Abstract
Central nervous system organization of masticatory muscles determines the magnitude of joint and muscle forces. Validated computer-assisted models of neuromuscular organization during biting were used to determine organization in individuals with and without temporomandibular disorders (TMD). Ninety-one individuals (47 women, 44 men) were assigned to one of four diagnostic groups based on the presence (+) or absence (-) of pain (P) and bilateral temporomandibular joint disc displacement (DD). Electromyography and bite-forces were measured during right and left incisor and molar biting. Two three-dimensional models employing neuromuscular objectives of minimization of joint loads (MJL) or muscle effort (MME) simulated biting tasks. Evaluations of diagnostic group and gender effects on choice of best-fit model were by analysis of variance (ANOVA) and Tukey-Kramer post hoc tests, evaluations of right-left symmetry were by Chi-square and Fisher’s exact statistics, and evaluations of model accuracy were by within-subject linear regressions. MME was the best-fit during left molar biting in +DD individuals and incisor biting in men (all p < 0.03). Incisor biting symmetry in muscle organization was significantly higher (p < 0.03) in healthy individuals compared with those with TMD. Within-subject regressions showed that best-fit model errors were similar among groups: 8 to 15% (0.68 ≤ R2 ≤ 0.74). These computer-assisted models predicted muscle organization during static biting in humans with and without TMDs.
Keywords: human, modeling, neuromuscular, biting, temporomandibular disorders, masticatory muscles
Introduction
Like all synovial joint systems, the craniomandibular apparatus is mechanically indeterminate. Nevertheless, central nervous system organization of an individual’s muscles of mastication results in unique repeatable apportionment of muscle forces to produce bite-forces (Gonzalez et al., 2011). The effects of muscle forces on jaw biomechanics can be investigated effectively and non-invasively by computer-assisted modeling, which can overcome the problem of mechanical indeterminacy. Computer-assisted modeling, however, requires that some assumptions be made for a unique solution to be rendered. Local assumptions—such as assigning muscle forces based on muscle cross-sectional areas and averaged electromyographic (EMG) data, and constraining directions of joint loads (Hannam, 2011; Koolstra, 2002)—are one approach. In contrast, assumptions based on summarized population data produce results for static tasks that, while mathematically correct, may not accurately describe individual-specific biomechanics (Trainor et al., 1995).
Accuracy can be checked if the model uniquely predicts parameters that are measurable in vivo and, thus, are testable. An alternative computer-assisted modeling approach that accomplishes this uses three-dimensional numerical methods to render solutions based on an objective likely to be of biological importance, thus representing a theory of underlying neuromuscular control. Biologically important objectives such as maximization of bite-force and minimization of joint loads, joint loads-squared, muscle force, muscle effort, or muscle force-cubed have been investigated. During static biting, the organization of muscle forces appears to be consistent for healthy individuals in whom, for any given point of application and direction of mandibular load, the activation patterns of the musculature match neuromuscular objectives of minimization of joint loads or muscle effort (Iwasaki et al., 2003a,b, 2004; Nickel et al., 2003). Moreover, computer-assisted models of craniomandibular biomechanics have been useful tools to examine the growth (Nickel et al., 1988; de Zee et al., 2009) and shape (Iwasaki et al., 2010) of TMJ eminences and inter-individual differences in temporomandibular joint (TMJ) loads (Iwasaki et al., 2009a).
To date, computer-assisted models have primarily been used to study healthy individuals, and bilateral symmetry of muscle organization has been assumed. It is unknown if these models accurately predict muscle activation patterns in individuals with temporomandibular disorders (TMD). This project used validated computer-assisted numerical models that predicted masticatory muscle activation patterns based on objective functions of minimization of TMJ loads or muscle effort. The aim was to test if these objective functions accurately predicted masseter and temporalis muscle activities during static incisor and molar biting in healthy individuals and those with TMD.
Materials & Methods
Study Participants
Protocols were approved by the appropriate Institutional Review Boards, and each of 115 individuals gave informed consent. Ninety-one persons (47 women, 44 men) participated in the protocols. Based on results from qualified calibrated examiners using Research Diagnostic Criteria (Dworkin and LeResche, 1992) and computed-tomography and magnetic resonance (MR) images (Ahmad et al., 2009), study participants were assigned to one of four diagnostic groups according to the presence (+) or absence (-) of myofascial and/or TMJ pain (P) and bilateral TMJ disc displacement with reduction (DD). Those with a history of frank TMJ trauma, a diagnosed motor-neurological disease, or osseous TMJ degeneration were excluded from participating.
