IL-6–producing B cells contribute to EAE pathology and possibly human MS, whereas ablation of B cell IL-6 is associated with a reduced Th17 response.
Abstract
B cells have paradoxical roles in autoimmunity, exerting both pathogenic and protective effects. Pathogenesis may be antibody independent, as B cell depletion therapy (BCDT) leads to amelioration of disease irrespective of autoantibody ablation. However, the mechanisms of pathogenesis are poorly understood. We demonstrate that BCDT alleviates central nervous system autoimmunity through ablation of IL-6–secreting pathogenic B cells. B cells from mice with experimental autoimmune encephalomyelitis (EAE) secreted elevated levels of IL-6 compared with B cells from naive controls, and mice with a B cell–specific IL-6 deficiency showed less severe disease than mice with wild-type B cells. Moreover, BCDT ameliorated EAE only in mice with IL-6–sufficient B cells. This mechanism of pathogenesis may also operate in multiple sclerosis (MS) because B cells from MS patients produced more IL-6 than B cells from healthy controls, and this abnormality was normalized with B cell reconstitution after Rituximab treatment. This suggests that BCDT improved disease progression, at least partly, by eliminating IL-6–producing B cells in MS patients. Taking these data together, we conclude that IL-6 secretion is a major mechanism of B cell–driven pathogenesis in T cell–mediated autoimmune disease such as EAE and MS.
Recent studies have shown that B cell depletion therapy (BCDT) can efficiently reduce disease progression in relapsing-remitting multiple sclerosis (RR-MS) and in experimental autoimmune encephalomyelitis (EAE; Bar-Or et al., 2008; Hauser et al., 2008; Matsushita et al., 2008). Thus, in addition to their documented regulatory capacity (Mauri et al., 2003; Mann et al., 2007; Fillatreau et al., 2008; Lampropoulou et al., 2008), B cells also promote the inflammatory response in EAE and MS (Anderton and Fillatreau, 2008; Lampropoulou et al., 2010). RR-MS is a chronic inflammatory demyelinating disease of the central nervous system (CNS) associated with an accumulation of immune cells at lesion sites. Although polymorphisms in genes controlling T cell activation show the strongest association with disease susceptibility (Oksenberg et al., 2008), B cell activation is also a common abnormality in RR-MS, highlighted by the presence of intrathecal oligoclonal immunoglobulin bands in >90% of patients (Fillatreau and Anderton, 2007). It is therefore clear that B cells participate in this disease. However, the mechanisms by which B cells exert pathogenic effects in RR-MS are not understood.
B cells might promote tissue destruction through autoantibody production in RR-MS (Wekerle, 1999). Myelin-reactive autoantibodies are sometimes found in serum and CNS of RR-MS patients, and transfusion of autoantibody-containing serum exacerbates demyelination and axonal loss in rats (Zhou et al., 2006). However, clinical improvement in patients treated with Rituximab often precedes reduction in autoantibody levels (Edwards and Cambridge, 2006; Martin and Chan, 2006). More importantly, treatment with Atacicept, which reduces numbers of short- and long-lived plasma cells (Balázs et al., 2002; O’Connor et al., 2004; Belnoue et al., 2008), resulted in aggravation, not improvement, of RR-MS (Hartung and Kieseier, 2010). These observations concur to indicate that B cells propagate this autoimmune disease via antibody-independent mechanisms. If antibody is not the principal mediator of B cell pathogenesis, then we must ask what other aspects of B cell function are important? Rituximab treatment results in a noticeable decline of T cell numbers in CNS of treated patients (Cross et al., 2006), suggesting that B cells facilitate RR-MS progression by sustaining pathogenic T cell responses, possibly through presentation of antigen and/or secretion of cytokines (Bar-Or et al., 2010). The latter mechanism attracted our interest because cytokine blockade is often an effective treatment for autoimmune disease (Bar-Or et al., 2010). Furthermore, cytokines can be elicited from B cells irrespective of antigenic specificity (e.g., toll-like receptor [TLR]–activated B cells, microbe-specific B cells, or B cells reactive to other antigens). Antigen presentation to encephalitogenic T cells, in contrast, can be performed only by myelin-specific B cells. This is a highly pertinent consideration because an important proportion of the B cell response is not myelin reactive in RR-MS (Owens et al., 2009). A candidate cytokine for the pathogenic functions of B cells in RR-MS is IL-6, which is essential for the development of EAE (Eugster et al., 1998; Mendel et al., 1998; Okuda et al., 1998; Samoilova et al., 1998), the primary mouse model of RR-MS. B cells can secrete large amounts of IL-6 in response to polyclonal activating stimuli and subsequently enhance T cell proliferation in vitro (Lampropoulou et al., 2008) and Th17 responses in vivo (Barr et al., 2010), which have a pathogenic role in autoimmune disease (Korn et al., 2009). Based on this rationale, we evaluated the role of IL-6 production by B cells in EAE and MS.
RESULTS
B cells are a major source of IL-6, which is stimulatory for T cells
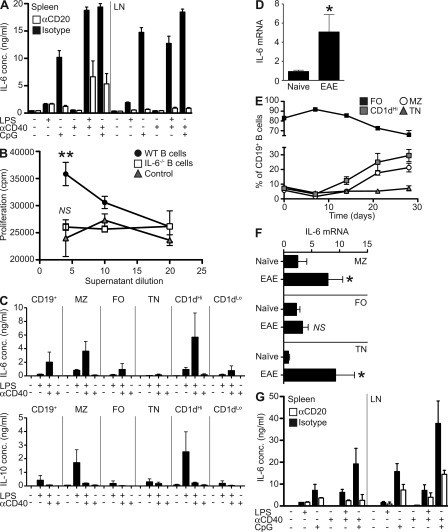
We first sought to determine the relative contribution of B cells to total IL-6 production in vivo. To address this, naive mice were ablated of B cells using anti-CD20. Whole (unsorted) spleen and lymph node cell cultures were then stimulated with LPS, CpG, and anti-CD40, which engage major receptors involved in activation of APCs. Anti-CD20 therapy depleted B cells with 95% or greater efficiency, with the knock-on consequence of enriching innate APC and T cells in the cultures (unpublished data). Despite this enrichment in many potential sources of IL-6 (e.g., macrophages, dendritic cells, neutrophils, and T cells), IL-6 production was massively reduced after anti-CD20 depletion (Fig. 1 A), and B cells accounted for 65–95% of IL-6, and, therefore, appeared to be a major source of IL-6 in secondary lymphoid tissues.
Figure 1.
