Synthetic a-Synuclein fibrils injected into the brain spread far beyond the injection site and are sufficient to accelerate Parkinson’s disease–like pathology in mice.
Abstract
The accumulation of misfolded proteins is a fundamental pathogenic process in neurodegenerative diseases. However, the factors that trigger aggregation of α-Synuclein (α-Syn), the principal component of the intraneuronal inclusions known as Lewy bodies (LBs), and Lewy neurites (LNs), which characterize Parkinson’s disease (PD) and dementia with LBs (DLB), are poorly understood. We show here that in young asymptomatic α-Syn transgenic (Tg) mice, intracerebral injections of brain homogenates derived from older Tg mice exhibiting α-Syn pathology accelerate both the formation of intracellular LB/LN-like inclusions and the onset of neurological symptoms in recipient animals. Pathological α-Syn propagated along major central nervous system (CNS) pathways to regions far beyond injection sites and reduced survival with a highly reproducible interval from injection to death in inoculated animals. Importantly, inoculation with α-Syn amyloid fibrils assembled from recombinant human α-Syn induced identical consequences. Furthermore, we show for the first time that synthetic α-Syn fibrils are wholly sufficient to initiate PD-like LBs/LNs and to transmit disease in vivo. Thus, our data point to a prion-like cascade in synucleinopathies whereby cell–cell transmission and propagation of misfolded α-Syn underlie the CNS spread of LBs/LNs. These findings open up new avenues for understanding the progression of PD and for developing novel therapeutics.
Accumulation of amyloid deposits is a defining feature of most neurodegenerative disorders. The highly soluble presynaptic protein α-Synuclein (α-Syn; Clayton and George, 1998) is the major component of Lewy bodies (LBs) and Lewy neurites (LNs), the intracellular inclusions that are the neuropathological hallmarks of dementia with LBs (DLBs), Parkinson’s disease (PD), and other α-synucleinopathies (Spillantini et al., 1998a). Although the progressive accumulation of aggregated α-Syn in patients parallel the decline in motor and/or cognitive function (Baba et al., 1998; Braak et al., 2003; Klucken et al., 2006), the events triggering α-Syn pathology in the central nervous system (CNS), and the processes linking LBs/LNs to neurodegeneration, are poorly understood. Importantly, the progression of α-Syn pathology in PD appears to follow a stereotypical pattern that commences in the brainstem and extends rostrally to neocortical regions (Braak et al., 2003; Fahn, 2003). This hierarchical and predictable pattern of disease progression suggests that cell–cell transmission of α-Syn pathology is the basis for the spreading, most likely affecting cells within interconnected neuronal pathways (Braak et al., 2003). Supporting this hypothesis is the observation that embryonic mesencephalic neurons grafted into the neostriatum of PD patients develop LBs (Kordower et al., 2008; Li et al., 2008). However, although cell–cell transfer of soluble α-Syn within the CNS has been reported (Desplats et al., 2009; Danzer et al., 2011; Hansen et al., 2011), the transmission of pathological α-Syn species and its potential role in the pathogenesis of DLB/PD and related α-synucleinopathies remain largely unexplored.
As with other neurodegenerative disease–related proteins, aggregation of α-Syn occurs as a nucleation-dependent process (Wood et al., 1999). Polymerization of α-Syn into amyloid fibrils is greatly accelerated by the presence of minute quantities of aggregated or fibrillar α-Syn serving as nucleation sites, indicating that the formation of intermediates or “seeds” represents an important rate-limiting step. We and others have recently demonstrated that fibrillar α-Syn assembled from recombinant α-Syn protein is internalized by cultured cells and neurons, where they seed the recruitment and conversion of soluble α-Syn into insoluble pathological LB/LN-like inclusions (Desplats et al., 2009; Luk et al., 2009; Volpicelli-Daley et al., 2011). Using a transgenic (Tg) model of α-synucleinopathies (Giasson et al., 2002), we demonstrate here that pathological α-Syn derived from diseased tissues and, more significantly, entirely synthetic α-Syn preformed fibrils (PFFs) greatly accelerate the formation and propagation of pathological inclusions throughout the murine CNS that are highly reminiscent of LBs/LNs. Indeed, we provide the first evidence that synthetic α-Syn PFFs alone can induce PD-like α-Syn pathology and transmit disease in vivo. Thus, both synthetic and disease-associated forms of α-Syn aggregates initiate a cascade of pathological events in vivo that are mediated by aggregation and transmission of this protein and which culminate in a highly lethal DLB-like phenotype.
