GVHD is associated with significant shifts in the composition of the intestinal microbiota in human and mouse models; manipulating the microbiota can alter the severity of GVHD in mice.
Abstract
Despite a growing understanding of the link between intestinal inflammation and resident gut microbes, longitudinal studies of human flora before initial onset of intestinal inflammation have not been reported. Here, we demonstrate in murine and human recipients of allogeneic bone marrow transplantation (BMT) that intestinal inflammation secondary to graft-versus-host disease (GVHD) is associated with major shifts in the composition of the intestinal microbiota. The microbiota, in turn, can modulate the severity of intestinal inflammation. In mouse models of GVHD, we observed loss of overall diversity and expansion of Lactobacillales and loss of Clostridiales. Eliminating Lactobacillales from the flora of mice before BMT aggravated GVHD, whereas reintroducing the predominant species of Lactobacillus mediated significant protection against GVHD. We then characterized gut flora of patients during onset of intestinal inflammation caused by GVHD and found patterns mirroring those in mice. We also identified increased microbial chaos early after allogeneic BMT as a potential risk factor for subsequent GVHD. Together, these data demonstrate regulation of flora by intestinal inflammation and suggest that flora manipulation may reduce intestinal inflammation and improve outcomes for allogeneic BMT recipients.
Regulators of the intestinal flora include diet (Kau et al., 2011), antibiotics (Willing et al., 2011), and, importantly, intestinal inflammation (Sekirov et al., 2010). As a result, the cause–effect relationships between intestinal inflammation and changes in microbiota have been difficult to define (Maloy and Powrie, 2011). The success of allogenic BM transplantation (BMT), a standard therapy for conditions such as hematopoietic malignancies and inherited hematopoietic disorders, is limited by graft-versus-host disease (GVHD) morbidity and mortality (Ferrara et al., 2009). With GVHD, vigorous activation of donor immune cells, most importantly T cells (Korngold and Sprent, 1978), leads to damage of skin, liver, hematopoietic system, and gut. The major sources of immune activation are histocompatibility complex differences between donor and recipient. Combinations of chemotherapy and radiation also contribute, as damage to the intestinal epithelium results in systemic exposure to microbial products normally sequestered in the intestinal lumen (Ferrara et al., 2009).
The impact of the microbiota on GVHD is known to be significant. Studies in mice have shown reduction of GVHD with gut-decontaminating antibiotics (van Bekkum et al., 1974) and transplantation in germ-free conditions (Jones et al., 1971). This led to efforts to eliminate bacterial colonization in allogenic BMT patients, combining gut decontamination with a near-sterile environment (Storb et al., 1983). Initial reports were promising, but subsequent studies could not confirm a benefit (Petersen et al., 1987; Passweg et al., 1998; Russell et al., 2000). Other approaches include targeting anaerobic bacteria (Beelen et al., 1999) and introducing potentially beneficial bacteria (Gerbitz et al., 2004), with some reduction of GVHD. These initial studies, however, have been few in number, and no consensus exists between BMT centers regarding how to target the flora. Until recently, a reliance on microbiological culture techniques to characterize flora composition limited these studies. Culture-independent techniques such as ribosomal RNA (rRNA) gene sequencing have demonstrated that a large majority of the estimated 500–1,000 bacterial species present in the human intestinal tract are not detected by culture techniques (Manson et al., 2008). In this study, we readdress the relationship between GVHD and the microbiota in murine and human allogenic BMT recipients.
RESULTS AND DISCUSSION
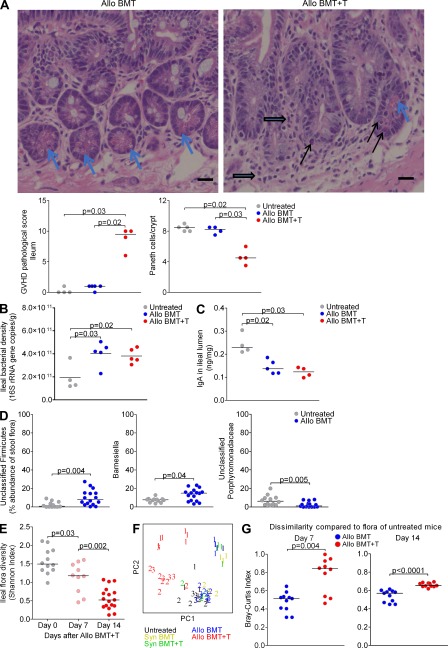
Studies of allogeneic BMT using mouse models have characterized exaggerated inflammatory mechanisms that lead to acute GVHD in target organs, including the intestine (Reddy and Ferrara, 2008). MHC-disparate donor/host combinations, including those used in this study, typically result in robust GVHD with full penetrance and rapid kinetics (Schroeder and DiPersio, 2011). The B10.BR→B6 model (H2k→H2b) used in most of our experiments is well-established and has been used in the past by us and others (Blazar et al., 1997, 2000; Penack et al., 2009). On day 14, we evaluated histologically for evidence of GVHD and found villous shortening, increased lymphocytic cell infiltration, crypt regeneration, crypt destruction, and epithelial apoptosis. The number of Paneth cells was also decreased, whereas goblet cells appear to be minimally affected (Fig. 1 A). We then quantified copies of 16S rRNA genes to determine bacterial load. After BMT, we noticed an increase in bacterial load in the ileum (Fig. 1 B), but not in the cecum (unpublished data). This occurred both in the absence and presence of GVHD, suggesting that bacterial expansion may result from reduced host-defense mechanisms in the post-BMT setting. Indeed, we found that levels of IgA in the ileal lumen were decreased after BMT, regardless of GVHD (Fig. 1 C).