B cells from naive and EAE mice are a major source of IL-6 which stimulates T cells in vitro. (A) Whole splenocyte and lymph node cells were isolated from mice after B cell depletion or isotype control treatment. IL-6 levels were quantified by ELISA after 72 h of stimulation with LPS, CpG, and/or anti-CD40. Bars represent the mean of groups of five mice and are representative of two independent experiments. (B) Supernatants from LPS- and anti-CD40–treated WT B cells, IL-6−/− B cells, or cell-free controls were added to sorted CD4+ T cells in the presence of anti-CD3 and anti-CD28. Proliferation was determined by thymidine incorporation after 72 h. Data points represent the means of triplicate cultures of cells pooled from two mice, with error bars the SEM. Results are representative of two independent experiments. Statistical analysis is by two-way ANOVA, where **, P < 0.01. (C) B cell subsets were isolated from spleens of naive mice by magnetic and FACS sorting based on CD19, CD21, CD23, and CD1d expression. After in vitro stimulation for 24 h with LPS and/or anti-CD40, IL-6 (top) and IL-10 (bottom) levels in supernatants were quantified by Bioplex. Histograms represent mean cytokine production from triplicate cultures of cells pooled from four to eight mice. Error bars represent SEM. Comparison between subsets stimulated with LPS and CD40 is by Student’s t test; MZ versus FO, P = 0.0156 and CD1dHi versus CD1dLo, P = 0.0352. Data presented are combined from nine independent experiments. (D) RT-PCR for IL-6 mRNA was performed on pooled B cells sorted directly ex vivo from naive and EAE (at day 14) mice. Data presented is combined from two independent experiments. Histograms represent mean mRNA expression from triplicate samples obtained from pooled cells from 10 mice. Error bars represent SEM. Statistical analysis is by Student’s t test, where *, P < 0.05. (E) Kinetics of B cell subset expansion during EAE were assessed by flow cytometry. Each point represents the mean of 6–12 mice, with error bars the SEM. Data are representative of two separate experiments. (F) RT-PCR for IL-6 mRNA was performed on sorted B cell subsets as indicated. Data presented is combined from two independent experiments. Comparisons between naive and EAE B cells for each subset was statistically analyzed by Student’s t test, where NS, P > 0.05 and *, P < 0.05. (G) Splenocyte and draining lymph node cells were isolated from EAE mice (at day 14) after treatment with isotype control or anti-CD20 at day 10. IL-6 was quantified Bioplex on supernatants after 72 h of stimulation with LPS, CpG, and anti-CD40. Bars represent the mean on duplicate samples pooled from five mice and are representative of three independent experiments.
In RR-MS, the disease-promoting activity of B cells might be related to a stimulatory effect on pathogenic T cells (Cross et al., 2006). We therefore tested if B cells could modulate T cell responses through IL-6. Naive T cells were stimulated in vitro in the presence of supernatants from B cells activated with LPS+CD40. Remarkably, T cell proliferation was significantly enhanced by supernatants from WT B cells (even though they were diluted at least fivefold) but not from IL-6–deficient B cells (Fig. 1 B; P = 0.0053 by two-way ANOVA). We next evaluated the capacity of various B cell subsets to secrete IL-6. Marginal zone (MZ), follicular (FO), and transitional (TN) B cell subsets were sorted by flow cytometric sorting based on expression of CD19, CD21, CD23, and CD1d. MZ B cells (CD21HiCD23Lo or CD1dHi) rapidly secreted IL-6 in response to TLR4 stimulation with LPS, and the amount of IL-6 produced was further increased to ∼4 ng/ml by provision of surrogate T cell help, in the form of anti-CD40 (Fig. 1 C). Intriguingly, this same subset also secreted the greatest amounts of IL-10, as we have reported previously (Barr et al., 2007; Lampropoulou et al., 2008; Fig. 1 C), although it is noteworthy that the signals driving maximal levels of these cytokines are distinct (i.e., IL-6 with CpG and IL-10 with LPS). The difference in IL-6 production by various B cell subsets was less apparent in longer term cultures (72 h stimulation; unpublished data).
To investigate whether B cells might contribute to EAE pathogenesis—which is T cell mediated—through production of IL-6, we first sought to determine if IL-6 production by B cells was increased during EAE. CD19+ B cells were isolated from naive mice, and from mice with EAE by magnetic and FACS sorting. IL-6 mRNA was expressed at significantly higher levels in B cells from EAE mice compared with B cells from naive animals (Fig. 1 D; P = 0.035 by Student’s t test). Given our demonstration that MZ/CD1dHi B cells represent the most significant source of B cell–derived IL-6, we next assessed the composition of the B cell compartment during the EAE response. The frequency of MZ B cells in EAE mice was elevated from day 14 onwards (Fig. 1 E). To assess the capacity of individual subsets to produce IL-6 in EAE, mRNA was quantified on FACS-sorted samples. Increased IL-6 mRNA expression was most noticeable in MZ (CD21HiCD23Lo; P = 0.0244) and TN (CD21LoCD23Lo; P = 0.024) but not in FO (CD21IntCD23Hi) B cell subsets (Fig. 1 F). Thus, both frequency of IL-6 producers and capacity of these subsets to produce IL-6 are elevated in EAE. As with naive mice (Fig. 1 A), B cells from EAE mice were found to be the major source of IL-6 in responses to TLR stimulation, as whole cell cultures from CD20-depleted animals with EAE showed up to 86% reduction in IL-6 secretion (Fig. 1 G). Increased IL-6 production by B cells is not restricted to CNS autoimmunity. B cells from mice immunized with a nonpathogenic peptide antigen (chicken egg OVA, pOVA) in complete Freund’s adjuvant showed similarly increased IL-6 secretion (unpublished data). We have also previously shown that B cells secrete elevated IL-6 during Salmonella infection (Barr et al., 2010). Thus, it appears that B cells respond via innate receptors rather than via antigen-specific BCR. Indeed, stimulation of B cells from myelin oligodendrocyte glycoprotein (MOG) or OVA-immunized mice does not produce IL-6 when restimulated with antigen, either alone or in conjunction with anti-CD40 (unpublished data). Collectively, our data show that B cells are an important source of IL-6 when cells from secondary lymphoid organs are exposed to stimuli controlling activation of immunity, and IL-6 from B cells can significantly increase T cell responses in vitro. Furthermore, IL-6 production by B cells is increased during EAE.
