seow WK. (1998). Biological mechanisms of early childhood caries. Community Dent Oral Epidemiol 26(1 Suppl):8S-27S. [DOI] [PubMed] [Google Scholar]
- Seow WK, Humphrys C, Tudehope DI. (1987). Increased prevalence of developmental dental defects in low birth-weight, prematurely born children: a controlled study. Pediatr Dent 9:221-225 [PubMed] [Google Scholar]
- Seow WK, Masel JP, Weir C, Tudehope DI. (1989). Mineral deficiency in the pathogenesis of enamel hypoplasia in prematurely born, very low birthweight children. Pediatr Dent 11:297-302 [PubMed] [Google Scholar]
- Seow WK, Shepherd RW, Ong TH. (1991). Oral changes associated with end-stage liver disease and liver transplantation: implications for dental management. ASDC J Dent Child 58:474-480 [PubMed] [Google Scholar]
- Seow WK, Young WG, Tsang AK, Daley T. (2005). A study of primary dental enamel from preterm and full-term children using light and scanning electron microscopy. Pediatr Dent 27:374-379 [PubMed] [Google Scholar]
- Seow WK, Clifford H, Battistutta D, Morawska A, Holcombe T. (2009). Case-control study of early childhood caries in Australia. Caries Res 43:25-35 [DOI] [PubMed] [Google Scholar]
- Suckling GW. (1989). Developmental defects of enamel—historical and present-day perspectives of their pathogenesis. Adv Dent Res 3:87-94 [DOI] [PubMed] [Google Scholar]
- Sweeney EA, Guzman M. (1966). Oral conditions in children from three highland villages in Guatemala. Arch Oral Biol 11:687-698 [DOI] [PubMed] [Google Scholar]
- Sweeney EA, Cabrera J, Urrutia J, Mata L. (1969). Factors associated with linear hypoplasia of human deciduous incisors. J Dent Res 48:1275-1279 [DOI] [PubMed] [Google Scholar]
- Sweeney EA, Saffir AJ, De Leon R. (1971). Linear hypoplasia of deciduous incisor teeth in malnourished children. Am J Clin Nutr 24:29-31 [DOI] [PubMed] [Google Scholar]
- Taji SS, Seow WK, Townsend GC, Holcombe T. (2011). Enamel hypoplasia in the primary dentition of monozygotic and dizygotic twins compared with singleton controls. Int J Paediatr Dent 21:175-184 [DOI] [PubMed] [Google Scholar]
- Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, et al. (2011). Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol 49:1464-1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targino AG, Rosenblatt A, Oliveira AF, Chaves AM, Santos VE. (2011). The relationship of enamel defects and caries: a cohort study. Oral Dis 17:420-426 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (2000). Oral Health in America: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health [Google Scholar]
- Vadiakas G. (2008). Case definition, aetiology and risk assessment of early childhood caries (ECC): a revisited review. Eur Arch Paediatr Dent 9:114-125 [DOI] [PubMed] [Google Scholar]
- van Houte J, Gibbs G, Butera C. (1982). Oral flora of children with ‘nursing bottle caries’. J Dent Res 61:382-385 [DOI] [PubMed] [Google Scholar]
- Vargas CM, Ronzio CR. (2006). Disparities in early childhood caries. BMC Oral Health 6(Suppl 1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan AK, Seow WK, Purdie DM, Bird PS, Walsh LJ, Tudehope DI. (2003). A longitudinal study of Streptococcus mutans colonization in infants after tooth eruption. J Dent Res 82:504-508 [DOI] [PubMed] [Google Scholar]
- Yang R, Argimon S, Li Y, Zhou X, Caufield PW. (2010). Determining the genetic diversity of lactobacilli from the oral cavity. J Microbiol Methods 82:163-169 [DOI] [PMC free article] [PubMed] [Google Scholar]



