Human skin-resident IL-10+ regulatory dendritic cells induce T reg cells that suppress allogeneic skin graft inflammation.
Abstract
Human skin immune homeostasis, and its regulation by specialized subsets of tissue-residing immune sentinels, is poorly understood. In this study, we identify an immunoregulatory tissue-resident dendritic cell (DC) in the dermis of human skin that is characterized by surface expression of CD141, CD14, and constitutive IL-10 secretion (CD141+ DDCs). CD141+ DDCs possess lymph node migratory capacity, induce T cell hyporesponsiveness, cross-present self-antigens to autoreactive T cells, and induce potent regulatory T cells that inhibit skin inflammation. Vitamin D3 (VitD3) promotes certain phenotypic and functional properties of tissue-resident CD141+ DDCs from human blood DCs. These CD141+ DDC-like cells can be generated in vitro and, once transferred in vivo, have the capacity to inhibit xeno-graft versus host disease and tumor alloimmunity. These findings suggest that CD141+ DDCs play an essential role in the maintenance of skin homeostasis and in the regulation of both systemic and tumor alloimmunity. Finally, VitD3-induced CD141+ DDC-like cells have potential clinical use for their capacity to induce immune tolerance.
Efficient immunoregulation in peripheral tissues is essential to maintain tissue homeostasis (Swamy et al., 2010). This task is of significant importance to skin, a major first line immune defense organ that protects the body against pathogen-derived and environmental challenges (Nestle et al., 2009). In mice, several studies have highlighted the critical roles of DCs in the regulation of skin immunity and tissue homeostasis (Steinman et al., 2003; Reis e Sousa, 2006; Heath and Carbone, 2009; Merad and Manz, 2009). In human skin, studies have focused on the functional role of immunostimulatory DCs and their role during skin inflammation (Nestle et al., 2009); however, little is known about human tissue-resident DCs with regulatory properties. Human blood-derived immature DCs have been shown to induce IL-10–producing regulatory T (Tr1) cells or T cell hyporesponsiveness to antigenic stimulation (Jonuleit et al., 2000; Dhodapkar et al., 2001). It has also been reported that exposure to antiinflammatory or immunosuppressive agents can induce a regulatory DC phenotype (Adorini et al., 2004). Common features of human regulatory DCs include altered maturation status, reduced T cell stimulatory capacity, and induction of T regulatory cells (Penna et al., 2005).
In human skin, myeloid DCs that reside in the dermis represent a major subset of dermal DCs (DDCs) during tissue homeostasis (Nestle et al., 2009). Subpopulations of DDCs have been described under both normal and pathological conditions. Classically, DDCs are CD1c+ with a CD1a+ and CD14+ subpopulation (Lenz et al., 1993; Nestle et al., 1993). The functional roles of DDC subsets are only partly understood. Zaba et al. (2007) showed that CD1a+ DDCs are potent inducers of allogeneic CD4+ and CD8+ T cell proliferation, whereas CD14+ DDCs are less immunogenic and might have the potential to differentiate into Langerhans cells in response to TGF-β (Caux et al., 1996; Larregina et al., 2001; Klechevsky et al., 2008).
A genome-wide expression profiling study suggested that human blood CD141+ DCs may correlate to mouse CD8α+ DCs (Robbins et al., 2008) and are capable of cross-presentation. Although CD141+ DCs are present only in small numbers in circulating blood, they are found in various lymphoid and nonlymphoid tissues (Demedts et al., 2005; Narbutt et al., 2006; Tsoumakidou et al., 2006; Zaba et al., 2007; Fiore et al., 2008; Jongbloed et al., 2010; Poulin et al., 2010). The functional specialization of CD141+ DCs in human skin and other peripheral tissues remains elusive.
In this study, we show that CD141+ DDCs are a major IL-10–producing skin-resident DC subset. They induce T cell hyporesponsiveness and CD25hi regulatory T cells (T reg cells) that suppress skin inflammation. Vitamin D3 (VitD3)–induced CD141+ cells generated from blood DCs share phenotypic and functional features of skin-resident CD141+ DDCs and are powerful regulators of alloimmunity. Adoptive transfer of these cells inhibits xeno-graft versus host disease (GvHD) and tumor alloimmunity in vivo. Collectively, our data suggest that CD141+ DDCs are key immunoregulatory antigen-presenting cells playing a potentially important role in tissue homeostasis and for the induction of clinical tolerance.
RESULTS AND DISCUSSION
Identification and characterization of a skin-resident CD141+ DDC population
Comprehensive profiling of human skin DCs identified a significant percentage of CD141+ DDCs (Fig. 1 A) ranging from 14.13 to 53.36% (mean = 30%; SD = 12.08) within the viable lineage-negative FSChiSSChiCD45+ migratory dermal cell population (Fig. S1). CD141+ migratory DDCs expressed CD11c and CD1c at low levels, but lacked expression of CD1a (Fig. 1, A and B). Importantly, CD141+ DDCs migrating from dermal explants coexpressed CD14 on the cell surface. They also expressed skin-relevant myeloid markers such as Factor XIIIa and CD163, but not CD103 or C-type lectin receptors such as CD209 (DC-SIGN), CD205 (DEC205), and CD207 (langerin; unpublished data). CD141+HLA-DR+ DDCs were located in a perivascular location in the upper dermis (Fig. 1 C). In agreement with the literature, CD141 expression was also detected on keratinocytes (Raife et al., 1994). Immunofluorescence staining for filamentous actin of flow cytometry–sorted CD141+ DDCs demonstrated a typical DC morphology with multiple dendritic processes (Fig. 1 D). CD141+ DDCs spontaneously migrated out from human dermis in tissue cultures and maintained a semimature phenotype, indicated by the absence of CD83, but expression of significant levels of co-stimulatory molecules, CD80 and CD86, and MHC class I and class II molecules (Fig. 1 E). This phenotype resembled the steady-state migratory (Kissenpfennig et al., 2005) and semimature tolerizing DCs (Lutz and Schuler, 2002) that have been described in mice. To address in vivo migratory capacity of CD141+ DDCs, we established a humanized skin transplantation mouse model (Sagoo et al., 2011). Human CD45+ leukocytes were detected in lymph nodes, but not in spleens, of mice that had received skin transplants (Fig. 1 F). Immunofluorescent staining showed both human CD3+ and CD141+ cells (Fig. 1 G). Detection of human CD141+ transcript in skin-draining lymph nodes via PCR confirmed active migration of CD141+ DDCs in vivo (Fig. 1 H). Together, human skin-resident CD141+ cells express DC markers and display hallmarks of DCs, including dendritic morphology, nonplastic adherence, and migratory activity in vitro and in vivo.