Figure 1.
GVHD in mice produces marked changes in the microbiota. (A) B6 mice were lethally irradiated and transplanted with 5 × 106 B10.BR T cell–depleted BM supplemented with or without 1 × 106 splenic T cells. Features of GVHD are indicated on ileal histology sections from day 14, including lymphocytic infiltration (block arrows), crypt regeneration (enlarged crypts and hyperchromasia), and apoptosis (black arrows). Paneth cells are indicated (blue arrows). Bar, 20 µm. Representative images are shown from one of two independent experiments with similar results. Each dot represents an individual mouse, with bars indicating medians. (B) Quantitation of bacterial load of ileal contents on day 14 was performed by quantitative PCR of 16S rRNA gene copies. Results of a single experiment are shown. (C) Quantification of IgA levels in ileal contents on day 14 was performed by ELISA. Results of a single experiment are shown. (D) Comparison of representation by Unclassified Firmicutes, Barnesiella, and unclassified Porphyromonadaceae from ileal samples. Combined results from three experiments are shown. (E) Diversity of ileal floras from mice with GVHD was determined by the Shannon index. Combined results from two experiments are shown. (F) Principal coordinate analysis of unweighted UniFrac, of ileal floras from B6 mice transplanted with syngeneic (syn) or allogenic (allo) BM with or without T cells. Combined results from three experiments, with data points from each experiment indicated by number. Mice from experiment three were housed individually. (G) Dissimilarity of ileal floras of allo BMT recipient mice without and with GVHD compared with untreated mice by Bray-Curtis index. Combined results of two (Day 7) and 3 (Day 14) experiments are shown.
We evaluated for effects on the microbiota by performing 16S rRNA gene sequencing and evaluating microbial diversity, as measured by the Shannon index (Magurran, 2004). Loss of diversity has been found to occur with antibiotic use (Dethlefsen et al., 2008; Ubeda et al., 2010) and increasing age (Woodmansey, 2007), and may predispose mice to disease. Mice undergoing BMT without GVHD showed little change in diversity (unpublished data), but phylogenetic classification of 16S rRNA sequences did show some expansion of unclassified Firmicutes and Barnesiella and mild contraction of unclassified Porphyromonadaceae (Fig. 1 D), demonstrating that radiation does produce some changes in the intestinal flora composition.
In contrast, mice with GVHD showed a dramatic loss of bacterial diversity during the first 2 wk after BMT (Fig. 1 E). To quantify changes in the composition of the flora, we used unweighted UniFrac (Lozupone et al., 2006) analyzed by the principal coordinate analysis (PCoA). We found that ileal floras of mice with GVHD were distinct from both those of untreated mice and those of mice after BMT without GVHD (Fig. 1 F). Mice after BMT without GVHD clustered apart from untreated mice inconsistently (one of three experiments); however, comparing the floras using the Bray-Curtis index, we found that GVHD increases dissimilarity from baseline more than BMT alone (Fig. 1 G).
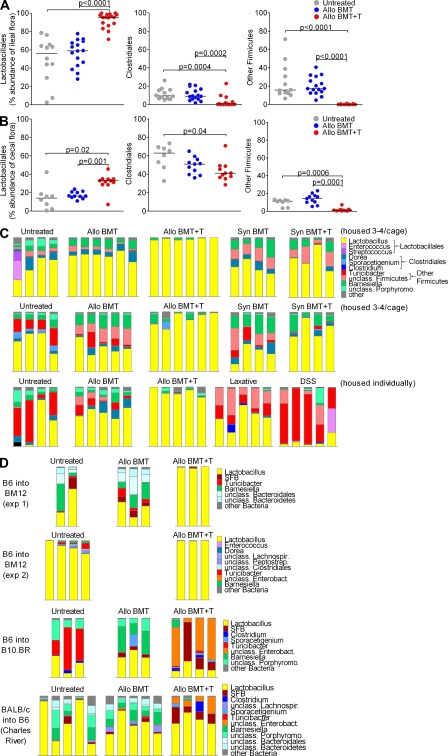
We then evaluated for changes in bacterial subpopulations in the setting of GVHD and found large shifts within the phylum Firmicutes, with a dramatic increase in Lactobacillales and decreases in Clostridiales and other Firmicutes in the ileum (Fig. 2 A). Previously, we have shown that the flora from the murine ileum is quite distinct from that of the large intestine, whereas samples within different compartments of the large intestine, including cecum and fresh stool pellets, are similar, although there are some minor changes in representation (Ubeda et al., 2010). Thus, we also evaluated for changes in the cecum with GVHD and found changes similar to those in the ileum, but of lesser magnitude (Fig. 2 B). At the genus level, we found marked ileal expansion of Lactobacillus, the dominant member of Lactobacillales (Fig. 2 C). Housing mice individually from the day of transplant to address the possibility of individual mice influencing the flora of cagemates produced identical results (Fig. 2 C). Within the 16S sequences assigned to the genus Lactobacillus, nearly all had identical sequence homology with Lactobacillus johnsonii, a species found as a commensal in humans (Pridmore et al., 2004) and rodents (Buhnik-Rosenblau et al., 2011), and also in probiotic preparations.