Mice with a B cell–specific IL-6 deficiency develop an attenuated form of EAE
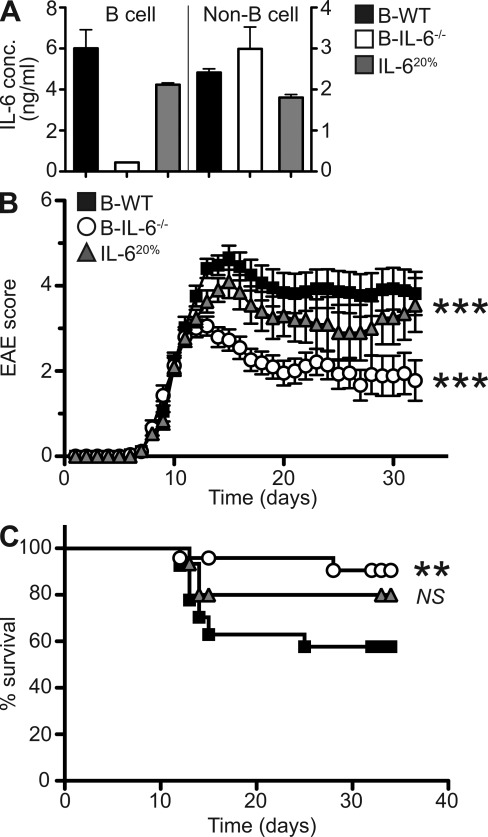
We next asked whether IL-6 production by B cells contributed to disease pathogenesis during EAE. To this end, we generated mixed bone marrow chimeric mice with a B cell–specific deficiency in IL-6 production (B-IL-6−/−), together with control chimeras (B-WT). The validation of these chimeras is outlined in Fig. 2 A. Of note, B-IL-6−/− mice contain a 20% IL-6 deficiency in all hematopoietic cells, in addition to a complete lack of IL-6 production by B cells. To control for the effect that such deficiency outside the B cell compartment could have on the disease course, we generated IL-620% chimeras, which have 20% IL-6 deficiency in all hematopoietic compartments, including in B cells. After 8–10 wk of reconstitution, chimeric mice were immunized with MOG to induce EAE. All groups of mice started to display disease signs with similar kinetics (mean day of disease onset B-WT = 9.2 ± 0.3, B-IL-6−/− = 9.0 ± 0.3, and IL-620% = 8.5 ± 0.3), indicating that IL-6 production by B cells was not required for initiation of the pathogenic process. However, B-IL-6−/− mice showed a markedly lower maximal disease score than control groups (B-IL-6−/− = 3.8 ± 0.2, IL-620% = 4.8 ± 0.3, and B-WT = 5.1 ± 0.3), implying that B cells drive disease exacerbation through the production of IL-6. Reflecting this, fewer B-IL-6−/− mice reached a disease severity imposing sacrifice (because of ethical regulations) compared with B-WT or IL-620% mice (P = 0.0077 and P = 0.1830, respectively; Fig. 2 C). In addition, B-IL-6−/− mice showed a better recovery from disease than the two control groups (Fig. 2 B). B-IL-6−/− mice also appeared to suffer a less severe EAE than B-WT mice when comparing global disease burdens. In such comparison, IL-620% mice also displayed a slightly milder overall disease than B-WT mice (P < 0.001), but the difference was clearly stronger for B-IL-6−/− mice (P < 0.001 B-IL-6−/− vs. B-WT, P < 0.001 B-IL-6−/− vs. IL-620%), indicating that lack of IL-6 production by B cells was the major cause of disease attenuation in B-IL-6−/− mice. Collectively, our results show that B cells specifically exacerbate ongoing disease through IL-6 production.
Figure 2.
Mice with a B cell–specific IL-6 deficiency develop an attenuated form of EAE. (A) Splenic B cells (left) and non–B cells (right) from B-WT, B-IL-6−/−, and IL-620% chimeric mice were MACS sorted and cultured for 72 h with PMA and ionomycin. Levels of IL-6 were then quantified by ELISA. Histograms represent the mean of triplicate cultures on pooled cells from 5–10 mice, with error bars indicating SEM. Data presented is representative of five separate experiments. (B) EAE progression was monitored for 32 d after immunization with MOG in B-WT, B-IL-6−/−, and IL-620% chimeric mice. Each point represents the mean disease score and error bars represent SEM. Statistical analysis is by two-way ANOVA, where ***, P < 0.001, compared with B-WT mice. (C) Survival curves of chimeric mice after induction of EAE. Statistical analysis by log-rank test where NS, P > 0.05 and **, P < 0.01. Data presented is combined from three independent experiments (B-WT, n = 27; B-IL-6−/−, n = 27; and IL-620%, n = 15).
BCDT acts by eliminating IL-6 production by B cells, irrespective of its effects on MOG-reactive antibodies
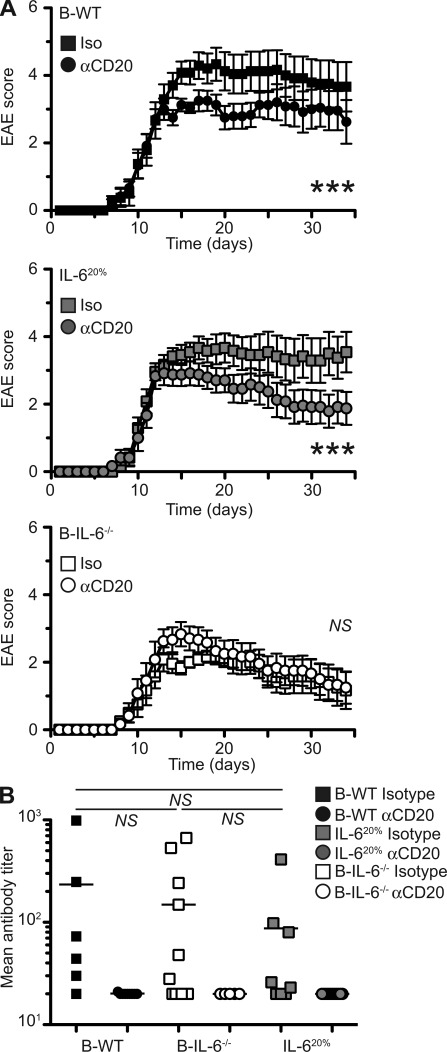
B cells might contribute to autoimmune pathogenesis via several mechanisms, in addition to IL-6 secretion, including through production of antibodies or other inflammatory cytokines. To test the importance of IL-6 production in the pathogenic functions of B cells during EAE, we treated B-IL-6−/− and B-WT chimeric mice with a B cell–depleting anti-CD20 antibody after initiation of disease. As previously described (Matsushita et al., 2008), such anti-CD20 treatment resulted in a marked amelioration of disease in mice with WT B cells (Fig. 3 A; B-WT, P < 0.0001; IL-620%, P < 0.0001). We then asked if this effect was related to the elimination of IL-6 production by B cells by treating B-IL-6−/− mice with anti-CD20 (Fig. 3 A). Remarkably, depletion of B cells had no impact on disease severity in B-IL-6−/− chimeras, suggesting that BCDT works primarily by eliminating IL-6–producing B cells. We then measured antibody responses in isotype control and anti-CD20–treated animals. Total MOG-specific antibody levels were similarly reduced in all the groups of chimeras treated with anti-CD20, irrespective of improvement of disease outcome (Fig. 3 B). This applied to all detectable antibody isotypes (IgG1 and IgM; unpublished data). From these results, we conclude that BCDT in mice acts through abrogating IL-6 production by B cells. The B cell IL-6–dependent mechanism of pathogenesis was unrelated to antibody production because mice with WT or IL-6–deficient B cells produced similar amounts of MOG-reactive antibodies during EAE.
Figure 3.
BCDT is effective only in mice with IL-6–sufficient B cells, irrespective of MOG-reactive antibody levels. (A) Clinical EAE scores of B-WT, IL-620%, and B-IL-6−/− chimeras after anti-CD20 depletion after disease onset (day 10). Curves represent the mean disease scores, with errors bars indicating SEM. Statistical analysis was done by two-way ANOVA, where NS, P > 0.05 and ***, P < 0.001. (B) MOG-specific antibody was quantified by ELISA. Each point represents antibody titer from individual mice, with the bar indicating the mean. Statistical analysis is by Student’s t test, where NS, P > 0.05. Data presented is combined from two independent experiments (n = 6–12).