Figure 1.
Identification and characterization of skin migratory CD141+ DDCs. (A and B) Healthy human skin DDCs were stained for CD1c, CD11c, CD14, and CD1a. (C) CD141+HLA-DR+ DDCs staining in the upper dermis of normal human skin (arrows). Dashed line indicates epidermal–dermal junction. (D) Filamentous actin (F-actin) staining of cell-sorted CD141+ DDCs. (E) CD141+ DDCs or CD1c+ DDCs stained for CD83, CD80, CD86, HLA-ABC, HLA-DR, and MMR. (F) Human CD45+ staining of lymph nodes and spleens of BALB/c Rag2−/−γc−/− mice bearing human skin transplants (Tx). (G) Human CD141+ and CD3 staining in lymph nodes of Tx mice. (H) mRNA expression for human CD141, cyclophilin, and mouse GAPDH in spleens and lymph nodes of Tx mice. Blood and skin CD141+ DCs expression of BATF3, Necl2, CLEC9A, and XCR1 (I) and CD141+ DDCs expression of CLEC9A (J) as compared with CD1c+ DDCs. (K) CD141+ DDCs or CD1c+ DDCs pulsed with PPI were incubated with autoreactive CD8+ T cell clones and analyzed for proliferation. Net CPM represents proliferation of the clone in pulsed DDC co-culture minus nonpulsed DDC co-culture. Error bars indicate SEM. Results are representative of at least 30 (A), at least three (B–E), and two (F–K) independent experiments. Two-way ANOVA test; *, P < 0.05.
An important aspect of maintaining tissue homeostasis is based on DC capacity to capture, process, and present tissue-derived self-antigens to self-reactive T cells via the MHC class I antigen presentation pathway under steady-state conditions (Liu et al., 2002). CD141+ DDCs expressed markers associated with cross-presentation, including BATF3, Necl2, the C-type lectin CLEC9A, and XC chemokine receptor 1 (XCR1), in a pattern similar to human blood CD141+ DCs and mouse CD8α+ DCs (Bachem et al., 2010; Crozat et al., 2010; Jongbloed et al., 2010; Poulin et al., 2010; Fig. 1 I and Table S1). In particular, macrophage mannose receptor (MMR) and CLEC9A were selectively expressed by CD141+ DDCs compared with CD1c+ DDCs (Fig. 1, E and J), which may play an important role in the cross-presentation of soluble and cell-associated antigens (Burgdorf et al., 2006; Sancho et al., 2009). A distinctive feature of CD141+ DDCs compared with CD141+ blood DCs was the expression of Toll-like receptor (TLR) 4, 7, and 9 (unpublished data). To determine cross-presentation, CD1c+ and CD141+ DDCs pulsed with the model self-antigen preproinsulin (PPI) or control protein were co-cultured with a human autoreactive CD8+ T cell clone specific for PPI (Skowera et al., 2008). PPI-pulsed CD141+ DDCs induced significantly higher proliferation of the T cell clone than PPI-pulsed CD1c+ DDCs (Fig. 1 K). Importantly, cross-presentation of PPI by CD141+ DDCs occurred in the absence of proinflammatory stimuli such as poly(I:C), which was previously shown to be required for cross-presentation by CD141+ blood DCs (Jongbloed et al., 2010; Poulin et al., 2010). This suggests an intrinsic and unique capacity of CD141+ DDCs to cross-present self-antigens and may allow regulation of autoreactive CD8+ T cell response under noninflammatory, steady-state conditions (Liu et al., 2002). Cross-presentation of tissue-associated self-antigens may significantly contribute to the maintenance of skin homeostasis.
CD141+ DDCs produce IL-10 and induce CD25hi T-regulatory cells
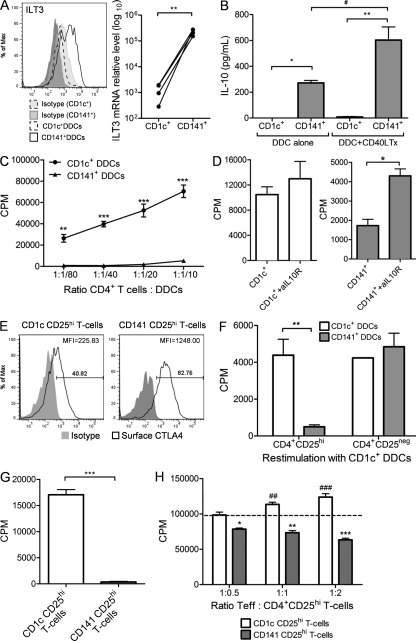
We next investigated the immunoregulatory properties of CD141+ DDCs. They expressed high levels of the immunoregulatory receptor immunoglobulin-like transcript 3 (ILT3; Chang et al., 2002) at the protein and mRNA levels in a consistently higher fashion than compared with CD1c+ DDCs (Fig. 2 A). In keeping with their immunoregulatory phenotype, CD141+ DDCs, but not CD1c+ DDCs, constitutively secreted high levels of IL-10 that was significantly enhanced after CD40 cross-linking (P < 0.05; Fig. 2 B). After CD40L stimulation, IL-10 production became detectable in CD1c+ DDCs and was boosted in CD141+ DDCs. When co-cultured with allogeneic CD4+ T cells, CD141+ DDCs were less proficient than CD1c+ DDCs in stimulating T cell proliferation (Fig. 2 C). This was not explained by significant differences in expression levels of MHC or co-stimulatory molecules (unpublished data). However, introducing a neutralizing anti–IL-10 receptor antibody significantly reversed the suppression of proliferation mediated by CD141+ DDCs, whereas no effect was observed in CD1c+ co-cultures (Fig. 2 D). During DC–T cell co-culture, alloreactive T cells became activated and up-regulated CD25 expression on the cell surface. Both CD141+ and CD1c+ DDCs induced a population of T cells that expressed high levels of CD25 (CD141 CD25hi T cells and CD1c CD25hi T cells, respectively). CD141+ DDCs induced higher surface expression of cytotoxic T lymphocyte antigen-4 (CTLA-4) on CD25hi T cells than CD1c+ DDCs (Fig. 2 E). In addition, CD141 CD25hi T cells, but not CD1c CD25hi T cells, were unresponsive to secondary alloantigen restimulation (Fig. 2 F). Our previous work has demonstrated correlation of in vitro hyporesponsiveness and regulatory function of T cells (Lombardi et al., 1994). We then expanded the cells in the presence of IL-2 to obtain sufficient cell numbers for in vivo functional studies (Sagoo et al., 2011). After cell expansion, CD141 CD25hi T cells and CD1c CD25hi T cells maintained their expression of CD25 and Foxp3 (unpublished data). CD141 CD25hi T cells were hyporesponsive to secondary TCR stimulation (Fig. 2 G) and suppressed CD4+CD25− T effector (T eff) cell proliferation, whereas CD1c CD25hi T cells enhanced T eff cell proliferation in a dose-dependent manner (Fig. 2 H).