In vivo Protocol (same repeated at two sessions)
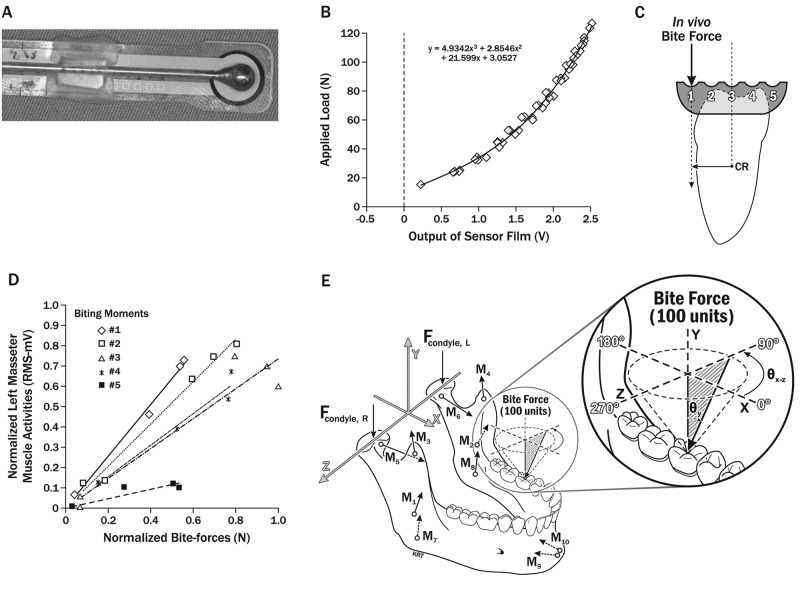
We tested accuracies of computer-assisted numerical models to predict individual-specific in vivo muscle activation patterns during biting tasks by comparing model results with measured masseter and anterior temporalis muscle activities per bite-force. The center of the muscle bulk was located by palpation, and bipolar surface electrodes were affixed to overlying skin as previously described (Nickel et al., 2003). Surface EMG data were recorded bilaterally during static biting tasks on a pre-calibrated bite-force transducer (Figs. 1A, 1B). The transducer was positioned between custom acrylic crowns on maxillary and mandibular right and left central incisors and first molars. Orientation of the transducer relative to the center of resistance of the mandibular teeth was controlled by 5 depressions aligned vestibulo-lingually on each mandibular crown (Fig. 1C). By this means, it was possible to control vestibulo-lingual direction and magnitude of a mechanical moment produced by the bite-force, as previously reported (Uchida et al., 2008). For each of four biting positions (left or right, incisors or molars), with opposing maxillary and mandibular crowns temporarily affixed (Band-Lok Blue, Reliance Orthodontic Products, Itasca, IL, USA), each individual was asked to produce a range of five comfortable bite-forces, each lasting for 5 sec, at each of the five depressions. Rest periods between bites were of approximately 10 seconds in duration. Muscle activities were amplified, viewed in real time, and stored. Data were analyzed for each bite, where muscle activities over a two-second period of approximately steady force were sampled at 2000 samples/second/channel and expressed as root-mean-square (RMS) values (microV), while bite-force (N) was averaged for the same period. For each individual, biting position, moment, and muscle, analyzed data from five bites were plotted, and slopes were calculated (RMS, mV/N; Fig. 1D) and normalized to peak slope. Within participants, normalized results from two sessions were compared with numerical model-predicted muscle activities relative to bite-force for the same biting positions and moments, for determination of model accuracy.
Figure 1.