Figure 2.
GVHD in mice produces marked changes in the microbiota. (A) B6 mice were transplanted with B10.BR donor BM and T cells as in Fig. 1. Comparison of representation by Lactobacillales, Clostridiales, and other Firmicutes from ileal samples. Combined results from three experiments are shown. (B) Comparison of representation by Lactobacillales, Clostridiales, and other Firmicutes from cecal samples. Combined results from two experiments are shown. (C) Bacterial composition at the genus level of ileal flora on day 14 after BMT are depicted with individual mice displayed in each bar. Results of three separate experiments, each displayed in a row, are shown. Additional untransplanted mice were treated with osmotic laxative or DSS starting on day 7 and also individually housed. (D) Mice were transplanted using the strain combinations indicated; mouse vendor was The Jackson Laboratory unless otherwise indicated. Bar graphs show bacterial composition of ileal contents at the genus level for individual mice on day 14.
We asked if GVHD-associated changes could be secondary to increased gut motility. We evaluated the effects of an osmotic laxative, as well as enteritis caused by dextran sodium sulfate, and found changes with both agents that were distinct from GVHD (Fig. 2 C). Together, these results suggest that GVHD changes the flora in a unique, reproducible pattern.
The flora of mice can vary widely from colony to colony. Our data presented thus far used B6 recipient mice from The Jackson Laboratory; we also performed BMT experiments using additional strains and vendors. With BALB/c host mice from The Jackson Laboratory, we found an abundance of Lactobacillus in mice with GVHD, although the floras of BALB/c mice are dominated by Lactobacillus at baseline (unpublished data). In the CD4-driven MHC II–disparate B6→BM12 model, we also found characteristic expansion of Lactobacillus with GVHD (Fig. 2 D). This indicated that alloreactive CD4 T cells are sufficient and do not require CD8 T cells to produce changes in the flora. Interestingly, in two additional models with B10.BR hosts from The Jackson Laboratory and B6 hosts from Charles River Laboratories, we noted expansion of Enterobacteriales with GVHD (Fig. 2 D). Enterobacteriales from both strains appears to be of the same type, an unclassified Enterobacteriaceae that, in our experience, is rarely detectable in B6 mice from The Jackson Laboratory (3 of 71 mice). Expansion of Enterobacteriaceae has been reported before in Japanese (Eriguchi, Y., S. Takashima, N. Miyake, Y. Nagasaki, N. Shimono, K. Akashi, and T. Teshima. 2010. ASH Annual Meeting Abstracts. Abstr. 244) and German (Heimesaat et al., 2010) mouse colonies. Collectively, these data suggest that Lactobacillales and Enterobacteriales (both capable of surviving in aerobic environments) may populate a niche that expands with GVHD at the expense of obligate anaerobes, including Clostridiales and other Firmicutes. Whether Lactobacillales or Enterobacteriales expand appears to depend on the presence of these organisms in the baseline flora. The potential impact of these expanding populations on GVHD has not been well-described.
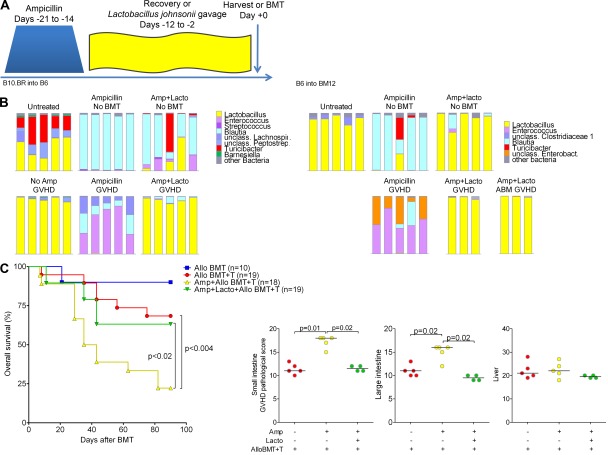
Treatment of B6 mice with ampicillin, followed by a recovery period, results in loss of Lactobacillus from the flora, with expansion of other commensal bacteria (Ubeda et al., 2010) such as Blautia (order Clostridiales; Fig. 3 A). We cultured the predominant L. johnsonii endogenous to B6 mice from The Jackson Laboratory and found that reintroduction after ampicillin treatment restores representation (Fig. 3 A). We then used ampicillin and L. johnsonii reintroduction as tools to test if expansion of Lactobacillales with GVHD could have clinical repercussions. Surprisingly, upon development of GVHD, mice treated with ampicillin before BMT showed loss of Blautia and emergence of Enterococcus (order Lactobacillales; Fig. 3 A). Mice that received L. johnsonii reintroduction after ampicillin showed domination with L. johnsonii and no expansion of Enterococcus (Fig. 3 A). We found similar results in the B6→BM12 model, though BM12 mice after ampicillin treatment demonstrated expansion of both Enterococcus and Enterobacteriaceae with GVHD (Fig. 3 B). BM12 mice that received L. johnsonii reintroduction after ampicillin also showed domination with L. johnsonii and no expansion of Enterococcus or Enterobacteriaceae. This occurred even when using monoclonal BM12-specific donor T cells from TCR transgenic ABM (Sayegh et al., 2003) RAG-1 deficient mice, suggesting that a broad alloreactive T cell repertoire is not required to produce changes in the microbiota with GVHD.