Figure 2.
CD141+ DDCs are immunoregulatory. (A) CD141+ DDCs or CD1c+ DDCs expression of ILT3 and (B) production of IL-10 in the absence or presence of CD40 ligand-transfected L cells (CD40L Tx). (C) CD141+ DDCs or CD1c+ DDCs stimulation of allogeneic CD4+ T cell proliferation. (D) CD141+ DDCs or CD1c+ DDCs co-cultured with allogeneic CD4+ T cells in the presence and absence of anti–IL-10 receptor antibody. CD4+CD25hi T cells induced by CD1c+ DDCs (CD1c CD25hi T cells) or CD141+ DDCs (CD141 CD25hi T cells) stained for surface CTLA-4 (E) and proliferation in response to alloantigen stimulation (F). (G) IL-2–expanded CD25hi T cells proliferative response to secondary polyclonal stimulation and (H) suppression of CD4+CD25− T eff cell proliferation. Error bars indicate SEM. Results are representative of five (A–C, E, and F) and two (D, G, and H) independent experiments. Wilcoxon matched pairs test (A), one-way ANOVA (B), two-way ANOVA (C, F, and H), or unpaired Student’s t test (D, G). *, P < 0.05; **, P < 0.01; ***, P < 0.001; #, P < 0.05.
CD141+ DDCs induce CD25hi T reg cells that suppress alloimmune cell–mediated skin inflammation
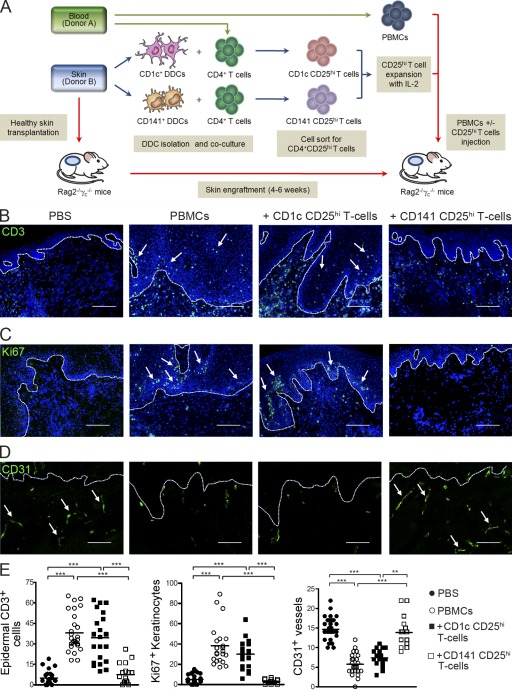
We assessed the in vivo immunoregulatory function of DDC-induced CD25hi T cells in our recently described model of human alloimmune cell–mediated skin inflammation (Fig. 3 A; Sagoo et al., 2011). Skin grafts from animals receiving allogeneic PBMCs showed typical inflammatory skin pathology with increased numbers of epidermal T cells, proliferating keratinocytes in the basal epidermal layer, and loss of intact CD31+ dermal microvasculature (Fig. 3, B–D). Co-transfer of CD141 CD25hi T cells protected from the induction of skin pathology in a significant manner, restoring markers of pathology to levels observed in control grafts (Fig. 3 E), whereas no significant protective effect was seen in mice treated with CD1c CD25hi T cells. Together, these data show that CD141+ DDCs display both phenotypic and functional characteristics of immunoregulatory DCs and induce T reg cells with immunosuppressive capability in vitro and in vivo.
Figure 3.
CD141+ DDC-induced CD25hi T reg cells inhibit human alloimmune cell–mediated skin inflammation. (A) Experimental strategy to assess CD25hi T cell suppression. BALB/c Rag2−/−γc−/− mice transplanted with human skin were injected with PBS, allogeneic PBMCs alone, or in combination with CD141 CD25hi T cells or CD1c CD25hi T cells. Representative fields from stained skin grafts for epidermal CD3+ T cell infiltration (B, arrows), epidermal keratinocyte expression of the proliferation marker Ki67 (C, arrows), and intact human CD31 superficial dermal microvasculature (D, arrows). Dashed lines indicate epidermal–dermal junction. Nuclei are stained with DAPI (blue). Bars, 100 µm. (E) Quantitative histological analysis of at least three independent visual fields per skin graft. Lines represent the mean (n = 5–9 animals per treatment group). Results are combined data from three independent experiments. One-way ANOVA test; **, P < 0.01; ***, P < 0.001.
VitD3 induces a CD141+ DDC-like phenotype and function in blood DCs
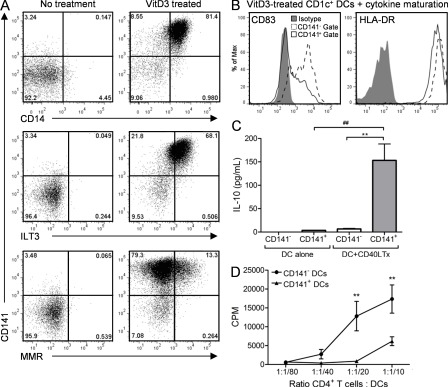
VitD3 has been linked to the immunosuppressive capacity of UV light, but human cellular effectors are ill defined (Schwarz and Schwarz, 2011). We addressed whether VitD3 could induce the unique immunoregulatory characteristics of CD141+ DDCs in blood DCs. Culture of CD141−CD1c+ blood DCs with the active form of VitD3, 1α,25-dihydroxyvitamin D3 (1,25[OH]2D3), induced the coexpression of CD141, CD14, ILT3, and MMR (Fig. 4 A). A core module of molecular markers present in cross-presenting DCs including BATF3, CLEC9A, and Necl2 was also induced in CD141+ VitD3 blood DCs (Table S1). VitD3-induced CD141+ DCs had a stable CD83low immature phenotype after exposure to a potent DC maturation cocktail consisting of TNF, IL-1β, IL-6, and prostaglandin E2 (Fig. 4 B; Jonuleit et al., 1997). This was in contrast to VitD3-induced CD141− DCs that readily up-regulated CD83 (Fig. 4 B). VitD3-induced CD141+ DCs produced significantly higher amounts of IL-10 than CD141− DCs after CD40 cross-linking (P < 0.01; Fig. 4 C) and were weak stimulators of allogeneic CD4+ T cell proliferation (Fig. 4 D). VitD3-induced CD141+ DCs and CD141+ DDCs have many similarities, including CD14 and ILT3 coexpression, semimature phenotype, IL-10 production, and poor T cell stimulatory capacity. Our findings provide a potential cellular link between VitD3 production and skin immune regulation.