RESULTS
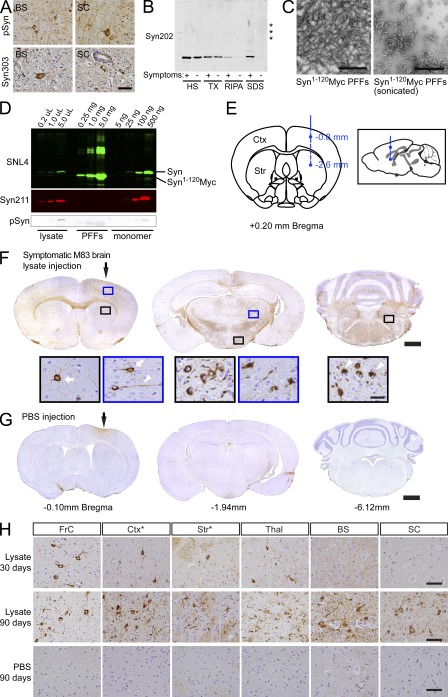
Tg mice expressing human α-Syn bearing the familial PD-related A53T mutation (M83 line) develop neurological symptoms, including abnormal posture, seizures, and paralysis, after ∼12 mo of age (Giasson et al., 2002). To investigate whether disease-associated aggregated α-Syn can seed pathology in vivo, we injected healthy 2–5-mo-old M83 mice with homogenates prepared from brainstem and spinal cord of aged (>12-mo-old) symptomatic M83 animals that contained abundant LB/LN-like α-Syn pathology (Fig. 1, A, B, and D). Lysates were stereotaxically injected into the neocortex and striatum (Fig. 1 E), regions that are affected in PD and have extensive afferent and efferent connections with other CNS areas (Bernheimer et al., 1973; Nieuwenhuys et al., 1982; MacDonald and Halliday, 2002).
Figure 1.
Transmission of α-Syn pathology after intracerebral inoculation of symptomatic aged M83 brain lysates harboring aggregated α-Syn. (A) Brainstem (BS) and spinal cord (SC) from symptomatic M83 mice used to prepare lysates for stereotaxic injections were stained with antibodies against pSyn (hyperphosphorylated α-Syn) or Syn303 (misfolded conformation of α-Syn) to detect α-Syn pathology. (B) Syn202 immunoblotting of lysates from symptomatic and asymptomatic M83 animals a sequential extraction with high-salt buffer (HS), HS + 1% Triton-X100 (TX), RIPA, and SDS buffer. Asterisks indicate high molecular weight α-Syn species. (C) Electron micrographs of α-Syn1-120Myc PFFs before and after sonication. (D) Immunoblot of α-Syn material used for intracerebral inoculation. The indicated amounts of symptomatic brain lysate (low-spin fraction) and recombinant α-Syn1-120Myc PFFs were separated and probed using either antibodies recognizing the N-terminal (SNL4) or C-terminal (Syn211) region of α-Syn, or against pSyn. Recombinant full-length human α-Syn monomer was used as standard. (E) Diagram illustrating route of stereotaxic injections. Pathological α-Syn was inoculated into the right hemisphere at subdural depths, indicated using a single needle tract. Exactly 2.5 µl of inoculum (either brain lysate or recombinant PFFs) was deposited into the striatum (Str) and cortex (Ctx). Inset shows sagittal view of injection sites. (F and G) Representative coronal brain sections from a young M83 mouse injected 90 d prior with symptomatic M83 brain lysate (F) or PBS (G). White arrows and arrowheads in insets show staining with anti-pSyn antibodies and LB/LN-like pathology. Bregma values denote the rostral/caudal level shown. Injection sites are indicated by black arrows. (H) pSyn pathology in young M83 mice injected with symptomatic aged M83 brain lysates 30 or 90 d prior, or with PBS 90 d prior. Injection sites are indicated by asterisks. Micrographs are representative of multiple lysate-injected M83 mice analyzed at 30 dpi (n = 3), 90 dpi (n = 12), or with PBS (n = 7). FrC (frontal cortex); Ctx (somatosensory cortex); Str (neostriatum); Thal (thalamus); BS (brainstem); SC (spinal cord). Bars: 50 µm (A and H); 500 nm (C); 1 mm (F and G); 25 µm (insets in F).