Ameliorated EAE in B-IL-6−/− chimeras is associated with a reduced Th17 response
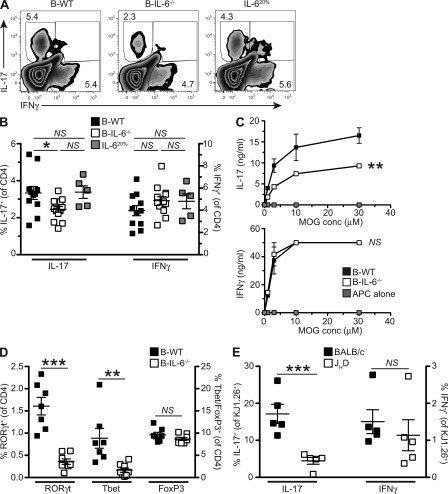
IL-6 has a critical role for the maintenance of Th17 cells (Korn et al., 2009), a T cell subset implicated in pathogenesis of EAE (Bettelli et al., 2006). We tested the impact of IL-6 production by B cells on the Th17 response during EAE. Intracellular cytokine staining of spleen cells from mice with EAE ex vivo revealed a significantly impaired IL-17 production by T cells in B-IL-6−/− mice, compared with B-WT controls (Fig. 4, A and B; P = 0.0236), whereas IFN-γ secretion was unaffected. The reduction in IL-17 expression was not complete, indicating that other cells and factors also contributed to Th17 polarization in this model, besides IL-6–producing B cells. In addition to ex vivo intracellular cytokine, we also restimulated purified splenic CD4+ T cells from EAE mice with MOG in the presence of WT irradiated APC. In agreement with the ex vivo data, T cells from B-IL-6−/− mice secreted less IL-17 than those from B-WT chimeras, whereas levels of IFN-γ were equivalent (Fig. 4 C; P = 0.0094). Given the modest reduction in IL-17 production by CD4+ T cells in B-IL-6−/− mice, we also quantified ex vivo expression of the Th17 master regulator RORγt by intracellular staining on circulating CD4+ T cells. In accordance with the secretion of IL-17 by splenic T cells, expression of RORγt was significantly reduced in B-IL-6−/− chimeras (Fig. 4 D). Intriguingly, Tbet expression was also impaired, whereas FoxP3 expression was not. This effect on T cell IL-17 production was noticeable systemically, in the spleen and in the blood. Although there was a trend toward reduced Th17 cells, numbers in the CNS and draining LN of B-WT and B-IL-6−/− mice at the peak of disease (day 14), and upon recovery (day 32) were not statistically different (unpublished data). We then asked if B cells also promoted Th17 responses in response to a distinct non–self-antigen such as OVA. For this purpose, transgenic OVA-specific DO11.10 CD4+ T cells were adoptively transferred into WT or B cell–deficient JHD hosts, and IL-17 expression by antigen-reactive CD4+ T cells was measured by flow cytometry after immunization with OVA. In agreement with the data obtained with MOG, DO11.10 T cell expression of IL-17 was greatly impaired in B cell-deficient mice, whereas IFN-γ was not affected (Fig. 4 E; P = 0.0017). Collectively, these data reveal that IL-6–producing B cells have an essential role in the propagation of Th17 responses in vivo, which might exacerbate CNS pathogenesis during EAE.
Figure 4.
Ameliorated EAE in B-IL-6−/− chimeras is associated with impaired Th17 responses in vivo. (A) Representative FACS plots showing frequencies of IL-17 and IFN-γ–secreting T cells by intracellular staining 32 d after MOG-induced EAE. (B) Combined data from two EAE experiments showing frequencies of IL-17–(left) and IFN-γ (right)–producing T cells by intracellular cytokine staining. Each point represents an individual mouse (n = 5–13), with the bar indicating the mean. Significance was determined by one-way ANOVA with Bonferroni’s post-testing, where NS, P > 0.05 and *, P < 0.05. (C) IL-17 and IFN-γ secretion by CD4+ splenic T cells from B-WT and B-IL-6−/− chimeric mice (at 32 d after EAE induction). Cytokines were quantified by ELISA after 3 d of culture with varying concentrations of MOG in the presence of WT irradiated APC. Curves represent the mean cytokine level with errors bars indicating SEM. Statistical analysis was by two-way ANOVA comparing B-WT with B-IL-6−/−, where NS, P > 0.05 and **, P < 0.01. Data presented is combined from two separate experiments and is representative of four independent experiments. (D) Transcription factor expression by circulating CD4+ T cells at day 14 after induction for RORγt, Tbet, and FoxP3. Each symbol represents an individual mouse, with the bar indicating the mean (n = 7–8), with data representative of two independent experiments. Statistical analysis is by Student’s t test, where ***, P < 0.001; **, P < 0.01, and NS, P > 0.05. (E) Frequencies of IL-17– and IFN-γ–secreting DO11.10 T cells were determined by intracellular staining 5 d after transfer and immunization of WT and JHD (B cell deficient) hosts. Each point represents an individual mouse (n = 5), with the bar indicating the mean. Significance was determined by Student’s t test where NS, P < 0.05 and ***, P < 0.001. Data presented is representative of two independent experiments.
B cells from RR-MS patients secrete elevated levels of IL-6, which is normalized after BCDT
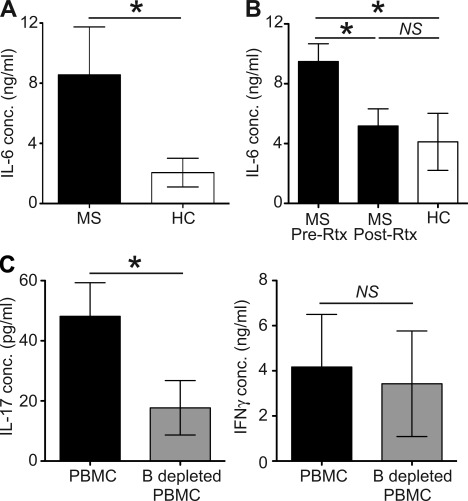
In our final series of experiments, we investigated the relevance of our findings on IL-6 production by B cells in EAE for the corresponding human disease, namely RR-MS. To achieve this, we isolated peripheral blood B cells from patients with RR-MS and healthy controls. After stimulation in vitro by engagement of BCR and CD40, with or without CpG DNA, IL-6 was quantified by ELISA. We found that B cells from RR-MS patients secreted IL-6 at four- to fivefold higher levels than B cells from healthy controls (Fig. 5 A; P = 0.0275). In a separate longitudinal study of RR-MS patients before and after BCDT with Rituximab, we found that B cells returning after depletion secreted reduced levels of IL-6, comparable with the healthy control group (Fig. 5 B; pre-Rtx vs. post-Rtx, P = 0.0349; pre-Rtx vs. HC, P = 0.0185; post-Rtx vs. HC, P = 0.1763). Finally, we tested IL-17 production in PBMC from RR-MS patients depleted of B cells or not, and found, in accordance with our EAE studies, that B cell depletion lead to a reduced IL-17 response without having an impact on IFN-γ production (Fig. 5 C; IL-17, P = 0.0400; IFN-γ, P = 0.7104). Collectively, these data suggest that, as in EAE, B cells contribute to pathogenesis of RR-MS through production of IL-6.
Figure 5.
B cells from patients with MS secrete elevated levels of IL-6, which is reduced after BCDT. (A) IL-6 production by B cells isolated from MS patients and healthy controls after in vitro stimulation. B cells were isolated ex vivo and stimulated by engagement of BCR and CD40 with or without addition of TLR9 ligand (CpG DNA) for 3 d and supernatants were harvested for cytokine analysis by ELISA. (B) Longitudinal study showing IL-6 production from B cells from MS patients before and after Rituximab treatment. Patients were given two infusions of 1,000 mg Rituximab, 2 wk apart and then followed prospectively. The data shown was obtained from CD19+ B cells isolated from PBMC obtained from the patients before treatment with Rituximab and at 12 mo after treatment, after in vitro stimulation via BCR, CD40, and TLR9. (C) IL-17 (left) and IFN-γ (right) production by whole PBMCs from RR-MS patients or PBMCs from the same individuals depleted of B cells. Data presented is from 8 (A) and 12 MS patients or sex- and age-matched healthy controls (B and C), with bars representing the mean and error bars SEM. Statistical analysis is by Mann-Whitney U test, where NS, P > 0.05 and *, P < 0.05.