Bite-force measurement and modeling. (A) Bite-force transducer consisting of a standardized spherical pseudo-bolus with a flattened side of approximately 5 mm diameter centered on sensor film (Tekscan Flexiforce®, Tekscan Inc., South Boston, MA, USA) which was attached to the wire handle of the pseudo-bolus with light-cured acrylic (modified from Gonzalez et al., 2011). (B) Calibration curve from pre-conditioned bite-force transducer (accuracy ± 1.5 N) showing applied ex vivo load vs. voltage output (V) from sensor film. (C) Line diagram: mesial view of right mandibular molar and custom acrylic crown with five spherical depressions, 5 mm apart, showing bite-force applied at depression #1 (most vestibular) and moment created relative to molar’s center of resistance (CR) (modified from Gonzalez et al., 2011). (D) Typical normalized data leading to slopes, showing regressions from five biting moments for a given participant’s muscle. The horizontal axis gives the normalized bite-forces (N), and the vertical axis gives the corresponding normalized root-mean-square (RMS-mV) muscle activities. (E) Force vectors involved in numerical models of static biting in humans: Applied bite-force (100 units), joints (Fcondyle), and representing five muscle pairs (M1,2 = masseter, M3,4 = anterior temporalis, M5,6 = lateral pterygoid, M7,8 = medial pterygoid, M9,10 = anterior digastric muscles), and the axis system used to characterize relative positions of the condyles, teeth, and muscle vectors, based on an individual’s anatomy, are shown (left). Enlargement (right) shows how bite-forces were modeled to mimic in vivo biting tasks and characterized by the azimuth angle (θXZ, 0-359°), measured parallel to the occlusal plane, and the angle relative to vertical (θY, where 0° is normal to the occlusal plane) (modified from Nickel et al., 2003).
Modeling Protocol
Two three-dimensional numerical models based on different objective functions (Trainor et al., 1995) predicted muscle and TMJ forces relative to applied bite-forces. Each model employed the individual’s anatomic data and an objective to produce unique solutions for static equilibrium. Anatomic data were determined according to previously described methods (Iwasaki et al., 2010) and consisted of the participant’s three-dimensional craniomandibular geometry (Fig. 1E) developed from standardized lateral and postero-anterior cephalometric radiographs and validated sagittal effective eminence shape established via previously described approaches (Iwasaki et al., 2010). Objectives were: (1) minimization and equalization of right and left TMJ loads (MJL), and (2) minimization of muscle effort (MME), defined as minimization of the sum of muscle forces squared. MJL and MME models calculated muscle forces for ranges of bite-force angles on mandibular incisors and first molars that mimicked moments produced in vivo (Figs. 1C, 1E). Each predicted muscle force was expressed as a percentage of the applied bite-force and normalized to peak predicted muscle force for participant, biting position, and angle.
Data and Statistical Analyses
We used customizable software (TestPoint V7, Measurement Computing Corporation, Norton, MA, USA) to identify normalized MJL and MME model results that best matched normalized in vivo results. The software compared normalized in vivo muscle data for a biting moment with model-predicted data for all reasonable biting angles. RMS errors between measured and predicted data were calculated. The combination of model-predicted/in vivo-measured data with minimum RMS error was identified as the “best-match” result. This process was done for all biting moments.
Analyses of best-match normalized model data with normalized in vivo data from two recording sessions of four muscles and all moments, for a given participant and biting position, began with the development of linear regressions between predicted and measured data. Regression slopes, confidence intervals, and coefficients of determination (R2) were calculated. Accuracy of the best-match model-prediction was calculated by the equation:
which determined the number of standard deviations the regression slope was away from a perfect predicted slope of 1.0. analysis of variance (ANOVA) and Tukey-Kramer post hoc tests examined the combined effects of diagnostic group, gender, position, side, and model. To test for accuracy of model predictions within participants, we identified the smaller of MME or MJL standardized slopes, obtained from the equation above, as the “best-fit” model. If the same model was best for both right and left, then the individual/position combination was considered “symmetric”. However, if best-fit models were different for right and left, then the individual/position was considered “asymmetric”. Chi-square and Fisher’s exact tests evaluated differences between healthy individuals (–P/-DD) and those with TMD (+P/+DD, +P/-DD, -P/+DD) with respect to right-left symmetry in muscle organization during incisor and molar biting.