When examined 90 d postinjection (dpi), abundant α-Syn lesions were detected throughout the CNS of these mice by immunohistochemistry for hyperphosphorylated α-Syn (pSyn; Fig. 1 F), a marker of pathological α-Syn (Fujiwara et al., 2002; Waxman and Giasson, 2008). In stark contrast, α-Syn pathology was completely undetectable in age-matched M83 mice 90 dpi with PBS (Fig. 1, G and H, bottom), indicating that this α-Syn pathology did not result from the surgical procedure or reflect the α-Syn transgene-induced pathology, which typically occurs at least 2 mo later. Despite the fact that inoculations were unilateral, LB/LN-like intraneuronal α-Syn deposits were widely distributed bilaterally and present throughout the anterior/posterior extent of the neural axis spanning the CNS from olfactory bulb to spinal cord (Fig. 1, F and H, middle). In addition to the injection sites, other severely affected areas included frontal cortex, thalamus, hypothalamus, brainstem nuclei, and major white-matter tracts (e.g., callosal and commissural fibers). In contrast, lysate-injected mice examined at 30 dpi showed significantly less severe pSyn pathology that was primarily restricted to the vicinity of the injection sites (Fig. 1 H, top), indicating that α-Syn pathology amplifies and expands through the CNS in a time-dependent fashion.
Host expression of soluble α-Syn is absolutely required for de novo formation of these PD-like inclusions because inoculation of the same symptomatic M83 mouse brain lysates into α-Syn–null (α-Syn−/−) animals, which express neither mouse nor human α-Syn, resulted in weak α-Syn and pSyn immunostaining at 7 dpi only at injection sites consistent with residual inoculated material (unpublished data). Furthermore, no α-Syn immunoreactivity was detected in these α-Syn−/− mice by 90 dpi, suggesting the injected α-Syn seeds were degraded by this time. Inoculation of M83 animals with brain homogenates from young asymptomatic M83 mice also failed to elicit α-Syn pathology for up to 164 dpi. Thus, pathological α-Syn in symptomatic brain lysates from M83 mice is the agent capable of initiating and propagating α-Syn pathology in these Tg mice.
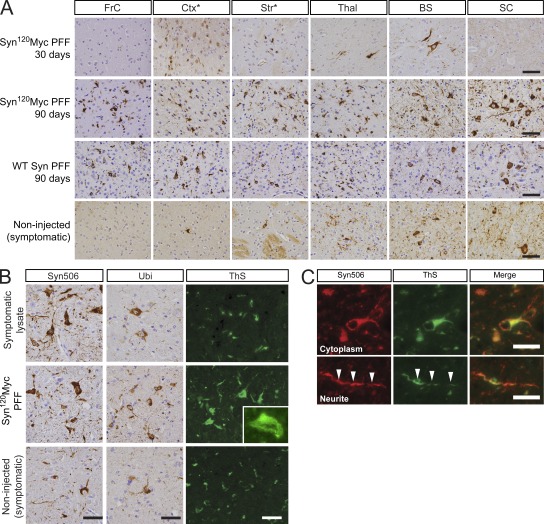
Because insoluble α-Syn species occur only in symptomatic M83 brains (Giasson et al., 2002) and could be responsible for transmission of α-Syn pathology, we hypothesized that α-Syn amyloid fibrils alone are responsible for the initiation and propagation of this pathology. To this end, we generated PFFs in vitro from recombinant α-Syn proteins (Murray et al., 2003) and examined whether they exhibited pathology-seeding activity similar to brain lysates from affected M83 mice (Fig. 1, C–E). Remarkably, injection of healthy M83 mice with PFFs assembled from human α-Syn1-120Myc, a C-terminal truncated form of WT human α-Syn containing the Myc epitope (Luk et al., 2009), also elicited robust LB/LN-like pSyn pathology with a neuroanatomical distribution identical to that seen in lysate-injected animals (Fig. 2 A). Like their lysate-injected counterparts, the α-Syn pathology in these PFF-injected M83 mice also spread progressively with time to distal CNS regions (Fig. 2 A and Fig. S1). Inoculation with PFFs generated from the full-length WT α-Syn protein also led to the development of pathology that was equivalent to α-Syn1-120Myc PFFs with respect to both severity and CNS distribution (Fig. 2 A). Thus, both synthetic WT full-length α-Syn and α-Syn1-120Myc PFFs are sufficient to initiate and propagate α-Syn pathology in vivo in the same manner as brain lysates obtained from symptomatic M83 animals.
Figure 2.
Recombinant α-Syn PFFs induce LBs and LNs in the CNS. (A) pSyn immunohistochemistry of various CNS regions in young M83 mice 30 or 90 d after intracerebral injection with either α-Syn1-120Myc or WT α-Syn PFFs. Asterisks indicate level of injection sites. Bottom panels show pSyn staining in aged symptomatic M83 mice. Immunohistochemical analyses were performed on multiple M83 mice after injection with PFFs assembled from α-Syn1-120Myc (30 d, n = 3; 90 d, n = 10) or from WT α-Syn (n = 4). (B) Brainstem sections from symptomatic lysate- or PFF-injected mice immunostained with antibodies to pathological α-Syn conformers (Syn506) or ubiquitin (Ubi). Thioflavin-S (ThS) staining was also performed to detect LB/LN-like pathology (inset, 60× magnification). (C) Colocalization between Syn506 and ThS in cytoplasmic and neuritic inclusions. Arrowheads denote a neurite positive for both markers. Bars: 50 µm (A); 25 µm (B); 5 µm (inset).