Figure 3.
Composition of intestinal flora can impact on severity of intestinal GVHD. (A) Schematic of treatment: B6 mice received ampicillin for 1 wk, followed by a 2-wk recovery period with unmodified drinking water; some were gavaged every 2 d with L. johnsonii (Lacto) of B6 flora origin during recovery, followed by harvest or BMT. Ileal contents were evaluated on days 0 (no BMT) and 14 after BMT. Bar graphs show bacterial composition of ileal contents at the genus level for individual mice. (B) Similar to as in A, BM12 mice received ampicillin followed by recovery; some also received L. johnsonii reintroduction. GVHD was induced upon transplantation with BM and either wild-type CD4 T cells (500K) or ABM RAG1 KO TCR transgenic CD4 T cells (100K). (C) B6 mice were treated with ampicillin, and then were or were not gavaged with L. johnsonii and transplanted with B10.BR BM and T cells. (top) Survival data combined from two experiments with similar results. (bottom) Pathological scores of GVHD target organs on day +21.
We then evaluated effects of flora manipulation on GVHD severity, focusing on the B10.BR→B6 model. Notably, ampicillin treatment before BMT resulted in worsened GVHD survival. Histologically, these mice had evidence for increased GVHD pathology in the small and large intestines, including epithelial damage and increased inflammation. Remarkably, L. johnsonii reintroduction prevented increased GVHD lethality and pathology (Fig. 3 C). Enterococcus has not been described as a potential contributor to gut GVHD, though enterococcal bacteremia occurs often in patients with GVHD (Dubberke et al., 2006). In mouse models, Enterococcus can contribute to gut inflammation by compromising epithelial barrier integrity (Steck et al., 2011) and stimulating TNF production from macrophages (Kim et al., 2006). Thus, one mechanism by which L. johnsonii may reduce GVHD severity could be prevention of Enterococcus expansion which may exacerbate GVHD-associated intestinal damage and inflammation.
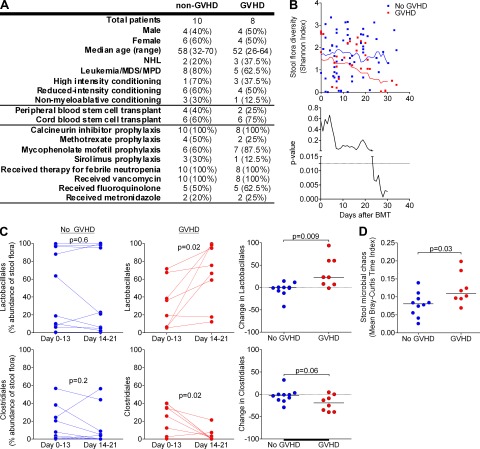
We then studied the relationship between the flora and GVHD in humans. We collected weekly stool samples from allogenic BMT patients during transplant hospitalization at our center. Of 9 patients who developed gut GVHD during hospitalization, 8 developed symptoms early, with GVHD onset clustering between days 18 and 21; these 8 were selected for our GVHD cohort. 18 additional patients provided weekly samples through day 21; of these, 10 met our prospective eligibility for inclusion in our non-GVHD cohort, with survival to at least day 30 and absence of GVHD in any target organ through day 100. Clinical parameters for included patients are summarized in Fig. 4 A. Importantly, non-GVHD and GVHD patients had similar exposures to antibiotics during the period of stool collection.
Figure 4.
GVHD produces marked changes in the microbiota of humans, and the microbiota may affect risk of developing GVHD. (A) Summary of clinical parameters of non-GVHD and GVHD patients. (B) Flora diversity, by Shannon index, of stool samples after BMT. Individual measurements of diversity are displayed, as well as moving averages and P values calculated for 10-d intervals. (C) Contribution of bacterial populations in samples during two time periods, days 0 to 13 and 14 to 21 after BMT. (D) Microbial chaos of stool samples by mean Bray-Curtis time index from pre-BMT to day 13 after BMT.
We first examined the effects of GVHD on flora diversity. We found that before GVHD, patients had flora diversity similar to controls but lost diversity over time, particularly after GVHD onset (Fig. 4 B). Thus, GVHD is associated with loss of flora diversity in humans, similar to in mice.
We then looked for bacterial populations that changed with the onset of GVHD. Interestingly, we discovered increases in Lactobacillales and decreases in Clostridiales, a pattern identical to our findings in mice. Other populations, as well as classifications at the family or genus level, were otherwise not significantly changed (unpublished data). Importantly, we did not identify these shifts in non-GVHD patients (Fig. 4 C), suggesting that these flora changes were indeed a result of GVHD rather than BMT or antibiotic exposure.