Results
Gender composition (average age ± standard deviation) of the four diagnostic groups was: (-P/-DD) 10 females (34 ± 10 yrs), 10 males (31 ± 14 yrs); (+P/+DD) 13 females (34 ± 12 yrs), 13 males (28 ± 7 yrs); (+P/-DD) 8 females (29 ± 12 yrs), 8 males (27 ± 6 yrs); and (-P/+DD) 16 females (35 ± 14 yrs), 13 males (27 ± 8 yrs). Characteristic of Pain Intensity scores for +P individuals ranged from 3 to 86 out of 100. Based on a cut-point of 50 to delineate between low and high scores, 26% of +P individuals rated pain intensity as high around the time of EMG recording. Although two diagnostic groups reported pain (+P/+DD; +P/-DD), all participants voluntarily completed the biting tasks. Average peak forces for incisor and molar biting in +P participants were 49 and 112 N, respectively, whereas in –P participants, forces were 30 and 85 N, respectively.
In vivo data represent 336 sets of biting tasks repeated at two sessions out of a potential 364 sets, because early recording problems caused failure of 4 sets, while 24 sets were not performed because of sizeable restorations or missing teeth at a given position in some participants (detailed in Appendix Tables 1-8).
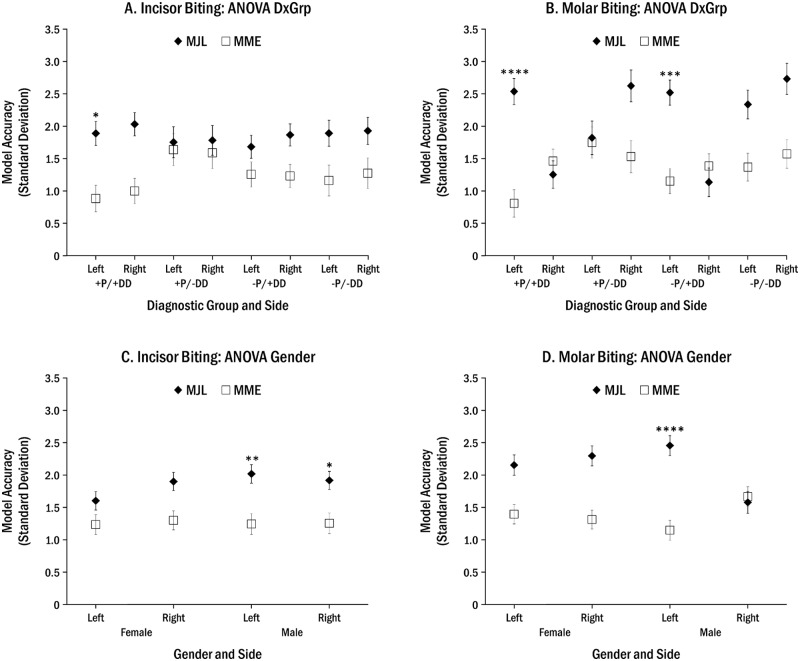
Significant combined effects were found for diagnostic group or gender, and position, side, and model (p < 0.02, Appendix Tables 9, 10). MME was found to be more accurate during left incisor biting in +P/+DD individuals (Fig. 2A), left molar biting in those with disc displacement (+P/+DD, -P/+DD; Fig. 2B), and incisor and left molar biting in men (all P < 0.03, Figs. 2C, 2D).
Figure 2.
ANOVA-post hoc adjusted four-way analyses of diagnostic group or gender effects on choice of “best-fit” model, where: *p < 0.03, **p < 0.01, ***p < 0.001, and ****p < 0.0001. In all cases where significant results were found, MME was the best fit. The combined effects of diagnostic group, side, and model are shown for incisor (A) and molar (B) biting, and those of gender, side, and model are shown for incisor (C) and molar (D) biting.
For within-participant analyses, we used the equation above to identify the most accurate model. As a result, average absolute errors between best numerical model-predicted and in vivo muscle activities were ≤ 15% overall and were similar among diagnostic groups. Errors ranged from 11 to 13% and 8 to 15% for incisor and molar biting, respectively (Table 1; participant-specific data: Appendix Tables 1-8). Average coefficients of determination (R2) demonstrated that predicted and in vivo data generally matched well and similarly among diagnostic groups, ranging from 0.70 to 0.74 and 0.68 to 0.74 for incisor and molar biting, respectively.
Table 1.