The α-Syn pathology in M83 mice injected with either symptomatic lysates or α-Syn PFFs was consistently more severe and widely distributed than that seen in noninjected Tg mice that had become symptomatic with age (Fig. 2 A, bottom; Giasson et al., 2002). Although the youngest age at which α-Syn pathology was detectable in noninjected M83 mice was 8 mo, but more typically after 12 mo of age, profuse α-Syn pathologies could be found in 100% of animals ≥30 dpi with symptomatic lysate (n = 21) or recombinant PFFs (n = 13; Fig. 1 H, Fig. 2 A, and Fig. S1). In contrast, pathology was undetectable in M83 mice up to 90 d after inoculation with PBS (n = 11) or brain lysate prepared from asymptomatic animals (n = 4). Thus, the pathological α-Syn species are highly potent in rapidly seeding aggregation of α-Syn in living animals. Indeed, inoculation with as little as 5 ng of pathological α-Syn in the form of PFFs was sufficient to induce visible pSyn accumulations in M83 mice at the level of the injection sites, whereas higher quantities of PFFs resulted in CNS-wide α-Syn pathology (unpublished data).
The α-Syn inclusions that formed in lysate- or PFF-inoculated mice resembled LBs and LNs found in PD and DLB brains, as they showed strong immunoreactivity to antibodies recognizing disease-specific conformations of α-Syn and ubiquitin (Fig. 2 B; Spillantini et al., 1998b; Sampathu et al., 2003). Abnormal α-Syn in both perikaryal and neuritic inclusions colocalized with Thioflavin-S staining (Fig. 2 C) indicating that they were comprised of amyloid fibrils formed by α-Syn.
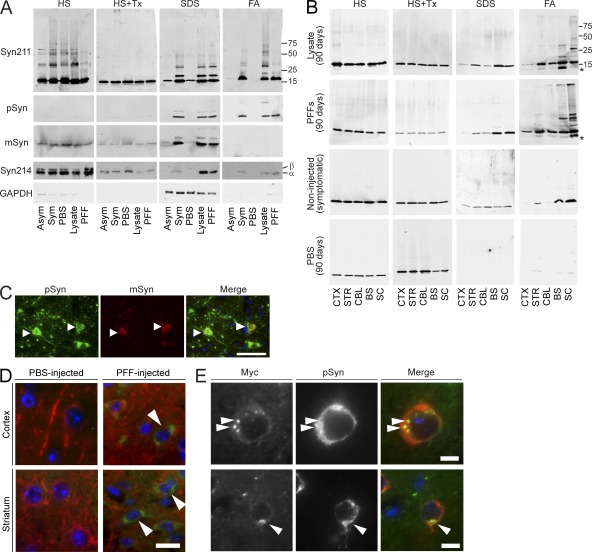
Consistent with this massive burden of α-Syn pathology revealed by immunostaining, biochemical analysis of CNS homogenates from α-Syn–inoculated mice showed a marked increase in detergent-insoluble α-Syn compared with uninjected symptomatic M83 mice (Fig. 3, A and B). A mixture of monomer and high molecular weight species suggestive of multimers and/or ubiquitinated α-Syn were recovered in SDS- and formic acid-soluble fractions. In addition to human A53T α-Syn, endogenous murine α-Syn was also recruited into the insoluble aggregates, whereas the solubility of β-Syn, a closely related member of the synuclein family, remained unchanged (Fig. 3 A), suggesting that recruitment and conversion is specific to α-Syn itself. The relative levels of α-Syn within formic acid–soluble fractions from different CNS regions in M83 mice injected with pathological α-Syn also mirrored the extent of pathology detected histologically (Fig. 3 B). Thus, the accelerated formation of α-Syn pathology observed in animals inoculated with a pathogenic form of the protein correlates with biochemical and posttranslational modifications of α-Syn that are characteristic of PD and related α-synucleinopathies.
Figure 3.