Figure 4.
VitD3 induces CD141+ DDC-like phenotype in blood DCs. (A) VitD3-treated blood CD1c+ DC expression of CD141 with CD14, ILT3, and MMR. VitD3-induced CD141+ DCs or VitD3-induced CD141− DCs were assessed for their expression of CD83 and HLA-DR in the presence of a cytokine maturation cocktail (B), production of IL-10 in the presence and absence of CD40L Tx stimulation (C), and ability to stimulate allogeneic CD4+ T cell proliferation (D). Results are representative of at least 12 (A) and 3 (B–D) independent experiments. One-way ANOVA (C) or two-way ANOVA (D); **, P < 0.01; ##, P < 0.01.
Adoptively transferred VitD3–induced CD141hi DCs suppress alloimmunity in vivo
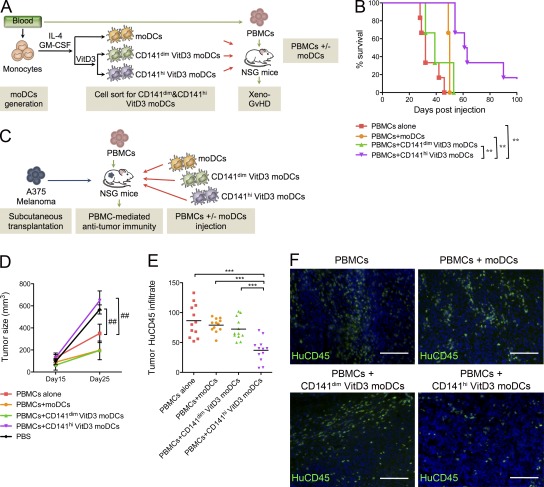
Establishment of a method to generate large numbers of cells with the immunoregulatory properties of CD141+ DDCs would allow the development of adoptive regulatory DC therapy in in vivo preclinical models and for development of therapeutic applications in patients. We thus investigated a source of DCs generated from blood monocytes after incubation with IL-4 and GM-CSF (moDCs). Incubation with VitD3 up-regulated CD141 expression on moDCs (VitD3 moDCs; unpublished data) and induced the expression of a molecular profile that resembles CD141+ DDCs (Table S1). VitD3 moDCs highly expressing CD141 (CD141hi VitD3 moDCs) produced high levels of IL-10 after CD40 cross-linking and demonstrated a reduced T cell stimulatory capacity, as compared with CD141dim VitD3 moDC or moDCs (unpublished data). PPI cross-presentation in the presence of poly(I:C) stimulation was enhanced in VitD3 moDCs compared with control moDCs (unpublished data). We assessed the in vivo regulatory capacity of CD141hi VitD3 moDCs in a human xeno-GvHD model (King et al., 2009; Fig. 5 A). Injection of human PBMCs induced xeno-GvHD in NSG mice (median survival time [MST] = 32). Co-transfer of CD141hi VitD3 moDCs with PBMCs significantly prolonged survival time (P < 0.001 versus PBMCs alone; MST = 62), whereas no significant protection was conferred by transfer of CD141dim VitD3 moDCs (MST = 39; Fig. 5 B). We next assessed the immunoregulatory role of CD141hi VitD3 moDCs in an alloimmune cell–dependent human melanoma model (Fig. 5 C). Injection of PBMCs reduced tumor size significantly compared with PBS-injected mice. However, tumor size was significantly increased in mice co-injected with PBMCs and CD141hi VitD3 moDCs, but not with CD141dim VitD3 moDCs or moDCs (Fig. 5 D), suggesting suppression of tumor alloimmunity. In line with these findings, we observed a significantly reduced human CD45+ cell infiltrate in tumors recovered from CD141hi VitD3 moDC–injected mice (Fig. 5, E and F). These results indicate that VitD3-induced CD141hi blood DCs have the ability to suppress alloimmunity in vivo and are possible candidates for regulatory DC therapy approaches.
Figure 5.
VitD3-induced CD141hi DCs suppress xeno-GvHD and antitumor immunity. (A) Strategy for preclinical assessment of adoptive cell therapy in xeno-GvHD. (B) NSG mice were injected with either PBMCs alone or in combination with moDCs, CD141dim VitD3 moDCs, or CD141hi VitD3 moDCs to induce xeno-GVHD. Survival was monitored over time. (C) Strategy to determine regulation of antitumor alloimmunity. Tumor size (D) and CD45+ infiltration (E and F) were assessed in animals injected with PBMCs alone or in combination with moDCs, CD141dim VitD3 moDCs or CD141hi VitD3 moDCs to induce antitumor alloimmunity (E) Quantitative histological analysis of tumor CD45+ infiltration from at least three independent visual fields per skin graft. Lines represent the mean (n = 3–6 animals per treatment group). (F) Representative pictures of human CD45+ infiltrates in tumors. Nuclei are stained with DAPI (blue). Bars, 100 µm. Results are pooled from three independent experiments (n = 3–6 animals per treatment group). One-way ANOVA (E), two-way ANOVA (D), or Mantel-Cox (B) test; **, P < 0.01; ***, P < 0.001; ##, P < 0.01.
Immunoregulation at tissue sites is tightly regulated and involves key immune sentinels, such as DCs. Although major progress has been made in understanding the immunoregulatory role of DCs residing in mouse tissues, their human counterparts are less well characterized. In this study, we identify an immunoregulatory CD141+ DC population in dermis of healthy human skin and establish culture methods to generate sufficient numbers of such cells for future regulatory DC therapy.
CD141+ DDCs coexpressed CD14 with low CD1c expression, a phenotypically distinct profile from blood CD141+ DCs. Although CD14 is expressed on the cells of the monocyte/macrophage lineage in the blood, it is also considered a marker of skin DC subsets (Nestle et al., 1993; Larregina et al., 2001; Klechevsky et al., 2008). In future studies, it will be interesting to investigate whether CD141+ DDCs activate follicular helper T cells, as has been shown for CD14+ interstitial DDCs (Klechevsky et al., 2008). We propose that the coexpression of CD141 and CD14 may be useful markers to define tissue-resident regulatory DC populations. DC populations with a similar phenotype have been identified in lung (Demedts et al., 2005), suggesting that our findings might extend to CD141+ DCs residing in other peripheral tissue sites.