Summary of Best-fit Model Results for Incisor and Molar Biting Data by Diagnostic Group in Terms of Average Absolute Errors, Coefficients of Determination (R2), and Frequencies (%) of Model Type
| Best-fit Model vs. Incisor Biting Data |
Best-fit Model vs. Molar Biting Data |
|||||||
|---|---|---|---|---|---|---|---|---|
| Frequency (%) [N] |
Frequency (%) [N] |
|||||||
| Diagnostic Group [N: females, males] | Absolute Error (%) | R2 | MJL | MME | Absolute Error (%) | R2 | MJL | MME |
| +Pain/+Disc Displacement [13, 13] | 11 | 0.71 | 15 [7a] | 85 [41a] | 8 | 0.74 | 34 [19a] | 66 [28a] |
| +Pain/-Disc Displacement [8, 8] | 13 | 0.70 | 28 [7a] | 72 [22a] | 15 | 0.68 | 37 [14a] | 63 [16a] |
| -Pain/+Disc Displacement [16, 13] | 13 | 0.70 | 30 [17a] | 70 [37a] | 10 | 0.73 | 37 [25a] | 63 [27a] |
| -Pain/-Disc Displacement [10, 10] | 12 | 0.74 | 11 [8a] | 89 [30a] | 13 | 0.72 | 36 [16a] | 64 [23a] |
No in vivo data for ≤ six teeth within the group. See Appendix Tables 1-8 for details.
Which model matched best with in vivo data was not markedly different among diagnostic groups. MME model predictions matched best with in vivo data more frequently for all biting positions for all diagnostic groups than did MJL model predictions. This was more so for incisor than molar biting, where frequencies of MME as best-fit model were 70 to 89% compared with 63 to 66%, respectively (Table 1).
Chi-square and Fisher exact statistics tested for differences between healthy participants (–P/-DD) and those with TMD (+P/+DD, +P/-DD, -P/+DD) with respect to right-left symmetry in muscle organization. Significant (P < 0.03, Table 2) group differences in symmetry of muscle organization occurred during incisor biting, where 89% of healthy participants were symmetric compared with 60% of those with TMD.
Table 2.
Chi-square (degrees of freedom = 1) and Fisher’s Exact Statistics of Diagnostic Group Differences in Right-Left Symmetry of Muscle Organization*
| Statistical Test |
||||||
|---|---|---|---|---|---|---|
| Frequency |
Chi-square |
|||||
| Biting Position | Group | Symmetry | Asymmetry | Value | p | Fisher’s Exact, p |
| Incisor | Healthy | 16 (89%) | 2 (11%) | 5.26 | 0.022 | 0.025 |
| TMD | 39 (60%) | 26 (40%) | ||||
| Molar | Healthy | 9 (47%) | 10 (53%) | 0.42 | 0.518 | 0.599 |
| TMD | 25 (39%) | 39 (61%) | ||||
Participants were grouped into healthy (-P/-DD) and TMD categories (+P/+DD, +P/-DD, -P/+DD). Analyses determined if there were significant differences in symmetry vs. asymmetry of muscle organization between the two study groups for incisor and molar biting.
Discussion
Average absolute model prediction errors for the diagnostic groups were ≤ 15% and were relatively similar among groups. The finding that right-left asymmetry of the best-fit model was more frequent overall for molar biting was not expected and may reflect the central nervous system organization of a preferred chewing side (Christensen and Radue, 1985; Nissan et al., 2004). Preferred chewing sides during mastication have been identified in children (Gisel, 1988). In adults, this sidedness can be demonstrated in the sensorimotor cortex by functional MR imaging (Jiang et al., 2010). The preferred side for chewing or biting was not determined in the current study. Hence, whether MME or MJL is more representative of muscle organization during preferred side loading of the mandible remains to be determined. We are not aware of reports of sidedness for biting on incisor teeth.
The symmetry of neuromuscular organization during incisor biting in healthy individuals may reflect mechanical coupling of incisor teeth (Trulsson and Johansson, 2002; Trulsson, 2007), which results in bilateral afferent input from periodontal ligament mechanoreceptors to the central nervous system. That individuals with TMD were more likely to have asymmetry in muscle organization during incisor biting may be considered as supportive evidence of the Pain Adaptation model of muscle recruitment (Peck et al., 2008), where pain results in a new recruitment strategy of motor units. The general consequences of asymmetry of neuromuscular objectives are that whenever MME is invoked, loads increase on at least one TMJ (Iwasaki et al., 2009a). Conversely, when MJL is utilized, higher muscle forces occur to achieve minimization and equalization of TMJ loads (Trainor et al., 1995).