Our sample size did not identify specific populations as potential risk factors for subsequent GVHD. Patients who later developed GVHD, however, did have significantly greater microbial chaos early after BMT (before our observed GVHD-associated changes), which we quantified using the Bray-Curtis dissimilarity index (Magurran, 2004) over time (Fig. 4 D). This suggests that large fluctuations in the microbiota early on may lead to an increased risk of GVHD.
In conclusion, our findings demonstrate the influence of inflammation on the structure of the intestinal microbiota after allogenic BMT in both mice and humans. The flora, in turn, can modulate severity of intestinal inflammation. Our mouse experiments indicate that antibiotic exposure before BMT, which occurs commonly in patients with hematologic malignancies, may be a risk factor for subsequent intestinal GVHD. This may be remedied with targeted flora reintroduction to potentially reduce the severity of gut GVHD.
MATERIALS AND METHODS
Mouse BMT experiments.
All mouse procedures were performed in accordance with institutional protocol guidelines at Memorial Sloan-Kettering Cancer Center (MSKCC). Mice were maintained according to National Institutes of Health Animal Care guidelines, under protocols approved by the MSKCC Institutional Animal Care Committee describing experiments specific to this study. Mouse BMT experiments were performed as previously described (Penack et al., 2010). Mice received 11 Gy divided in 2 split doses 3–4 h apart. All BMT experiments were performed at Memorial Sloan-Kettering with the exception of the BALB/c into B6/CR experiment in Fig. 2 D, which was performed at University of Minnesota. All mice were obtained from The Jackson Laboratory, with the exception of B6 mice from Charles River in Fig. 2 D, and ABM mice (Sayegh et al., 2003) in Fig. 3 B, which were provided by M. Sayegh (Brigham and Women’s Hospital and Children’s Hospital Boston, Boston, MA) and had been backcrossed onto a B6 background for at least 20 generations, and then crossed on a RAG-1–deficient background derived from The Jackson Laboratory, previously backcrossed 10 times to B6 background. Mice were either co-housed three to five mice/cage in all experiments, or were housed individually as indicated in experiment three of Fig. 1 F and Fig. 2 C.
GVHD clinical and histological scoring.
Mice were monitored daily for survival and weekly for GVHD clinical scores (Cooke et al., 1996). Small intestine, large intestine, and liver samples were evaluated histologically for evidence of GVHD and scored as previously described (Hill et al., 1997).
Laxative and dextran sodium sulfate (DSS) treatments.
Mice were treated with drinking water containing osmotic laxative (60 g/l polyethylene glycol 3350, 1.46 g/l NaCl, 0.745 g/l KCl, 1.68 g/l NaHCO3, and 5.698 g/l Na2SO4) or DSS 3.5% for 7 d. A 7-d course of treatment was selected to better compare with GVHD-induced intestinal changes, which first requires alloactivation and expansion of donor T cells.
Ampicillin treatment, Lactobacillus isolation, and reintroduction.
Mice were given 1 g/l ampicillin in their drinking water during 7 d, followed by a recovery period with normal drinking water for 14 d. The dominant Lactobacillus strain from the small intestine of B6 mice (The Jackson Laboratory) was isolated by plating contents under anaerobic conditions on plates with Lactobacilli MRS agar (BD). 16S rRNA was sequenced and classified using the ribosomal RDP classifier, and confirmed using MOTHUR to be identical to the operational taxonomic unit (OTU) most predominant in B6 mice (The Jackson Laboratory). 1 d after stopping ampicillin treatment, 108 CFUs of the isolated Lactobacillus strain were given to mice by oral gavage every other day during the 14-d recovery period.
Patient selection.
We collected stool samples on a weekly basis from allogenic BMT patients from 8/29/09 to 5/24/11. Patients were identified that developed upper gut GVHD symptoms (nausea, vomiting, and loss of appetite) or lower gut GVHD symptoms (abdominal discomfort and diarrhea). 8 had onset of symptoms at a similar time point, between days 18–21, and were selected for our GVHD cohort; the ninth patient had GVHD onset on day 27. 7 underwent confirmatory biopsy; 1 could not be biopsied because of thrombocytopenia. Treatment for GVHD began on days ranging from 26 to 48, and thus the majority of samples that were analyzed were collected before initiation of corticosteroids. Six had symptoms of upper intestinal GVHD and were all treated with the oral corticosteroid budesonide; two also had symptoms of lower intestinal GVHD and were treated with intravenous methylprednisolone. Antibiotic exposures were tabulated from day −7 to 21 after BMT. Standard antibiotic guidelines were followed, including prophylaxis with intravenous vancomycin starting at day −2, and empirical treatment of neutropenic fever with piperacillin/tazobactam, or in patients with allergies, cefepime, or aztreonam. The study protocol was approved by the Memorial Sloan-Kettering Cancer Center Institutional Review Board; informed consent was obtained from all subjects before collection procedures.
Sample collection and DNA extraction.
Stool samples from patients were stored at 4°C for <24 h before freezing at −80°C. Ileal and cecal samples from mice were frozen at −80°C. DNA was extracted using one of the two methods, which give similar results. In Fig. 1, Fig. 2 (A–C), Fig. 3, and Fig. 4, DNA was extracted using a phenol-chloroform extraction technique (Ubeda et al., 2010). In Fig. 2 D, DNA was extracted from samples using Power Soil DNA isolation kit (MO BIO Laboratories).