DISCUSSION
In the clinic, BCDT can markedly ameliorate the course of autoimmune diseases in patients, but the mechanisms underlying these beneficial effects are poorly understood, as they are thought, in many cases, to operate irrespective of autoantibody levels. Here, we demonstrate that IL-6 production is the major mechanism of B cell–mediated pathogenesis during EAE, and we show that this inflammatory pathway is markedly increased in RR-MS patients. Autoantibody levels were unaffected by IL-6 production by B cells. This is the first demonstration of a nonantibody-mediated mechanism of B cell pathogenesis in EAE and MS. Remarkably, the elevated production of IL-6 by B cells from MS patients was effectively normalized by Rituximab treatment. In both patients and mice the reduced disease severity after B cell depletion was accompanied by a decrease in the autoreactive Th17 response, and so we believe that B cells are making an all-important contribution to the IL-6–dependent promotion of pathogenic Th17 differentiation (Bettelli et al., 2006). The role of IL-17 in EAE/MS pathogenesis has undergone controversy (Komiyama et al., 2006; Haak et al., 2009) since the first observation (Bettelli et al., 2006); however, the current consensus is that Th17 as well as Th1 cells contribute to disease progression (Kroenke et al., 2008; O’Connor et al., 2008; Zepp et al., 2011). We should also not forget that IL-6 has many other actions that contribute to pathology in autoimmune or chronic inflammatory conditions (Neurath and Finotto, 2011), but we do not know yet if B cell–derived IL-6 contributes more widely to pathogenesis than supporting the Th17 response. This seems likely, as IL-6 controls proliferation and resistance to apoptosis of activated T cells (Neurath and Finotto, 2011), it switches off the suppressive effects of T reg cells (Pasare and Medzhitov, 2003), and IL-6 blockade inhibits the development of myelin-specific Th1 as well as Th17 cells in EAE (Serada et al., 2008). Of note, B cells can also secrete other proinflammatory cytokines in RR-MS (Duddy et al., 2007; Bar-Or et al., 2010), and it will be important to evaluate directly the pathogenic contribution of these functions to the disease process in future studies, even though our data clearly emphasize IL-6 as a major B cell–derived driver of disease.
Interestingly, the B cell IL-6 response does not influence the onset of EAE, suggesting a later acting role. The late contribution of B cells to pathogenesis coincides with the expansion of CD1dHi MZ B cells, the most potent producers of B cell–derived IL-6. In contrast, we have previously shown that the B cell IL-10 response and regulatory function is set up early during immune responses, in both autoimmune and infection models (Lampropoulou et al., 2008; Barr et al., 2010; Neves et al., 2010). This early IL-10 response sets up the opportunity of a negative feed-forward loop, and this not only has the benefit of preventing excessive inflammation but also, surprisingly, of accelerating the immune response (Lampropoulou et al., 2010; Neves et al., 2010). We have proposed that an impairment or lack of this early-acting B cell regulation is the reason why autoimmune activation leads to chronic nonresolving lesions that progress to full-blown disease (Fillatreau et al., 2008). The prediction of an imbalance in the B cell cytokine response in autoimmune conditions is borne out by the observation that B cells from MS patients make less IL-10 (Duddy et al., 2007). Another aspect of this imbalance can be seen here in MS patients compared with healthy volunteers and also in the patients after BCDT. The levels of IL-6 made by B cells from MS patients are much higher than from healthy volunteers and there is a dramatic fall to normal IL-6 secretory activity after BCDT. This would suggest that the B cells repopulating after Rituximab are normal and have not been influenced by conditions that led to the pathogenic cytokine imbalance. In other words, the B cell cytokine response has been reset. As memory B cells have been reported to secrete elevated levels of IL-6 (Burdin et al., 1995; Good et al., 2006; Cognasse et al., 2008), a speculative interpretation would be that alleviation of disease by BCDT is associated with depletion of these cells. The phenotype and repopulation kinetics of this patient cohort have been previously published, and they show that the B cells reemerging after BCDT are almost exclusively naive CD27− cells (Duddy et al., 2007). However, frequencies of CD27+ cells before depletion were similar between healthy controls and MS patients, and so elevated IL-6 in MS patients may not be solely attributed to the frequency of this subset. Future studies are aimed at identifying the IL-6–producing cells in humans. Whether this resetting of the B cell cytokine environment will be lasting or short-lived still needs to be investigated. We suggest that measuring IL-6 production by B cells from patients might be a useful means for predicting (and monitoring) their response to BCDT.
The role of B cells in the generation and maintenance, but also control of pathogenic T cell responses in autoimmunity is a complex one that we have barely begun to understand. One issue is whether particular subsets of B cells have specific roles to play. However, in the area of regulation where most work has been done, there is a lack of clarity. The so-called B reg cells identified by some groups (e.g., B10 cells [Yanaba et al., 2008] or TN B cells [Blair et al., 2010]) may simply represent the most active IL-10 producers ex vivo; however, under stimulatory or inflammatory conditions other cells may come into play in vivo (e.g., MZ B cells, plasmablasts, or human memory-type cells [Barr et al., 2007; Lampropoulou et al., 2008; Bouaziz et al., 2010; Neves et al., 2010]). In this study, we find that the very same cells, namely CD1dHi MZ B cells, can produce IL-6 and IL-10 upon short-term stimulation in vitro. Our data suggest that the same B cells might promote either pathogenesis or regulation depending on the prevailing immunological context in which they find themselves (Gray and Gray, 2010; Lampropoulou et al., 2010). A possible scenario could be that in vivo the pathogenic (IL-6) and regulatory (IL-10) responses are generated with different kinetics as a result of stimuli dose variations or the combinatorial quality of the signals driving one or other response. Of particular note is the role of CD40 stimulation in IL-6 and IL-10 production by B cells. IL-6 is augmented by CD40 stimulation, whereas IL-10 is inhibited. This suggests that B cells responding solely to TLRs become regulatory, whereas upon receiving T cell help B cells promote inflammation through secretion of IL-6. With human B cells, IL-10 may also augmented by CD40 stimulation (Burdin et al., 1995), and so it will be important to ascertain the stimuli that lead to IL-6 expression in vivo. Thus we propose that B cells become inflammatory only upon interaction with appropriately primed helper T cells (i.e., late in the response) and regulatory in the absence of specific T cell help (i.e., early in the response).