A population of CD103+ DCs coexpressing CD207 has recently been identified in the dermis of murine skin as the primary migratory DDC subset capable of antigen capturing and (cross-)presentation (Bedoui et al., 2009; Henri et al., 2010). CD103+CD207+ DCs have been shown to mediate T cell tolerance, possibly in the context of their cross-presentation capability (Waithman et al., 2007) and their ability to induce T reg cells (Azukizawa et al., 2011). Given the skin origin, migratory capacity, cross-presentation ability, and immunoregulatory characteristics of the CD141+ DDC population described here, they may be related to this migratory cross-presenting CD103+ DC population. A population of CD26+SIRPα− DCs that shares transcriptomic and functional similarities with mouse CD8α+ DCs has also been identified in sheep skin (Contreras et al., 2010). This suggests a unique functional specialization of skin DCs across mammalian species for the maintenance of skin tissue homeostasis.
One of the most striking findings was the potent in vivo regulatory function of CD141+ DDCs and their induced T reg cells, as demonstrated in three independent human in vivo models of disease. A common denominator across these models was the efficient regulation of alloimmune-mediated pathology providing a framework for the future use of CD141+ DDCs or their VitD3-induced functional counterparts in the context of GvHD in organ transplantation. On the other hand, specific blockade of CD141+ DDCs might be beneficial in the cancer setting.
CD141+ DDCs express various TLRs and CD14, which is thought to facilitate microbial clearance and to amplify cellular responses (Anas et al., 2010). It is possible that CD141+ DDCs may exert antimicrobial or antiviral immunity when encountering viral/microbial products or under specific environmental conditions. TLR9 ligation was reported to reverse the regulatory function of CD8α+ DCs and promote T cell immunity in mice (Rizzitelli et al., 2005). Thus, our study does not exclude the possibility that CD141+ DDCs may be stimulatory in the context of viral/microbial infection and inflammatory skin pathology.
We identified VitD3 as a potent inducer of CD141+ DDC phenotype and function. This allowed us to generate large numbers of CD141+ DDC-like cells for future use in cell therapy applications. Collectively, our data identify a potent regulatory DC that is strategically positioned at epithelial tissue sites poised to play an important role in maintaining barrier immune homeostasis and to serve as a future therapeutic target in conditions of alloimmunity.
MATERIALS AND METHODS
Human samples.
Human buffy coats were provided by the National Blood Transfusion Centre (South Thames, Tooting, England, UK). Discarded skin was obtained from routine plastic surgery of healthy individuals. Human studies were conducted in accordance with the Helsinki Declaration and approved by the Institutional Review Board of Guy’s Hospital. Informed consent was obtained from all patients and healthy controls before enrollment into the study.
Isolation of DDCs.
DDC isolation was performed as previously described (Nestle et al., 1993). Skin was sliced into long strips, followed by 5 mg/ml dispase digestion (StemCell) for 2 h at 37°C. Epidermis and loose connective tissues in the lower dermis was removed, and dermis was sliced into 1–2-mm-thick strips and cultured in RPMI 1640 supplemented with 50 IU/ml penicillin, 50 µg/ml streptomycin, 2 mM l-glutamine (complete RPMI medium, all from Invitrogen), and 10% human AB serum (HS; Sigma-Aldrich). Nonplastic adherent cells that had migrated out of dermis 48–72 h after culture were harvested for flow cytometric analysis. Cell sorting of DDC subsets was performed by staining dermal cells with CD45-PECy7 (eBioscience), CD1c/BDCA1-PE (Miltenyi Biotec), CD141/BDCA3-APC (Miltenyi Biotec), and DAPI (Invitrogen), followed by sorting with a FACSAria II cell sorter (BD).
Immunodeficient mice.
Male and female NOD/scid/IL-2Rγ−/− mice (NOD.cg-PrkdcscidIl2rgtm1Wjl/SzJ [NSG]; The Jackson Laboratory) and BALB/c RAG2−/−γc−/− mice (provided by A. Hayday, The London Research Institute, Cancer Research UK, London, England, UK; and P. Gorer, Department of Immunobiology, King’s College School of Medicine at Guy’s Hospital, London, England, UK) were used between 8 and 12 wk of age. Mice were maintained under specific pathogen–free conditions and handled in accordance with the Institutional Committees on Animal Welfare of the UK Home Office (the Home Office Animals Scientific Procedures Act, 1986)
Humanized mouse model of skin DC lymph node migration.
Human skin samples were first keratomed using a hand-held air-powered dermatome (Zimmer) to obtain 500–700-µm split-thickness explants consisting of the epidermis and superficial dermis. Explants were kept at 4°C in complete RPMI medium and subsequently cut into 1–1.5-cm2 pieces and transplanted orthotopically onto the backs of BALB/c RAG2−/−γc−/− mice, as previously described (Sagoo et al., 2011). Spleens and skin-draining lymph nodes were harvested 8–12 wk after transplantation for analysis of DDC migration. Cell suspensions were obtained by dissociating spleens and lymph nodes through 70-µm nylon mesh strainers (BD). Mouse spleens and lymph node cell suspensions were preincubated with Mouse Fc Block (BD), and then human leukocytes identified by co-staining with anti-human CD45-PECy7 (eBioscience) and mouse CD45-APC/eFluor780 (eBioscience).
Flow cytometry.
The following antibodies were used in different combinations: CD1c-PE and CD141-APC (Miltenyi Biotec); CD45-PECy7 (eBioscience); CD11c-PerCp/Cy5.5 (BioLegend); CD14-FITC, CD80-PE, CD83-FITC, CD86-FITC, and HLA-DR-PE (Invitrogen); CD1a-PE, CD25-PE, CD45RO-PE, CD152/CTLA-4-APC, CD206/MMR-PECy5, and HLA-ABC-PE (BD); ILT3-PC5 (Beckman Coulter). Samples were acquired with a FACSCanto (BD), and data were analyzed by FlowJo (Tree Star) software.
Immunofluorescence staining.
Laser confocal microscopy was performed (TCS SP2; Leica). The following antibodies were used to stain OCT-embedded cryosections: CD141 (AbD Serotec); CD3 (Dako); human CD45 (eBioscience); Ki-67 (Abcam); CD31 (Abcam); HLA-DR (BD); goat anti–mouse IgG Alexa Fluor 555 or 488 and goat anti–rabbit IgG Alexa Fluor 555 or 488 (Invitrogen). To determine DC morphology, sorted CD141+ DDCs were immobilized on poly-l-lysine coated coverslips (BioCoat; BD) and stained with Fluorescent phallotoxins (Invitrogen) according to manufacturer’s specification. ProLong Gold antifade reagent with DAPI (Invitrogen) was used for nuclear staining.
Blood DCs, monocyte-derived DCs (moDCs), T cell isolation, and culturing.