Growth of the temporomandibular joint eminence appears to be consistent with the neuromuscular objective of MJL (Nickel et al., 1988; de Zee et al., 2009). No data are available which describe central nervous system organization of masticatory muscles in children during static biting. Future work may test the hypotheses that the predominant objective for muscle organization is MJL in children and may change to MME in some individuals as they mature.
By definition, models simplify, and hence, cannot exactly simulate in vivo conditions. For example, modeled biting at depressions #1-2 and #4-5 (Fig. 1C) used angular bite-forces that mimicked specific moments associated with vertical biting at each of these depressions in vivo (Iwasaki et al., 2004). This introduced loads in the occlusal (XZ) plane (Fig. 1E) during computer modeling, transverse (± FZ) during molar biting, and vestibulo-lingual (± FX) during incisor biting, whereas in vivo bite-forces were primarily vertical (Iwasaki et al., 2003b; Nickel et al., 2003). Since model- defined molar FZ and incisor FX loads increased biting angle, the greatest likelihood of error between model-predicted and measured muscle activities occurred in the simulation of extreme mechanical moments produced by biting at depressions #1 and #5.
Another source of error may exist, given that no EMG data were recorded from lateral pterygoid muscles during biting. Temporalis and lateral pterygoid muscle activities vs. bite-force relations have previously been shown to vary significantly with small changes in sign and magnitude of tooth-tipping moments (Uchida et al., 2008). Therefore, it is possible that data from the lateral pterygoid muscles may affect model accuracies and determination of MJL or MME as good descriptors of CNS organization of masticatory muscles in individuals with pain and/or bilateral disc displacement. Nevertheless, predominance of MME for muscle organization during incisor biting (70-89%, Table 1) is consistent with our previous reports, where muscle activities were measured in masticatory muscles, including the lateral pterygoid, in a total of 14 healthy individuals (Iwasaki et al., 2003b, 2009b). Also, surface EMG from masseter, temporalis, and suprahyoid muscles without the lateral pterygoid muscles have previously shown clear discrimination among specific tasks involving the jaws in those with and without temporomandibular disorders (Ohrbach et al., 2008).
In conclusion, results of this study support the hypothesis that neuromuscular organization of human masseter and temporalis muscle activities during static biting was consistent with MJL and/or MME models for all diagnostic groups. Model predictions matched best with measured data more frequently from MME than MJL for all groups and biting positions. Overall, symmetry of neuromuscular organization was more common during incisor biting in healthy individuals. Asymmetry of muscle organization was common during molar biting in all diagnostic groups. Given that average errors between model-predicted and in vivo muscle activities ranged from 8 to 15%, computer-assisted numerical modeling offers a unique and relatively accurate method to investigate group and individual differences in muscle activities and TMJ loads during static biting.
Acknowledgments
The study participants and contributions from Theresa Speers, Emily Eigensee, Michael Crosby, Gena McGivern, and Kim Theesen are gratefully acknowledged.