With regard to the signals that drive different B cell cytokine responses in vivo during disease induction no comprehensive study exists, the closest is a study published by Duddy et al. (2007) comparing patients with RR-MS and controls. Interestingly, they find that the IL-10 derives from naive B cells as opposed to activated/memory B cells, whereas the converse is true of the inflammatory cytokines such as TNF and lymphotoxin. This study of MS patients (Duddy et al., 2007) used signals mimicking adaptive stimuli (anti-BCR and anti-CD40), and in mice with EAE, it is also true that the regulatory B cell IL-10 response required T cell–derived (CD40L) and antigen-specific signals (Fillatreau et al., 2002). However, the production of IL-10 in mice (Lampropoulou et al., 2008; Barr et al., 2010; Neves et al., 2010) and humans (Bouaziz et al., 2010) is strongly augmented by TLR signals and, indeed, these innate signals are required in vivo for the full expression of regulatory activity (Lampropoulou et al., 2008; Neves et al., 2010) and T cell programming by B cells in general (Barr et al., 2010). We know less about the control of the B cell IL-6 response; however, it seems to mirror the IL-10 response in that it is elicited both by innate and adaptive stimuli and it is secreted most actively by cells within the same subpopulation (MZ). In the experiments shown here and in previous studies (Barr et al., 2007; Lampropoulou et al., 2008), anti-CD40 and TLR signals (LPS) synergize in their stimulation of IL-6 secretion. Intriguingly, no signal has been identified that selectively instructs pro- or anti-inflammatory functions in B cells. We have previously shown that B cells activated via TLR produce a cytokine milieu that can inhibit T cell stimulation by dendritic cells (via IL-10) while facilitating T cell activation by anti-CD3 (via IL-6; Lampropoulou et al., 2008). Activated B cells can therefore concomitantly secrete cytokines having opposite effects on immune responses so that their net influence on immunity profoundly depends on their immediate cellular microenvironment, which is likely to change during the course of the immune response.
Our findings might be particularly relevant for treatment of RR-MS. Both IFN-β and glatiramer acetate therapies induce increased IL-10 secretion by B cells (Kala et al., 2010; Ramgolam et al., 2011), suggesting that part of their beneficial effects relates to tipping the balance from pathogenic to regulatory B cell function. IFN-β has broader effects on cytokine production by B cells, reducing their secretion of IL-1β and IL-23 while increasing expression of IL-12 and IL-27 (Ramgolam et al., 2011), an activity likely to reduce Th17 differentiation. Our study supports the notion that the pathogenic role for B cells in MS (and other autoimmune diseases) relates largely to their production of cytokines (Bar-Or et al., 2010). Here, we demonstrate that IL-6 production is a primary mechanism of B cell–mediated pathogenesis during EAE, and we provide evidence that this inflammatory pathway is profoundly deregulated in RR-MS. It will be important to evaluate whether current RR-MS therapies modulate the production of IL-6 by B cells during RR-MS. Remarkably, the repopulating B cells after BCDT no longer have a pathogenic cytokine profile, suggesting that the mode of action of such treatment relies on the elimination of inflammatory B cell activities. Novel approaches that selectively modulate cytokine production by activated B cells might provide interesting alternatives to control pathogenic inflammation in autoimmune diseases.
MATERIALS AND METHODS
Mice.
Mice were bred and maintained in specific pathogen-free conditions at the School of Biological Sciences facility at the University of Edinburgh. Mice were aged 6–8 wk at the start of procedures. Experiments were covered by a UK Home Office Project License, granted under the Animal (Scientific Procedures) Act 1986 and approved locally by the University of Edinburgh Ethical Review Committee.
B cell subset sorting and stimulation.
B cells were isolated by CD43 depletion from spleens of naive or EAE day 14 mice, using MACS magnetic beads (Miltenyi Biotec). The negative fraction was stained with CD19, CD21, CD1d, and CD23, and then FACS sorted for MZ, FO, and double-negative TN populations. 5–10 × 105 cells were lysed in RNAZol for mRNA extraction and cDNA synthesis. 5 × 105 cells per well were stimulated with 5 µg/ml LPS or 10 µg/ml anti-CD40, in combination or alone. Controls cells were left unstimulated. Supernatants were harvested 24 or 72 h later. The following primers were used for RT-PCR: actin sense, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′; actin antisense, 5′-TAAAACGCAGCTCAGTAACAGTCC-3′; IL-6 sense, 5′-GTTCTCTGGGAAATCGTGGA-3′; IL-6 antisense, 5′-TGTACTCCAGGTAGCTATGG-3′; IL-10 sense, 5′-AGCCGGGAAGACAATAACTG-3′; and IL-10 antisense, 5′-CATTTCCGATAAGGCTTGG-3′.
Mixed bone marrow chimeras.
Mice with a B cell–specific IL-6 deficiency were generated using the mixed bone marrow chimera system as described previously (Fillatreau et al., 2002; Barr et al., 2010). In brief, irradiated μMT mice were reconstituted with a mixed inoculum of bone marrow (20% IL-6−/− or WT and 80% μMT). The hematopoietic compartment was left for 8–10 wk to repopulate. Thus, all B cells arising from these inocula originated from the IL-6−/− (or WT) portion, with all other hematopoietic lineages deriving predominantly from the μMT portion (i.e., IL-6 sufficient). These mice were designated B-IL-6−/− and B-WT chimeras. To control for a partial IL-6 deficiency in the non–B cell compartment, a control group with a 20% deficiency in all hematopoietic cells was made (designated IL-620%). Chimerism was confirmed by IL-6 ELISA on supernatants from MACS sorted B cell (CD43−) and non–B cell compartments (CD43+) after PMA and ionomycin stimulation. Characterization of chimeras is outlined in Fig. 2 A.
Induction of EAE.
Mice were immunized subcutaneously with 100 µg MOG (35–55) peptide emulsified in complete Freund’s adjuvant and 200 ng pertussis toxin (Ptx) intraperitoneally on day 0, as previously described (Fillatreau et al., 2002). Mice received a second dose of Ptx 2 d later. Clinical signs of EAE were assessed as follows: 0, no signs; 1, flaccid tail; 2, impaired gait/righting reflex; 3, partial hind limb paralysis; 4, total hind limb paralysis; 5, hind limb paralysis with partial front limb paralysis; 6, moribund or dead.
Mouse B cell depletion.
The anti-CD20 antibody 18B12 and its isotype control (IgG2a) were provided by M. Kehry and R. Dunn (Biogen Idec, Weston, MA). B cells were depleted with a single 250 µg dose via the intravenous route at day 10. Depletion was confirmed by staining circulating CD19+ cells in blood taken 14–21 d after EAE induction.
Intracellular cytokine staining.
Splenocytes from EAE mice were stimulated with 10 ng/ml PMA and 1 µg/ml ionomycin (Sigma-Aldrich) in the presence of 1 µg/ml brefeldin A (GolgiStop; BD) for 4 h. Cells were washed in FACS buffer (PBS, 2% FCS, and 0.02% NaN3) and stained for CD4 (GK1.5; BioLegend). Samples were treated with fixative/permeabilization buffer (BD) and then stained with anti–IFN-γ (XMG1.2) and anti–IL-17 (TC11.18H10; BioLegend). Samples were acquired using FACSCanto and LSR-II flow cytometers (BD). Data were analyzed using FlowJo software (Tree Star).
In vitro restimulation assays.
106 cells/ml MACS-sorted CD4+ T cells from spleens of EAE mice were cultured with 30 µM–0.1 µM MOG peptide. WT naive irradiated splenocytes were used as APC at a 1:1 ratio in a total volume of 200 µl. Supernatants were harvested after 3 d and cytokine quantified by ELISA (BD) or BioPlex (Bio-Rad Laboratories) using commercially available antibody sets for IL-6, IL-10, IL-17, and IFN-γ in accordance with the manufacturer’s instructions.