Biochemical analysis of α-Syn inoculated CNS tissue. (A) Immunoblots of brainstem samples sequentially extracted with the following: high-salt buffer (HS), HS+1% Triton-X (HS+Tx), 1% SDS (SDS) and formic acid (FA). Tissue was from untreated asymptomatic (Asym) M83 mice, aged symptomatic mice (Sym), or mice injected with PBS, symptomatic lysate, or α-Syn1-120Myc PFFs for 90 d. Immunoblots were probed with anti–α-Syn antibodies including anti–C terminus (Syn211), anti-phosphorylated Ser129 (pSyn), anti–mouse α-Syn (mSyn), or anti–α/β-Syn (Syn214). GAPDH is the loading control. Immunoblots are representative of two independent extraction experiments (n = 3 brains per group). (B) Various CNS regions from M83 mice injected with either symptomatic lysate or α-Syn1-120Myc PFFs for 90 d were sequentially extracted and probed using SNL4, which recognizes the N terminus of α-Syn. Samples from aged symptomatic M83 mice or mice injected with PBS are shown as positive controls. (C) Brainstem neurons of M83 mice injected with α-Syn1-120Myc PFFs (90 d) were double-immunostained using anti-pSyn and a rabbit polyclonal antibody specific for murine α-Syn (mSyn). Arrowheads show endogenous mSyn in pathological inclusions. (D) M83 were mice sacrificed 7 dpi with α-Syn1-120Myc PFFs. Tissue was double-immunostained for MAP-2 (red) and Myc (green) to detect internalization of exogenous Myc-tagged α-Syn by neurons. Cell nuclei were stained with DAPI (blue). Arrowheads denote intracellular Myc staining in cortical and striatal neurons. (E) Sections from α-Syn1-120Myc PFF-injected animals were double-immunolabeled against Myc and pSyn. Arrowheads denote accumulation of pSyn around internalized α-Syn. Bars: 50 µm (C); 15 µm (D); 10 µm (E).
Given that PFFs generated from α-Syn1-120Myc lack the Ser129 phosphoepitope recognized by anti-pSyn, the detection of LB/LN-like pSyn pathology after their injection into M83 mice overexpressing full-length human α-Syn confirms that inclusions induced by the inoculations contain both human α-Syn and endogenously expressed mouse α-Syn (Fig. 3, A and C). The observation by immunoblot that the bulk of detergent-insoluble α-Syn was detectable by antibodies specifically recognizing the C terminus of human α-Syn (Fig. 3 A; e.g., Syn211) gives further support to the notion that soluble α-Syn expressed by M83 animals is recruited to inclusions.
As the exogenous pathogenic α-Syn species likely act as a seed for recruiting endogenous α-Syn into intracellular inclusions, we examined whether α-Syn1-120Myc PFFs were internalized by neurons after stereotaxic injection. Indeed, focal pSyn immunostaining surrounding α-Syn1-120Myc internalized by neurons in the cortex and striatum was apparent in PFF-injected M83 mice at 7 dpi (Fig. 3, D and E). In contrast, Syn−/− mice showed only negligible Myc-immunostaining at PFF injection sites at 7 dpi, suggesting that the recruitment and conversion of α-Syn expressed by M83 animals to exogenously introduced seeds represents an early step in the formation of α-Syn inclusions (unpublished data).
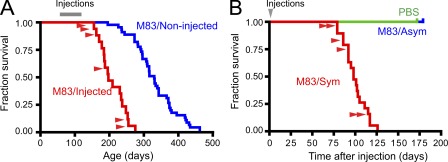
The acceleration and increased accumulation of α-Syn pathology in M83 mice injected with either symptomatic lysate or α-Syn PFFs was associated with a dramatic reduction in survival when compared with uninjected M83 animals (median of 204 d for M83-injected vs. 316 d for M83-uninjected control mice), all of which eventually succumb to disease from the transgene-driven expression of A53T mutant α-Syn (Fig. 4 A). Interestingly, the lag period between inoculation with pathological α-Syn and the appearance of motor symptoms was highly uniform (median of 100 d) irrespective of the animal’s age at injection (Fig. 4 B), correlating closely with the emergence of extensive α-Syn pathology (i.e., ∼90 dpi). In contrast, neurological deficits in uninjected M83 mice or those that were treated with either PBS or homogenates from asymptomatic animals did not emerge until the appearance of α-Syn inclusions resulting solely from the transgene-driven expression of mutant A53T α-Syn, which occurred variably between 226–462 d of age, with none succumbing to disease before 175 dpi (Fig. 4 A; Giasson et al., 2002). Thus, the amplification and transmission of pathological α-Syn in the M83 mice injected with pathological α-Syn is directly associated with a rapid and predictable disease process linked to the onset of motoric symptoms. The similarity in phenotype between α-Syn–inoculated M83 mice and those that developed symptoms through advanced aging (e.g., paralysis beginning in the hindlimbs) corresponded to the massive brainstem and spinal cord α-Syn pathology observed and is consistent with CNS damage previously reported in older Tg mice expressing A53T α-Syn (Giasson et al., 2002; Martin et al., 2006).