Footnotes
This work was supported in part by NIDCR (R01 DE016417).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, et al. (2009). Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107:844-860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LV, Radue JT. (1985). Lateral preference in mastication: relation to pain. J Oral Rehabil 12:461-467 [DOI] [PubMed] [Google Scholar]
- de Zee M, Cattaneo PM, Svensson P, Pedersen TK, Melsen B, Rasmussen J, et al. (2009). Prediction of the articular eminence shape in a patient with unilateral hypoplasia of the right mandibular ramus before and after distraction osteogenesis—A simulation study. J Biomech 42:1049-1053 [DOI] [PubMed] [Google Scholar]
- Dworkin SF, LeResche L. (1992). Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 6:301-355 [PubMed] [Google Scholar]
- Gisel EG. (1988). Development of oral side preference during chewing and its relation to hand preference in normal 2- to 8-year-old children. Am J Occup Ther 42:378-383 [DOI] [PubMed] [Google Scholar]
- Gonzalez Y, Iwasaki LR, McCall WD, Jr, Ohrbach R, Lozier E, Nickel JC. (2011). Reliability of electromyographic activity vs. bite-force from human masticatory muscles. Eur J Oral Sci 119:219-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannam AG. (2011). Current computational modelling trends in craniomandibular biomechanics and their clinical implications. J Oral Rehabil 38:217-234 [DOI] [PubMed] [Google Scholar]
- Iwasaki LR, Baird BW, McCall WD, Jr, Nickel JC. (2003a). Muscle and temporomandibular joint forces associated with chincup loading predicted by numerical modeling. Am J Orthod Dentofacial Orthop 124:530-540 [DOI] [PubMed] [Google Scholar]
- Iwasaki LR, Petsche PE, McCall WD, Jr, Marx D, Nickel JC. (2003b). Neuromuscular objectives of the human masticatory apparatus during static biting. Arch Oral Biol 48:767-777 [DOI] [PubMed] [Google Scholar]
- Iwasaki LR, Thornton BR, McCall WD, Jr, Nickel JC. (2004). Individual variations in numerically modeled human muscle and temporomandibular joint forces during static biting. J Orofac Pain 18:235-245 [PubMed] [Google Scholar]
- Iwasaki LR, Crosby MJ, Gonzalez Y, McCall WD, Marx DB, Ohrbach R, et al. (2009a). Temporomandibular joint loads in subjects with and without disc displacement. Orthop Rev (Pavia) 1:90-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Uchida S, Marx DB, Yotsui Y, Maeda T, Inoue H, et al. (2009b). Ipsilateral and contralateral human TMJ loads compared via validated numerical models. In: Temporomandibular disorders and orofacial pain - Separating controversy from consensus. Kapila SD, McNamara JA. editors. Ann Arbor, MI: Needham Press, pp. 405-425 [Google Scholar]
- Iwasaki LR, Crosby MJ, Marx DB, Gonzalez Y, McCall WD, Jr, Ohrbach R, et al. (2010). Human temporomandibular joint eminence shape and load minimization. J Dent Res 89:722-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Liu H, Liu G, Jin Z, Liu X. (2010). The effects of chewing-side preference on human brain activity during tooth clenching: an fMRI study. J Oral Rehabil 37:877-883 [DOI] [PubMed] [Google Scholar]
- Koolstra JH. (2002). Dynamics of the human masticatory system. Crit Rev Oral Biol Med 13:366-376 [DOI] [PubMed] [Google Scholar]
- Nickel JC, McLachlan KR, Smith DM. (1988). Eminence development of the post-natal human temporomandibular joint. J Dent Res 67:896-902 [DOI] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Walker RD, McLachlan KR, McCall WD., Jr (2003). Human masticatory muscle forces during static biting. J Dent Res 82:212-217 [DOI] [PubMed] [Google Scholar]
- Nissan J, Gross MD, Shifman A, Tzadok L, Assif D. (2004). Chewing side preference as a type of hemispheric laterality. J Oral Rehabil 31:412-416 [DOI] [PubMed] [Google Scholar]
- Ohrbach R, Markiewicz MR, McCall WD., Jr (2008). Waking-state oral parafunctional behaviors: specificity and validity as assessed by electromyography. Eur J Oral Sci 116:438-444 [DOI] [PubMed] [Google Scholar]
- Peck CC, Murray GM, Gerzina TM. (2008). How does pain affect jaw muscle activity? The Integrated Pain Adaptation Model. Aust Dent J 53:201-207 [DOI] [PubMed] [Google Scholar]
- Trainor PG, McLachlan KR, McCall WD. (1995). Modelling of forces in the human masticatory system with optimization of the angulations of the joint loads. J Biomech 28:829-843 [DOI] [PubMed] [Google Scholar]
- Trulsson M. (2007). Force encoding by human periodontal mechanoreceptors during mastication. Arch Oral Biol 52:357-360 [DOI] [PubMed] [Google Scholar]
- Trulsson M, Johansson RS. (2002). Orofacial mechanoreceptors in humans: encoding characteristics and responses during natural orofacial behaviors. Behav Brain Res 135:27-33 [DOI] [PubMed] [Google Scholar]
- Uchida S, Iwasaki LR, Marx DB, Yotsui Y, Inoue H, Nickel JC. (2008). Variations in activities of human jaw muscles depend on tooth-tipping moments. Arch Oral Biol 53:199-205 [DOI] [PubMed] [Google Scholar]