RR-MS patient study groups.
All patients in this study were participants in the clinical trial program of Rituximab in MS and were subject to inclusion criteria outlined previously. These include the phase II placebo controlled study by Hauser et al. (2008) and the open-label phase I RR-MS study by Bar-Or et al. (2008). These placebo-controlled randomized trials were performed at 32 different centers in the United States and Canada. Protocols were approved by the institutional review board and the ethics reviews committee of each institution. For Fig. 5 A, there were five female (aged 26, 28, 37, 51, and 66 yr) and three male (aged 27, 33, and 51 yr) patients, with the same number of age/sex-matched healthy control volunteers. For Fig. 5 B, there were seven female (aged 55, 42, 61, 41, 49, 52, and 39 yr) and five male (aged 51, 55, 59, 28, and 54 yr) patients. Patients were not HLA typed and were receiving no treatments other than Rituximab.
Rituximab treatment of RR-MS.
Patients were given two infusions of 1,000 mg Rituximab, 2 wk apart, and then followed prospectively. CD19+ B cells were isolated from PBMC obtained from the patients before treatment with Rituximab and at 12 mo after treatment as outlined in the next section.
Human PBMC cultures.
Human B cells and PBMC were isolated and stimulated as previously described (Duddy et al., 2007). In brief, after Ficoll-Plaque separation and CD19+ MACS sorting, B cells were plated at 7.5 × 105 cells/ml in a total volume of 200 µl. The cells were cultured with CD154-transfected L cells together with anti-BCR (cross-linking anti-IgM/IgG), with and without the addition of CpG DNA. After 3 d, supernatants were harvested for cytokine analysis by ELISA. Cytokine production was assessed by ELISA using OptEIA ELISA kits (BD).
Adoptive transfer of DO11.10 T cells.
T cells from spleens of pOVA-specific transgenic mice (DO11.10) were sorted by CD4+ selection using MACS. 106 cells were injected intravenously into BALB/c or B cell–deficient hosts (JHD). Animals were immunized subcutaneously with 100 µg pOVA emulsified in CFA 24 h later. Animals were sacrificed 5 d after immunization, and cytokines were quantified in splenocytes by flow cytometry as outlined in Intracellular cytokine staining. Transferred T cells were identified using the anti-idiotype antibody KJ1.26.
Statistics.
Student’s t test and Mann Whitney U test were used to calculate p-values between groups with normal and non-Gaussian distributions, respectively. Comparison between three or more groups was by one-way ANOVA with Bonferroni’s post-test analyses. Where comparing curves with two variables (e.g., time and disease score) two-way ANOVA was used. Survival curves were analyzed by log-rank test.
Acknowledgments
We would like to thank Marilyn Kehry (Biogen Idec) for provision of the anti-CD20 antibody. We also thank members of the Experimental Therapeutics Program at the Montreal Neurological Institute.
This work was funded by the Wellcome Trust (D. Gray), Hertie Stiftung and the Deutsche Forschungsgemeinschaft (SFB-650; S. Fillatreau), Canadian Institutes of Health Research/Multiple Sclerosis Society of Canada New Emerging Team in Clinical Autoimmunity, and the Centers for Excellence and Commercialization of Research (A. Bar-Or) and the Medical Research Council (S.M. Anderton).
The authors have no conflicts of interest to declare.
Footnotes
Abbreviations used:
- BCDT
- B cell depletion therapy
- CNS
- central nervous system
- EAE
- experimental autoimmune encephalomyelitis
- FO
- follicular
- MOG
- myelin oligodendrocyte glycoprotein
- MS
- multiple sclerosis
- MZ
- marginal zone
- RR-MS
- relapsing-remitting MS
- TLR
- toll-like receptor
- TN
- transitional
References
- Anderton S.M., Fillatreau S. 2008. Activated B cells in autoimmune diseases: the case for a regulatory role. Nat. Clin. Pract. Rheumatol. 4:657–666 10.1038/ncprheum0950 [DOI] [PubMed] [Google Scholar]
- Balázs M., Martin F., Zhou T., Kearney J. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 17:341–352 10.1016/S1074-7613(02)00389-8 [DOI] [PubMed] [Google Scholar]
- Bar-Or A., Calabresi P.A., Arnold D., Markowitz C., Shafer S., Kasper L.H., Waubant E., Gazda S., Fox R.J., Panzara M., et al. 2008. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann. Neurol. 63:395–400 10.1002/ana.21363 [DOI] [PubMed] [Google Scholar]
- Bar-Or A., Fawaz L., Fan B., Darlington P.J., Rieger A., Ghorayeb C., Calabresi P.A., Waubant E., Hauser S.L., Zhang J., Smith C.H. 2010. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann. Neurol. 67:452–461 10.1002/ana.21939 [DOI] [PubMed] [Google Scholar]
- Barr T.A., Brown S., Ryan G., Zhao J., Gray D. 2007. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur. J. Immunol. 37:3040–3053 10.1002/eji.200636483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr T.A., Brown S., Mastroeni P., Gray D. 2010. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J. Immunol. 185:2783–2789 10.4049/jimmunol.1001431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belnoue E., Pihlgren M., McGaha T.L., Tougne C., Rochat A.F., Bossen C., Schneider P., Huard B., Lambert P.H., Siegrist C.A. 2008. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 111:2755–2764 10.1182/blood-2007-09-110858 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Blair P.A., Noreña L.Y., Flores-Borja F., Rawlings D.J., Isenberg D.A., Ehrenstein M.R., Mauri C. 2010. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 32:129–140 10.1016/j.immuni.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Bouaziz J.D., Calbo S., Maho-Vaillant M., Saussine A., Bagot M., Bensussan A., Musette P. 2010. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur. J. Immunol. 40:2686–2691 10.1002/eji.201040673 [DOI] [PubMed] [Google Scholar]
- Burdin N., Van Kooten C., Galibert L., Abrams J.S., Wijdenes J., Banchereau J., Rousset F. 1995. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J. Immunol. 154:2533–2544 [PubMed] [Google Scholar]
- Cognasse F., Hamzeh-Cognasse H., Lafarge S., Chavarin P., Pozzetto B., Richard Y., Garraud O. 2008. Identification of two subpopulations of purified human blood B cells, CD27- CD23+ and CD27high CD80+, that strongly express cell surface Toll-like receptor 9 and secrete high levels of interleukin-6. Immunology. 125:430–437 10.1111/j.1365-2567.2008.02844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A.H., Stark J.L., Lauber J., Ramsbottom M.J., Lyons J.A. 2006. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 180:63–70 10.1016/j.jneuroim.2006.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy M., Niino M., Adatia F., Hebert S., Freedman M., Atkins H., Kim H.J., Bar-Or A. 2007. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 178:6092–6099 [DOI] [PubMed] [Google Scholar]
- Edwards J.C., Cambridge G. 2006. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat. Rev. Immunol. 6:394–403 10.1038/nri1838 [DOI] [PubMed] [Google Scholar]
- Eugster H.P., Frei K., Kopf M., Lassmann H., Fontana A. 1998. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur. J. Immunol. 28:2178–2187 [DOI] [PubMed] [Google Scholar]