Figure 4.
Intracerebral inoculation with pathological α-Syn reduces survival in M83 Tg mice. (A) Kaplan-Meier survival plots comparing lifespans of M83 mice injected (red) with either symptomatic brain lysate (n = 13) or α-Syn PFFs (n = 6). Uninjected M83 mice are shown in blue (n = 47). Gray bar indicates time of injection and age is shown on the horizontal axis. (P < 0.0001; χ2, 51.08; DF, 1). (B) Time until demise of young M83 mice after inoculation with pathological α-Syn (Sym, n = 19), asymptomatic M83 brain lysates (Asym), or PBS. Red arrowheads denote animals that received PFF injections. The demise of all mice inoculated with symptomatic M83 brain lysates or α-Syn PFFs occurred within 126 dpi (median 101 dpi), whereas mice injected with asymptomatic lysate- and PBS-treated animals (n = 4 each) remained disease free (P < 0.0001; χ2, 20.42; DF, 2).
To further understand the relationship between transmission of α-Syn pathology and this disease phenotype, we characterized the anatomical distribution of α-Syn inclusions at 30 and 90 dpi with either symptomatic lysate or α-Syn PFFs (Fig. 5). At 30 dpi, inclusions were confined to the injection site and immediate surrounding areas, in agreement with the recruitment of α-Syn expressed by M83 mice into seeded aggregates (Fig. 5 A). By 90 dpi, α-Syn pathology was far more widespread and abundant, even when compared with aged M83 mice. Moreover, lysate- and PFF-injected mice displayed nearly identical spatial distributions of pSyn pathology at both time points examined (Fig. 5 A), indicating that recombinant α-Syn PFFs are qualitatively equivalent to lysates derived from symptomatic M83 brain tissue in their capacity to propagate α-Syn pathology in vivo.
Figure 5.
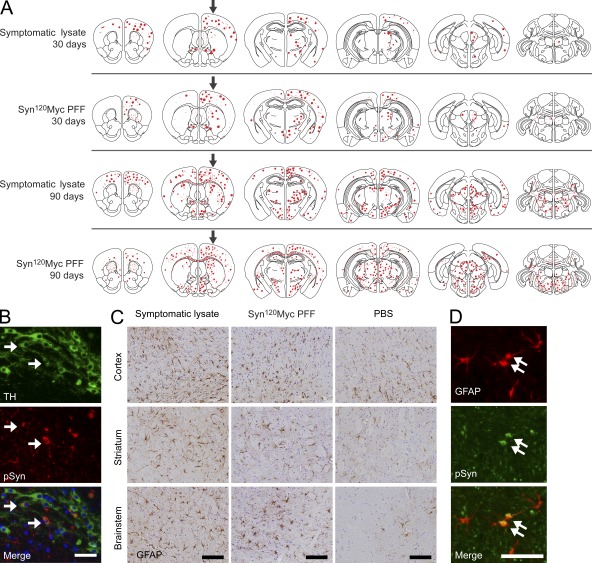
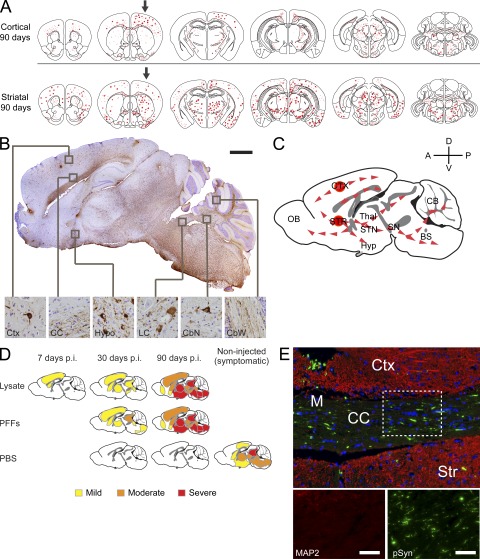
Distribution of α-Syn pathology after inoculation with pathological α-Syn. (A) Injections of symptomatic brain lysate or α-Syn1-120Myc PFFs were made to the right cortex and striatum (black arrows) of young healthy M83 mice. Maps denote the distribution of α-Syn LB- and LN-like pathology (red dots and lines, respectively) in coronal sections from injected mice sacrificed at either 30 or 90 dpi and immunostained with anti-pSyn. Representative plots are shown for mice injected with symptomatic lysate or α-Syn1-120Myc PFFs (n = 3–5 per group). (B) α-Syn pathology in dopaminergic neurons of inoculated M83 mice. Double-immunolabeling of tyrosine hydroxylase (TH, green) and pSyn (red) in the substantia nigra pars compacta of animals injected with α-Syn1-120Myc PFFs and sacrificed 90 d later. A subpopulation of dopaminergic neurons containing intracellular pSyn accumulations are indicated by arrows. (C) Glial fibrillary acidic protein (GFAP) immunostaining of the cortex, striatum, and brainstem of M83 mice injected with either symptomatic lysate, α-Syn1-120Myc PFFs, or PBS. Animals were sacrificed 90 dpi. (D) Double-immunostaining for pSyn and GFAP in the thalamus of M83 mouse 90 dpi with α-Syn1-120Myc PFFs. Arrows indicate astrocytes containing intracellular pSyn inclusions. Bars: 35 µm (B); 40 µm (C); 50 µm (D).