Quantification of gut flora bacterial density.
Gut flora bacterial density was quantified as previously described (Ubeda et al., 2010).
IgA quantification.
Ileum contents were resuspended in 1 ml of a 3:1 mixture of PBS/0.1 M EDTA containing soybean trypsin inhibitor (type II-S; Sigma-Aldrich) at a concentration of 0.1 mg/ml. The mixture was centrifuged at 12.000 rpm for 10 min, and the supernatant was collected for the assay. Plates were coated with 100 µl/well of rat anti–mouse IgA (SouthernBiotech) at a dilution of 1:1,000 in 50 mM carbonate buffer, pH 9.6. After blocking and washing of plates, 100 µl/well serial dilutions of the previously prepared mouse intestinal samples were added and plates were incubated overnight at room temperature. Bounded antibody was detected by incubating plates at 37°C for 1 h with goat anti–mouse IgA-HRP conjugate at a dilution of 1:1,000 in PBS-T-0.1% BSA. Plates were developed with 2, 2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (Sigma-Aldrich) and 0.03% H2O2 (Sigma-Aldrich), and optical density was determined using a Vmax microplate reader (Molecular Devices) at 405 nm kinetically for 20 min at 14-s intervals. Total ileum content IgA was calculated using a mouse IgA standard (Kappa TEPC 15; Sigma-Aldrich).
16S rRNA gene amplification, 454 pyrosequencing.
For each sample, 3 replicate 25-µl PCRs were performed. Each PCR contained 50 ng of purified DNA, 0.2 mM dNTPs, 1.5 mM MgCl2, 1.25 units of Platinum Taq DNA polymerase, 2.5 µl of 10× PCR buffer, and 0.2 µM of each primer designed to amplify the V1-V2 (Ubeda et al., 2010; Fig. 1 and Fig. 2) or V1-V3 (Fig. 3 and Fig. 4) 16S rRNA variable regions, as described in the Human Microbiome Project Provisional 16S 454 Protocol (http://www.hmpdacc.org/tools_protocols/tools_protocols.php). The cycling conditions used were: 94°C for 3 min, followed by 25 cycles (cecum and fecal samples) or 28 cycles (ileum samples) of 94°C for 30 s, 52°C (V1-V2) or 56°C (V1-V3) for 30 s, and 72°C for 1 min. Replicate PCRs were pooled and amplicons were purified using the QIAquick PCR Purification kit (QIAGEN). PCR products were sequenced on a 454 GS FLX or 454 GS FLX Titanium platform following the recommended procedures (Roche).
Sequence analysis.
Sequence data were compiled and processed using MOTHUR (Schloss et al., 2009). Sequences were aligned to the 16S rRNA gene, using as a template the SILVA reference alignment and the Needleman-Wunsch algorithm with the default scoring options. Potentially chimeric sequences were removed using the ChimeraSlayer program. To minimize the effect of pyrosequencing errors in overestimating microbial diversity, rare abundance sequences that differ in 1 or 2 nt from a high abundant sequence were merged to the high abundant sequence using the pre.cluster option in MOTHUR. Sequences were grouped into OTUs using the average neighbor algorithm. Sequences with distance-based similarity of 97% or greater were assigned to the same OTU. Mouse samples were processed and sequenced as individual experiments, and resulting sequences were analyzed together with all other samples within each figure panel. Human samples were processed and sequenced in batches, and resulting sequences were analyzed together. Sequences from all experiments have been deposited in the Sequence Read Archive of National Center for Biotechnology Information, submission number SRA049925.
Determining diversity, phylogenetic classification, dissimilarity, microbial chaos, and UniFrac PCoA.
OTU-based microbial diversity was estimated by calculating the Shannon diversity index (Magurran, 2004) using MOTHUR. Phylogenetic classification was performed for each sequence, using the Bayesian classifier algorithm described by Wang et al. (2007) with the bootstrap cutoff at 60%. A phylogenetic tree was inferred using clearcut on the 16S sequence alignment generated by MOTHUR. Microbial chaos was quantified by mean Bray-Curtis time index, calculated as follows: Bray-Curtis dissimilarity index (Magurran, 2004) between temporally adjacent samples was quantified using MOTHUR and divided by the length of the time interval (in days) between samples, starting with the last sample obtained before the transplant and all samples obtained until day 13. Unweighted UniFrac was run using the resulting tree (Lozupone et al., 2006). PCoA was performed on the resulting matrix of distances between each pair of samples.
Statistical comparisons.
Shannon diversity index in Fig. 4 B for 10-d intervals compared using unpaired two-sided Student’s t tests with a more stringent cut-off of 0.0125 given multiple comparisons, by the Bonferroni correction for 4 time periods of independent comparisons. Comparisons of bacterial populations in Fig. 3 C using paired two-sided Wilcoxon matched pairs test for individual patients. In Fig. 4 C, Change in Clostridiales was compared using a two-sided Student’s t test, with normality confirmed by D’Agostino and Pearson omnibus test with α = 0.05. All other comparisons were done using two-sided Mann-Whitney tests.