- Fillatreau S., Anderton S.M. 2007. B-cell function in CNS inflammatory demyelinating disease: a complexity of roles and a wealth of possibilities. Expert Rev. Clin. Immunol. 3:565–578 10.1586/1744666X.3.4.565 [DOI] [PubMed] [Google Scholar]
- Fillatreau S., Sweenie C.H., McGeachy M.J., Gray D., Anderton S.M. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944–950 10.1038/ni833 [DOI] [PubMed] [Google Scholar]
- Fillatreau S., Gray D., Anderton S.M. 2008. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat. Rev. Immunol. 8:391–397 10.1038/nri2315 [DOI] [PubMed] [Google Scholar]
- Good K.L., Bryant V.L., Tangye S.G. 2006. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J. Immunol. 177:5236–5247 [DOI] [PubMed] [Google Scholar]
- Gray D., Gray M. 2010. What are regulatory B cells? Eur. J. Immunol. 40:2677–2679 10.1002/eji.201040961 [DOI] [PubMed] [Google Scholar]
- Haak S., Croxford A.L., Kreymborg K., Heppner F.L., Pouly S., Becher B., Waisman A. 2009. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 119:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H.P., Kieseier B.C. 2010. Atacicept: targeting B cells in multiple sclerosis. Ther. Adv. Neurol. Disord. 3:205–216 10.1177/1756285610371146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Waubant E., Arnold D.L., Vollmer T., Antel J., Fox R.J., Bar-Or A., Panzara M., Sarkar N., Agarwal S., et al. ; HERMES Trial Group 2008. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 358:676–688 10.1056/NEJMoa0706383 [DOI] [PubMed] [Google Scholar]
- Kala M., Rhodes S.N., Piao W.H., Shi F.D., Campagnolo D.I., Vollmer T.L. 2010. B cells from glatiramer acetate-treated mice suppress experimental autoimmune encephalomyelitis. Exp. Neurol. 221:136–145 10.1016/j.expneurol.2009.10.015 [DOI] [PubMed] [Google Scholar]
- Komiyama Y., Nakae S., Matsuki T., Nambu A., Ishigame H., Kakuta S., Sudo K., Iwakura Y. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566–573 [DOI] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. 2009. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27:485–517 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- Kroenke M.A., Carlson T.J., Andjelkovic A.V., Segal B.M. 2008. IL-12– and IL-23–modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 205:1535–1541 10.1084/jem.20080159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampropoulou V., Hoehlig K., Roch T., Neves P., Calderón Gómez E., Sweenie C.H., Hao Y., Freitas A.A., Steinhoff U., Anderton S.M., Fillatreau S. 2008. TLR-activated B cells suppress T cell-mediated autoimmunity. J. Immunol. 180:4763–4773 [DOI] [PubMed] [Google Scholar]
- Lampropoulou V., Calderon-Gomez E., Roch T., Neves P., Shen P., Stervbo U., Boudinot P., Anderton S.M., Fillatreau S. 2010. Suppressive functions of activated B cells in autoimmune diseases reveal the dual roles of Toll-like receptors in immunity. Immunol. Rev. 233:146–161 10.1111/j.0105-2896.2009.00855.x [DOI] [PubMed] [Google Scholar]
- Mann M.K., Maresz K., Shriver L.P., Tan Y., Dittel B.N. 2007. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J. Immunol. 178:3447–3456 [DOI] [PubMed] [Google Scholar]
- Martin F., Chan A.C. 2006. B cell immunobiology in disease: evolving concepts from the clinic. Annu. Rev. Immunol. 24:467–496 10.1146/annurev.immunol.24.021605.090517 [DOI] [PubMed] [Google Scholar]
- Matsushita T., Yanaba K., Bouaziz J.D., Fujimoto M., Tedder T.F. 2008. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 118:3420–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C., Gray D., Mushtaq N., Londei M. 2003. Prevention of arthritis by interleukin 10–producing B cells. J. Exp. Med. 197:489–501 10.1084/jem.20021293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel I., Katz A., Kozak N., Ben-Nun A., Revel M. 1998. Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur. J. Immunol. 28:1727–1737 [DOI] [PubMed] [Google Scholar]
- Neurath M.F., Finotto S. 2011. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 22:83–89 10.1016/j.cytogfr.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Neves P., Lampropoulou V., Calderon-Gomez E., Roch T., Stervbo U., Shen P., Kühl A.A., Loddenkemper C., Haury M., Nedospasov S.A., et al. 2010. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 33:777–790 10.1016/j.immuni.2010.10.016 [DOI] [PubMed] [Google Scholar]
- O’Connor B.P., Raman V.S., Erickson L.D., Cook W.J., Weaver L.K., Ahonen C., Lin L.L., Mantchev G.T., Bram R.J., Noelle R.J. 2004. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199:91–98 10.1084/jem.20031330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor R.A., Prendergast C.T., Sabatos C.A., Lau C.W., Leech M.D., Wraith D.C., Anderton S.M. 2008. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J. Immunol. 181:3750–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksenberg J.R., Baranzini S.E., Sawcer S., Hauser S.L. 2008. The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat. Rev. Genet. 9:516–526 10.1038/nrg2395 [DOI] [PubMed] [Google Scholar]
- Okuda Y., Sakoda S., Bernard C.C., Fujimura H., Saeki Y., Kishimoto T., Yanagihara T. 1998. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int. Immunol. 10:703–708 10.1093/intimm/10.5.703 [DOI] [PubMed] [Google Scholar]
- Owens G.P., Bennett J.L., Lassmann H., O’Connor K.C., Ritchie A.M., Shearer A., Lam C., Yu X., Birlea M., DuPree C., et al. 2009. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann. Neurol. 65:639–649 10.1002/ana.21641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C., Medzhitov R. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036 10.1126/science.1078231 [DOI] [PubMed] [Google Scholar]
- Ramgolam V.S., Sha Y., Marcus K.L., Choudhary N., Troiani L., Chopra M., Markovic-Plese S. 2011. B cells as a therapeutic target for IFN-β in relapsing-remitting multiple sclerosis. J. Immunol. 186:4518–4526 10.4049/jimmunol.1000271 [DOI] [PubMed] [Google Scholar]
- Samoilova E.B., Horton J.L., Hilliard B., Liu T.S., Chen Y. 1998. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 161:6480–6486 [PubMed] [Google Scholar]
- Serada S., Fujimoto M., Mihara M., Koike N., Ohsugi Y., Nomura S., Yoshida H., Nishikawa T., Terabe F., Ohkawara T., et al. 2008. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 105:9041–9046 10.1073/pnas.0802218105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H. 1999. Remembering MOG: autoantibody mediated demyelination in multiple sclerosis? Nat. Med. 5:153–154 10.1038/5514 [DOI] [PubMed] [Google Scholar]
- Yanaba K., Bouaziz J.D., Haas K.M., Poe J.C., Fujimoto M., Tedder T.F. 2008. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 28:639–650 10.1016/j.immuni.2008.03.017 [DOI] [PubMed] [Google Scholar]
- Zepp J., Wu L., Li X. 2011. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol. 32:232–239 10.1016/j.it.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Srivastava R., Nessler S., Grummel V., Sommer N., Brück W., Hartung H.P., Stadelmann C., Hemmer B. 2006. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc. Natl. Acad. Sci. USA. 103:19057–19062 10.1073/pnas.0607242103 [DOI] [PMC free article] [PubMed] [Google Scholar]