These mapping studies further revealed that regions which developed the most prominent α-Syn pathology after injection of symptomatic lysate or α-Syn PFFs were those containing neurons that project to, or receive input from, the inoculation sites (e.g., frontal cortex and thalamus). A CNS-wide survey of all regions exhibiting α-Syn inclusions in lysate- and PFF-inoculated M83 mice revealed that regions sharing significant interconnections displayed the most severe pathology (Fig. S1), suggesting that propagation of pathological α-Syn occurs most readily between associated neuronal populations. Supporting this hypothesis, accumulation of hyperphosphorylated α-Syn was also apparent in neurons of the substantia nigra pars compacta, a population of neurons that provides dopaminergic innervation to the dorsal striatum and is highly susceptible to accumulation of LBs/LNs in human PD (Fig. 5 B). Nigral neurons bearing α-Syn inclusions also showed visible reductions in tyrosine-hydroxylase staining, suggesting impaired dopamine production in these cells. Finally, the α-Syn pathology in the injected M83 mice was also accompanied by astrogliosis and microgliosis, which are indicative of progressive neurodegeneration (Fig. 5 C). In addition, brain lysates from symptomatic M83 mice injected separately into either the neocortex or striatum induced distinct, yet complementary, distributions of α-Syn pathology that were consistent with their known afferent and efferent pathways (Fig. 6 A). When superimposed, these two patterns recapitulated the full extent of pathology seen in M83 mice that were injected at both sites (Fig. 5 A), indicating that the neuronal connectivity of the initial seeding site is a major determinant of the route of α-Syn propagation. Interestingly, a subset of astrocytes that express α-Syn in this Tg line contained robust pSyn pathology, suggesting that nonneuronal cells are also capable of internalizing pathological α-Syn and forming seeded α-Syn inclusions (Fig. 5 D).
Figure 6.
Propagation and transmission of pathological α-Syn species in CNS. (A) Representative maps showing distribution of pSyn pathology 90 d after a single injection (black arrows) with symptomatic aged M83 brain lysate to the cortex or striatum in young M83 mice (n = 3 animals per group). (B) Sagittal brain section from a symptomatic lysate-injected M83 animal 90 dpi immunostained with anti-pSyn. Neocortex (Ctx), corpus callosum (CC), hypothalamus (Hypo), locus coeruleus (LC), cerebellar nuclei (CbN), and cerebellar white matter (CbW) are highlighted (insets of boxed areas, 40X magnification). (C) Possible routes of α-Syn propagation and transmission are illustrated. Gray denotes white matter tracts. (D) Diagram illustrating anatomical pattern of α-Syn accumulation in M83 mice 7, 30, or 90 dpi with either symptomatic brain lysate, α-Syn1-120Myc PFFs, or PBS. The distribution of α-Syn pathology in uninjected aged M83 animals is also shown. (E) Coronal section of axons in the corpus callosum (CC) and surrounding cortical (Ctx) and striatal (Str) tissue from a M83 mouse injected with symptomatic lysate. Immunostaining for pSyn (green) and microtubule-associated protein 2 (MAP2, red) were performed 90 dpi. The midline is also indicated (M). Bars: 1 mm (B); 50 µm (E).
Although we cannot rule out the possibility that small quantities of pathological α-Syn from the inoculum itself or from affected cells infiltrated the ventricular space, the distinct segregation between the distributions of pathology resulting from separate injections into neocortex versus striatum, along with the lack of any periventricular pathology, suggests that dissemination through the cerebrospinal fluid plays a minor role in transmission of pathological α-Syn. Moreover, several areas adjacent to heavily affected regions, most notably the hippocampus, showed only limited α-Syn pathology, suggesting that pathological α-Syn does not spread indiscriminately. Rather, consistent with the hypothesis that pathological α-Syn may be directly transmitted between neuronal populations, anti-pSyn immunohistochemistry in sagittal sections from lysate-injected mice showed that α-Syn pathology was concentrated along major telencephalic axonal pathways (e.g., lateral and medial forebrain bundles; Fig. 6, B–E). The detection of α-Syn inclusions in spinal cord neurons and deep cerebellar nuclei is further concordant with this hypothesis, although it remains to be established whether this occurred through transmission of pathological α-Syn through secondary or tertiary synaptic circuits such as pontine or medullary connections (Fig. 6, B and C).