PBMCs were isolated from buffy coats by density gradient centrifugation over Lymphocyte Separation Medium (PAA). To obtain CD1c+ myeloid DCs, DC populations were enriched using Dynal DC-enrichment kit according to the manufacturer’s specification (Invitrogen). CD1c+ DCs were further purified by cell sorting of lineage (CD3, CD14, CD19, and CD56)-negative and CD1c-positive populations. Monocytes were isolated from PBMCs using CD14 microbeads (Miltenyi Biotec) according to the manufacturer’s instructions, and cultured in complete RPMI medium supplemented with 1% single-donor plasma (NBS Totting), 500 IU/ml GM-CSF (PeproTech), and 500 IU/ml IL-4 (R&D Systems). Fresh GM-CSF and IL-4 were added on day 2 and 5 of a culture period of 7 d.
CD141+ DDC-like DCs were induced by treatment of CD1c+ blood DCs with 100 nM VitD3 for 2 d or on day 5 of moDC differentiation. In some cases, DCs were matured with TNF (10 ng/ml), IL-1β (10 ng/ml), IL-6 (1,000 IU/ml; all from R&D Systems), and prostaglandin E2 (1 µg/ml; Sigma-Aldrich) 24 h before harvesting. Where indicated, VitD3-induced CD141hi and CD141dim DCs were cell sorted by methods described above.
To study the allostimulatory capacity of DCs, allogeneic CD4+ T cells were prepared from buffy coats using RosetteSep human CD4+ T cell enrichment cocktail (STEMCELL Technologies) according to the manufacturers’ instructions. CD4+ T cells were cultured in 96-well round-bottom plates (5 × 104 cells/well) with graded numbers of allogeneic CD141+ and CD1c+ DDCs, or VitD3-induced CD141hi and CD141dim DCs. Proliferation of alloreactive T cells was assessed by [H3]thymidine incorporation (1 µCi/well; GE Healthcare) during the last 18 h of a 5-d culture. In some circumstances, because of limiting DDC numbers, responder T cells were scaled down accordingly resulting in lower thymidine counts. In some cultures, anti–IL-10R mAb (3F9; R&D Systems) was added. The optimal dose was determined to be 10 µg/ml. DDC-induced CD4+CD25hi T cells were isolated by cell sorting CD11c−CD4+CD25hi T cells from DDC co-cultures, resting overnight, and restimulation with CD1c+ DDCs derived from the primary allogeneic skin donor. To generate CD4+CD25hi lines, CD4+CD25hi T cells were cell-sorted directly into 96-well plates containing RPMI 10% HS and left to rest overnight. Subsequently, low dose IL-2 (R&D Systems) was added at 250 IU/ml and replenished every 2–3 d. After adequate expansion for a period of 4–5 wk, CD25hi T cells were harvested by cell sorting. To assess in vitro suppressive capacity, titrated numbers of CD25hi T cells were co-cultured with 5 × 104 autologous CD4+CD25− effector T cells for 5 d in the presence of CD3/CD28 T cell Expander Dynal Beads (Invitrogen). Cell proliferation was measured as described above.
Humanized mouse model of alloimmune cell–mediated skin inflammation.
BALB/c RAG2−/−γc−/− mice bearing human skin transplants were allowed to engraft for 5–6 wk before adoptive transfer of human cells. For the total duration of these experiments, purified anti–mouse Gr1 mAb (100 µg; BioXell) was injected intraperitoneally every 4–5 d to deplete mouse granulocytes. Allogeneic (to the skin) PBMCs depleted of CD25+ cells were then adoptively transferred intravenously via the tail vein to induce alloimmune skin inflammation, as previously described (Sagoo et al., 2011). The in vivo suppressive capacity of DDC induced CD25hi T cells was investigated by co-injecting CD141 CD25hi T cells or CD1c CD25hi T cells with PBMCs (autologous to the CD25hi T cells and allogeneic to the skin) depleted of CD25+ cells at a ratio of 1:10 (CD25hi T cells:CD3 composition of PBMCs inoculum); typically 2.5 × 106 human PBMCs alone or in combination with 1.5 × 105 CD25hi T cells. Skin grafts were harvested 3–4 wk after transfer of human cells and assessed histologically for markers of skin inflammation.
Humanized mouse model of xeno-GvHD.
GvHD was induced by intravenous transfer of 10 × 106 human PBMCs into adult NSG mice. Animals that developed clinical symptoms of GvHD (severe weight loss, hunched posture, fur loss, reduced mobility, and tachypnea) were sacrificed, and spleens were harvested for flow cytometric analysis of human cell engraftment. To assess the immunoregulatory potential of VitD3 moDCs in GvHD, human PBMCs were either injected alone or co-transferred with 5 × 105 cell-sorted CD141hi VitD3 moDCs, CD141dim VitD3 moDCs, or moDCs with an end point of survival recorded in all treatment groups.
Humanized mouse model of melanoma antitumor alloimmunity.
To establish a xenograft melanoma model, 5 × 105 human A375 melanoma cells (American Type Culture Collection) were injected subcutaneously into the flank of adult NSG mice. 5 d after tumor xenografting, 10 × 106 human PBMCs were transferred intravenously via the tail vein. To determine immunoregulatory ability of VitD3 moDCs, human PBMCs were either injected alone or co-transferred with 5 × 105 cell sorted CD141hi VitD3 moDCs, CD141dim VitD3 moDCs, or moDCs. Tumor size was measured at day 15 and day 25 after adoptive transfer and calculated using the formula: (short diameter)2 × (long diameter)/2.
Determination of IL-10 production.
2 × 104 DCs were cultured in 96-well flat-bottom plates with 100 µl of complete RPMI medium containing 10% HS for 2 d in the absence or presence of CD40 ligand-transfected cells (CD40L Tx, 5 × 104 cells/well). IL-10 levels in the supernatant were assayed by using the MILLIPLEX MAP Human Cytokine kit (Millipore) and acquired on a Luminex 100 flow-based sorting and detection analyzer (Luminex Corporation).
RNA extraction and quantitative RT-PCR (qRT-PCR).
RNA extraction was performed using NucleoSpin RNA XS kit (Macherey-Nagel GmbH and Co.) according to manufacturer’s instructions and retrotranscribed into cDNA. Human CD141, CLEC9A, ILT3, BATF3, Necl2, and TLR9 expression was assessed by multiplex real-time quantitative RT-PCR using TaqMan assays (Applied Biosystems) according to manufacturer’s instructions. For each sample, mRNA abundance was normalized to the amount of human cyclophilin. Data analysis was performed using the ΔΔCt method: results were expressed as relative mRNA levels in arbitrary units. Where indicated, qPCR products from singleplex assays were run on a 2% agarose gel followed by ethidium bromide.