Acknowledgments
This research was supported by National Institutes of Health award numbers R01-HL069929, R01-CA107096, and R01-AI080455 (M.R.M van den Brink); RO1-AI042135, R37-AI039031, and PO1-CA023766 (E.G. Pamer); and R01-AI34495 and R01-HL56067 (B.R. Blazar). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support was also received from the United States Department of Defense USAMRAA Award W81XWH-09-1-0294 (M.R.M van den Brink), the Radiation Effects Research Foundation (RERF-NIAID; M.R.M van den Brink), the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr. William H. Goodwin and Mrs. Alice Goodwin, The Lymphoma Foundation, Alex’s Lemonade Stand, The Geoffrey Beene Cancer Research Center at Memorial Sloan-Kettering Cancer Center, The Peter Solomon Fund, the Lucille Castori Center for Microbes, Inflammation, and Cancer, and the Leonard Tow Foundation.
Author contributions: R.R. Jenq, C. Ubeda, E.G. Pamer, and M.R.M. van den Brink designed the experiments, interpreted the data, and wrote the paper. R.R. Jenq and C. Ubeda carried out most of the mouse experiments, with assistance from J.A. Dudakov, M.L. West, N.V. Singer, M.J. Equinda, L.F. Young, O.M. Smith, A. Ghosh, and A.M. Hanash. C. Liu quantified GVHD pathology. K. Aoyama and B.R. Blazar designed and performed mouse experiments and helped write the paper. Human samples were processed and sequenced by Y. Taur, A. Ghosh, and L. Lipuma. Y. Taur and J. Ghosh assessed clinical parameters. R. Khanin performed computational analysis of human flora data.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- BMT
- BM transplantation
- DSS
- dextran sodium sulfate
- GVHD
- graft-versus-host disease
- OTU
- operational taxonomic unit
- PCoA
- principal coordinate analysis
- rRNA
- recombinant RNA
References
- Beelen D.W., Elmaagacli A., Müller K.D., Hirche H., Schaefer U.W. 1999. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 93:3267–3275 [PubMed] [Google Scholar]
- Blazar B.R., Taylor P.A., Boyer M.W., Panoskaltsis-Mortari A., Allison J.P., Vallera D.A. 1997. CD28/B7 interactions are required for sustaining the graft-versus-leukemia effect of delayed post-bone marrow transplantation splenocyte infusion in murine recipients of myeloid or lymphoid leukemia cells. J. Immunol. 159:3460–3473 [PubMed] [Google Scholar]
- Blazar B.R., Lees C.J., Martin P.J., Noelle R.J., Kwon B., Murphy W., Taylor P.A. 2000. Host T cells resist graft-versus-host disease mediated by donor leukocyte infusions. J. Immunol. 165:4901–4909 [DOI] [PubMed] [Google Scholar]
- Buhnik-Rosenblau K., Danin-Poleg Y., Kashi Y. 2011. Predominant effect of host genetics on levels of Lactobacillus johnsonii bacteria in the mouse gut. Appl. Environ. Microbiol. 77:6531–6538 10.1128/AEM.00324-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke K.R., Kobzik L., Martin T.R., Brewer J., Delmonte J., Jr, Crawford J.M., Ferrara J.L. 1996. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 88:3230–3239 [PubMed] [Google Scholar]
- Dethlefsen L., Huse S., Sogin M.L., Relman D.A. 2008. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 6:e280 10.1371/journal.pbio.0060280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubberke E.R., Hollands J.M., Georgantopoulos P., Augustin K., DiPersio J.F., Mundy L.M., Khoury H.J. 2006. Vancomycin-resistant enterococcal bloodstream infections on a hematopoietic stem cell transplant unit: are the sick getting sicker? Bone Marrow Transplant. 38:813–819 10.1038/sj.bmt.1705530 [DOI] [PubMed] [Google Scholar]
- Ferrara J.L., Levine J.E., Reddy P., Holler E. 2009. Graft-versus-host disease. Lancet. 373:1550–1561 10.1016/S0140-6736(09)60237-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbitz A., Schultz M., Wilke A., Linde H.J., Schölmerich J., Andreesen R., Holler E. 2004. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 103:4365–4367 10.1182/blood-2003-11-3769 [DOI] [PubMed] [Google Scholar]
- Heimesaat M.M., Nogai A., Bereswill S., Plickert R., Fischer A., Loddenkemper C., Steinhoff U., Tchaptchet S., Thiel E., Freudenberg M.A., et al. 2010. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 59:1079–1087 10.1136/gut.2009.197434 [DOI] [PubMed] [Google Scholar]
- Hill G.R., Crawford J.M., Cooke K.R., Brinson Y.S., Pan L., Ferrara J.L. 1997. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 90:3204–3213 [PubMed] [Google Scholar]
- Jones J.M., Wilson R., Bealmear P.M. 1971. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat. Res. 45:577–588 10.2307/3573066 [DOI] [PubMed] [Google Scholar]
- Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. 2011. Human nutrition, the gut microbiome and the immune system. Nature. 474:327–336 10.1038/nature10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.O., Sheikh H.I., Ha S.D., Martins A., Reid G. 2006. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell. Microbiol. 8:1958–1971 10.1111/j.1462-5822.2006.00763.x [DOI] [PubMed] [Google Scholar]