Cross-presentation assays.
20 µg/ml of PPI protein, prepared in-house (Skowera et al., 2008), or recombinant CMV protein (Miltenyi Biotec) were added to the DDC containing dermal culture prepared from a HLA-A2 donor. After 48 h, CD1c+ and CD141+DDCs were isolated by cell sorting, rested overnight, and then co-cultured with PPI15-24-specific CD8+ T cell clone, clone 1E6, as previously described (Skowera et al., 2008).
DDCs pulsed with PPI15-24 peptide 1 h before co-culture were used as positive control. Proliferation of clone 1E6 was assessed by [H3]thymidine incorporation (1 µCi/well) during the last 18 h of 3-d culture.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism version 4.0 (GraphPad Software). Results were assessed for normal Gaussian distribution, and then analyzed by Mann-Whitney nonparametric Student’s t test, Wilcoxon matched pairs test, one-way ANOVA test, two-way ANOVA, or Mantel-Cox as appropriate. For markers of skin inflammation quantification, at least one independent image was acquired from each individual, and every image was counted by two independent researchers blinded from treatment groups. Values of P < 0.05 were considered significant.
Online supplemental material.
Fig. S1 shows the gating strategy of skin DDCs. Table S1 shows the expression of common molecular markers by CD141+ DDCs and VitD3 induced blood DCs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20112583/DC1.
Acknowledgments
We thank Dr. Katie Lacy, Dr. Gayathri Perera, Dr. Rose Mak, Angela Clifford, Sharon Jones and Mrs. Jenny Geh and Mr. Ciaran Healy from plastic surgery for help with collecting clinical samples. In addition we thank Isabella Tosi for excellent management and coordination of our tissue bank. This work would not have been possible without the generous help of healthy volunteers and of patients from Guy’s and St. Thomas’ Hospital, London.
We acknowledge support by the following grant funding bodies: Wellcome Trust Program GR078173MA, Medical Research Council UK Program G0601387, Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. C.C. Chu is supported by St. John’s Institute of Dermatology Studentship and Overseas Research Students Awards Scheme (ORSAS). N. Ali is supported by a PhD Studentship from the MRC Centre for Transplantation.
A patent application has been submitted by King’s College London based on the findings presented in this study. The authors have no other competing financial interests.
Footnotes
Abbreviations used:
- DDC
- dermal DC
- GvHD
- graft versus host disease
- ILT3
- immunoglobulin-like transcript 3
- MMR
- macrophage mannose receptor
- moDC
- monocyte-derived DC
- MST
- median survival time
- PPI
- preproinsulin
- T eff cell
- effector T cell
- TLR
- Toll-like receptor
- T reg cell
- regulatory T cell
- VitD3
- vitamin D3
References
- Adorini L., Giarratana N., Penna G. 2004. Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin. Immunol. 16:127–134 10.1016/j.smim.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Anas A., van der Poll T., de Vos A.F. 2010. Role of CD14 in lung inflammation and infection. Crit. Care. 14:209 10.1186/cc8850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azukizawa H., Döhler A., Kanazawa N., Nayak A., Lipp M., Malissen B., Autenrieth I., Katayama I., Riemann M., Weih F., et al. 2011. Steady state migratory RelB+ langerin+ dermal dendritic cells mediate peripheral induction of antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells. Eur. J. Immunol. 41:1420–1434 10.1002/eji.201040930 [DOI] [PubMed] [Google Scholar]
- Bachem A., Güttler S., Hartung E., Ebstein F., Schaefer M., Tannert A., Salama A., Movassaghi K., Opitz C., Mages H.W., et al. 2010. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 207:1273–1281 10.1084/jem.20100348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoui S., Whitney P.G., Waithman J., Eidsmo L., Wakim L., Caminschi I., Allan R.S., Wojtasiak M., Shortman K., Carbone F.R., et al. 2009. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10:488–495 10.1038/ni.1724 [DOI] [PubMed] [Google Scholar]
- Burgdorf S., Lukacs-Kornek V., Kurts C. 2006. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J. Immunol. 176:6770–6776 [DOI] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Dezutter-Dambuyant C., de Saint-Vis B., Jacquet C., Yoneda K., Imamura S., Schmitt D., Banchereau J. 1996. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J. Exp. Med. 184:695–706 10.1084/jem.184.2.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Ciubotariu R., Manavalan J.S., Yuan J., Colovai A.I., Piazza F., Lederman S., Colonna M., Cortesini R., Dalla-Favera R., Suciu-Foca N. 2002. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat. Immunol. 3:237–243 10.1038/ni760 [DOI] [PubMed] [Google Scholar]
- Contreras V., Urien C., Guiton R., Alexandre Y., Vu Manh T.P., Andrieu T., Crozat K., Jouneau L., Bertho N., Epardaud M., et al. 2010. Existence of CD8α-like dendritic cells with a conserved functional specialization and a common molecular signature in distant mammalian species. J. Immunol. 185:3313–3325 10.4049/jimmunol.1000824 [DOI] [PubMed] [Google Scholar]
- Crozat K., Guiton R., Contreras V., Feuillet V., Dutertre C.A., Ventre E., Vu Manh T.P., Baranek T., Storset A.K., Marvel J., et al. 2010. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J. Exp. Med. 207:1283–1292 10.1084/jem.20100223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts I.K., Brusselle G.G., Vermaelen K.Y., Pauwels R.A. 2005. Identification and characterization of human pulmonary dendritic cells. Am. J. Respir. Cell Mol. Biol. 32:177–184 10.1165/rcmb.2004-0279OC [DOI] [PubMed] [Google Scholar]
- Dhodapkar M.V., Steinman R.M., Krasovsky J., Munz C., Bhardwaj N. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233–238 10.1084/jem.193.2.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore N., Castellano G., Blasi A., Capobianco C., Loverre A., Montinaro V., Netti S., Torres D., Manno C., Grandaliano G., et al. 2008. Immature myeloid and plasmacytoid dendritic cells infiltrate renal tubulointerstitium in patients with lupus nephritis. Mol. Immunol. 45:259–265 10.1016/j.molimm.2007.04.029 [DOI] [PubMed] [Google Scholar]
- Heath W.R., Carbone F.R. 2009. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 10:1237–1244 10.1038/ni.1822 [DOI] [PubMed] [Google Scholar]
- Henri S., Poulin L.F., Tamoutounour S., Ardouin L., Guilliams M., de Bovis B., Devilard E., Viret C., Azukizawa H., Kissenpfennig A., Malissen B. 2010. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 207:189–206 10.1084/jem.20091964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed S.L., Kassianos A.J., McDonald K.J., Clark G.J., Ju X., Angel C.E., Chen C.J., Dunbar P.R., Wadley R.B., Jeet V., et al. 2010. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 207:1247–1260 10.1084/jem.20092140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H., Kühn U., Müller G., Steinbrink K., Paragnik L., Schmitt E., Knop J., Enk A.H. 1997. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 27:3135–3142 10.1002/eji.1830271209 [DOI] [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. 2000. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222 10.1084/jem.192.9.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M.A., Covassin L., Brehm M.A., Racki W., Pearson T., Leif J., Laning J., Fodor W., Foreman O., Burzenski L., et al. 2009. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin. Exp. Immunol. 157:104–118 10.1111/j.1365-2249.2009.03933.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A., Henri S., Dubois B., Laplace-Builhé C., Perrin P., Romani N., Tripp C.H., Douillard P., Leserman L., Kaiserlian D., et al. 2005. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 22:643–654 10.1016/j.immuni.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Klechevsky E., Morita R., Liu M., Cao Y., Coquery S., Thompson-Snipes L., Briere F., Chaussabel D., Zurawski G., Palucka A.K., et al. 2008. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 29:497–510 10.1016/j.immuni.2008.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larregina A.T., Morelli A.E., Spencer L.A., Logar A.J., Watkins S.C., Thomson A.W., Falo L.D., Jr 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2:1151–1158 10.1038/ni731 [DOI] [PubMed] [Google Scholar]
- Lenz A., Heine M., Schuler G., Romani N. 1993. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J. Clin. Invest. 92:2587–2596 10.1172/JCI116873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Iyoda T., Saternus M., Kimura Y., Inaba K., Steinman R.M. 2002. Immune tolerance after delivery of dying cells to dendritic cells in situ. J. Exp. Med. 196:1091–1097 10.1084/jem.20021215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi G., Sidhu S., Batchelor R., Lechler R. 1994. Anergic T cells as suppressor cells in vitro. Science. 264:1587–1589 10.1126/science.8202711 [DOI] [PubMed] [Google Scholar]
- Lutz M.B., Schuler G. 2002. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 23:445–449 10.1016/S1471-4906(02)02281-0 [DOI] [PubMed] [Google Scholar]
- Merad M., Manz M.G. 2009. Dendritic cell homeostasis. Blood. 113:3418–3427 10.1182/blood-2008-12-180646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbutt J., Lesiak A., Sysa-Jedrzejowska A., Smolewski P., Robak T., Zalewska A. 2006. The number and distribution of blood dendritic cells in the epidermis and dermis of healthy human subjects. Folia Histochem. Cytobiol. 44:61–63 [PubMed] [Google Scholar]
- Nestle F.O., Zheng X.G., Thompson C.B., Turka L.A., Nickoloff B.J. 1993. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J. Immunol. 151:6535–6545 [PubMed] [Google Scholar]
- Nestle F.O., Di Meglio P., Qin J.Z., Nickoloff B.J. 2009. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9:679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna G., Giarratana N., Amuchastegui S., Mariani R., Daniel K.C., Adorini L. 2005. Manipulating dendritic cells to induce regulatory T cells. Microbes Infect. 7:1033–1039 10.1016/j.micinf.2005.03.025 [DOI] [PubMed] [Google Scholar]
- Poulin L.F., Salio M., Griessinger E., Anjos-Afonso F., Craciun L., Chen J.L., Keller A.M., Joffre O., Zelenay S., Nye E., et al. 2010. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J. Exp. Med. 207:1261–1271 10.1084/jem.20092618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raife T.J., Lager D.J., Madison K.C., Piette W.W., Howard E.J., Sturm M.T., Chen Y., Lentz S.R. 1994. Thrombomodulin expression by human keratinocytes. Induction of cofactor activity during epidermal differentiation. J. Clin. Invest. 93:1846–1851 10.1172/JCI117171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C. 2006. Dendritic cells in a mature age. Nat. Rev. Immunol. 6:476–483 10.1038/nri1845 [DOI] [PubMed] [Google Scholar]
- Rizzitelli A., Vremec D., Villadangos J.A., Mavaddat N., Wright M.D., Shortman K. 2005. Switching from a restricted to an effective CD4 T cell response by activating CD8+ murine dendritic cells with a Toll-like receptor 9 ligand. Eur. J. Immunol. 35:3209–3220 10.1002/eji.200526231 [DOI] [PubMed] [Google Scholar]
- Robbins S.H., Walzer T., Dembélé D., Thibault C., Defays A., Bessou G., Xu H., Vivier E., Sellars M., Pierre P., et al. 2008. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 9:R17 10.1186/gb-2008-9-1-r17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo P., Ali N., Garg G., Nestle F.O., Lechler R.I., Lombardi G. 2011. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci. Transl. Med. 3:ra42 10.1126/scitranslmed.3002076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D., Joffre O.P., Keller A.M., Rogers N.C., Martínez D., Hernanz-Falcón P., Rosewell I., Reis e Sousa C. 2009. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 458:899–903 10.1038/nature07750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T., Schwarz A. 2011. Molecular mechanisms of ultraviolet radiation-induced immunosuppression. Eur. J. Cell Biol. 90:560–564 10.1016/j.ejcb.2010.09.011 [DOI] [PubMed] [Google Scholar]
- Skowera A., Ellis R.J., Varela-Calviño R., Arif S., Huang G.C., Van-Krinks C., Zaremba A., Rackham C., Allen J.S., Tree T.I., et al. 2008. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J. Clin. Invest. 118:3390–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M., Hawiger D., Nussenzweig M.C. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711 10.1146/annurev.immunol.21.120601.141040 [DOI] [PubMed] [Google Scholar]
- Swamy M., Jamora C., Havran W., Hayday A. 2010. Epithelial decision makers: in search of the ‘epimmunome’. Nat. Immunol. 11:656–665 10.1038/ni.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoumakidou M., Tzanakis N., Papadaki H.A., Koutala H., Siafakas N.M. 2006. Isolation of myeloid and plasmacytoid dendritic cells from human bronchoalveolar lavage fluid. Immunol. Cell Biol. 84:267–273 10.1111/j.1440-1711.2006.01428.x [DOI] [PubMed] [Google Scholar]
- Waithman J., Allan R.S., Kosaka H., Azukizawa H., Shortman K., Lutz M.B., Heath W.R., Carbone F.R., Belz G.T. 2007. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J. Immunol. 179:4535–4541 [DOI] [PubMed] [Google Scholar]
- Zaba L.C., Fuentes-Duculan J., Steinman R.M., Krueger J.G., Lowes M.A. 2007. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J. Clin. Invest. 117:2517–2525 10.1172/JCI32282 [DOI] [PMC free article] [PubMed] [Google Scholar]