- Korngold R., Sprent J. 1978. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. J. Exp. Med. 148:1687–1698 10.1084/jem.148.6.1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., Hamady M., Knight R. 2006. UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 7:371 10.1186/1471-2105-7-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magurran A.E. 2004. Measuring Biological Diversity. Blackwell Pub., Malden, Ma. 256 pages. [Google Scholar]
- Maloy K.J., Powrie F. 2011. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 474:298–306 10.1038/nature10208 [DOI] [PubMed] [Google Scholar]
- Manson J.M., Rauch M., Gilmore M.S. 2008. The commensal microbiology of the gastrointestinal tract. Adv. Exp. Med. Biol. 635:15–28 10.1007/978-0-387-09550-9_2 [DOI] [PubMed] [Google Scholar]
- Passweg J.R., Rowlings P.A., Atkinson K.A., Barrett A.J., Gale R.P., Gratwohl A., Jacobsen N., Klein J.P., Ljungman P., Russell J.A., et al. 1998. Influence of protective isolation on outcome of allogeneic bone marrow transplantation for leukemia. Bone Marrow Transplant. 21:1231–1238 10.1038/sj.bmt.1701238 [DOI] [PubMed] [Google Scholar]
- Penack O., Smith O.M., Cunningham-Bussel A., Liu X., Rao U., Yim N., Na I.K., Holland A.M., Ghosh A., Lu S.X., et al. 2009. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J. Exp. Med. 206:2101–2110 10.1084/jem.20090623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penack O., Henke E., Suh D., King C.G., Smith O.M., Na I.K., Holland A.M., Ghosh A., Lu S.X., Jenq R.R., et al. 2010. Inhibition of neovascularization to simultaneously ameliorate graft-vs-host disease and decrease tumor growth. J. Natl. Cancer Inst. 102:894–908 10.1093/jnci/djq172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen F.B., Buckner C.D., Clift R.A., Nelson N., Counts G.W., Meyers J.D., Thomas E.D. 1987. Infectious complications in patients undergoing marrow transplantation: a prospective randomized study of the additional effect of decontamination and laminar air flow isolation among patients receiving prophylactic systemic antibiotics. Scand. J. Infect. Dis. 19:559–567 10.3109/00365548709032423 [DOI] [PubMed] [Google Scholar]
- Pridmore R.D., Berger B., Desiere F., Vilanova D., Barretto C., Pittet A.C., Zwahlen M.C., Rouvet M., Altermann E., Barrangou R., et al. 2004. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc. Natl. Acad. Sci. USA. 101:2512–2517 10.1073/pnas.0307327101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P., Ferrara J.L.M.2008. Mouse models of graft-versus-host disease. In StemBook. Lisa Gerard, editor. StemBook, Cambridge, MA. http://www.stembook.org/node/548.
- Russell J.A., Chaudhry A., Booth K., Brown C., Woodman R.C., Valentine K., Stewart D., Ruether J.D., Ruether B.A., Jones A.R., et al. 2000. Early outcomes after allogeneic stem cell transplantation for leukemia and myelodysplasia without protective isolation: a 10-year experience. Biol. Blood Marrow Transplant. 6:109–114 10.1016/S1083-8791(00)70073-5 [DOI] [PubMed] [Google Scholar]
- Sayegh M.H., Wu Z., Hancock W.W., Langmuir P.B., Mata M., Sandner S., Kishimoto K., Sho M., Palmer E., Mitchell R.N., Turka L.A. 2003. Allograft rejection in a new allospecific CD4+ TCR transgenic mouse. Am. J. Transplant. 3:381–389 10.1034/j.1600-6143.2003.00062.x [DOI] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder M.A., DiPersio J.F. 2011. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 4:318–333 10.1242/dmm.006668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. 2010. Gut microbiota in health and disease. Physiol. Rev. 90:859–904 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- Steck N., Hoffmann M., Sava I.G., Kim S.C., Hahne H., Tonkonogy S.L., Mair K., Krueger D., Pruteanu M., Shanahan F., et al. 2011. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology. 141:959–971 10.1053/j.gastro.2011.05.035 [DOI] [PubMed] [Google Scholar]
- Storb R., Prentice R.L., Buckner C.D., Clift R.A., Appelbaum F., Deeg J., Doney K., Hansen J.A., Mason M., Sanders J.E., et al. 1983. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N. Engl. J. Med. 308:302–307 10.1056/NEJM198302103080602 [DOI] [PubMed] [Google Scholar]
- Ubeda C., Taur Y., Jenq R.R., Equinda M.J., Son T., Samstein M., Viale A., Socci N.D., van den Brink M.R.M., Kamboj M., Pamer E.G. 2010. Intestinal domination by Vancomycin-resistant Enterococcus precedes bloodstream invasion in humans. J. Clin. Invest. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bekkum D.W., Roodenburg J., Heidt P.J., van der Waaij D. 1974. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J. Natl. Cancer Inst. 52:401–404 [DOI] [PubMed] [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261–5267 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing B.P., Russell S.L., Finlay B.B. 2011. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 9:233–243 10.1038/nrmicro2536 [DOI] [PubMed] [Google Scholar]
- Woodmansey E.J. 2007. Intestinal bacteria and ageing. J. Appl. Microbiol. 102:1178–1186 10.1111/j.1365-2672.2007.03400.x [DOI] [PubMed] [Google Scholar]