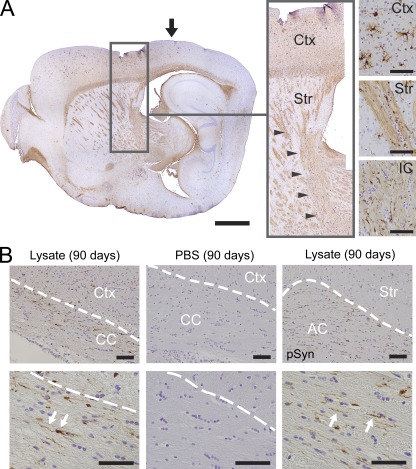
Immunoreactive α-Syn inclusions were detected in axons of the corpus callosum, anterior commissure, and corticospinal tract, suggestive of propagation of α-Syn pathology along major white matter tracts and that they may serve as significant conduits for disease transmission (Fig. 7, A and B). Because callosal and commissural fibers also extend bilaterally, they may also represent possible routes for the interhemispheric transmission of α-Syn pathology observed at later time points (Fig. 7 B). In addition, α-Syn pathology in thalamic and subthalamic areas could also be traced back to the striatal injection site via the internal capsule, a structure that fenestrates the dorsal striatum (Fig. 7 A, inset), further supporting the view that pathological α-Syn propagates along neuronal processes.
Figure 7.
Pathological α-Syn species in major CNS pathways and white matter tracts. (A) Sagittal section of lateral forebrain from lysate-injected M83 animal showing LB/LN-like pathology labeled with anti-pSyn (inset) within cortex, striatum (Str) and internal capsule (IC; arrowheads in inset). Black arrow, injection site. (B) Anti-pSyn immunohistochemistry demonstrates α-Syn pathology (arrows) in corpus callosum and anterior commissure (AC) of lysate-injected animals, but not PBS-treated controls. Dashed lines demarcate white matter tracts. Bars: 1 mm (A); 50 µm (insets in A and B).
DISCUSSION
Our data here demonstrate that a single inoculation of pathological α-Syn, including α-Syn PFFs assembled from recombinant protein, is sufficient to induce widespread CNS α-Syn pathology and accelerate disease in vivo. Importantly, the near-identical pathology initiated by α-Syn PFFs and diseased brain lysates suggests that α-Syn PFFs alone can act as a pathogenic agent that ultimately leads to a fatal neurological phenotype in the M83 Tg mouse model. Our findings here compliment recent experimental studies demonstrating the cell–cell transmission of α-Syn (Danzer et al., 2009; Desplats et al., 2009; Hansen et al., 2011) and that aggregated α-Syn induces PD-like pathology in recipient cells with damaging consequences (Luk et al., 2009; Waxman and Giasson, 2010; Volpicelli-Daley et al., 2011). Moreover, they are compatible with histopathological studies in humans suggesting that the transmission of misfolded α-Syn promotes the spreading of pathology in synucleinopathies such as PD and DLB (Braak et al., 2003; Kordower et al., 2008; Li et al., 2008), thereby contributing to disease progression. Our data also adds to a growing body of evidence that the prion-like transmission and propagation of misfolded proteins represents a common process in the development and progression of neurodegenerative diseases, including Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis, as well as PD and DLB (Aguzzi and Rajendran, 2009; Brundin et al., 2010; Polymenidou and Cleveland, 2011).
The efficiency and rapidity with which α-Syn PFFs induced pathology in M83 mice contrasts with studies of mouse models of other neurodegenerative diseases, most notably Alzheimer’s disease pathology, wherein homogenates of diseased brain tissue, but not synthetic Aβ peptide or recombinant tau protein, efficiently seed plaque and tangle-like pathology after intracerebral or peripheral injection (Meyer-Luehmann et al., 2006; Clavaguera et al., 2009; Eisele et al., 2010). Although amyloid fibrils represent the predominant form of α-Syn in synthetic preparations, the possibility that other α-Syn species may also transmit pathology and disease cannot be excluded at this point. Nonetheless, the in vivo pathogenic activity of synthetic α-Syn PFF preparations required neither templating with disease-derived material nor serial propagation in a cellular host, indicating that the conformations responsible for transmission were generated in vitro. Although the de novo generation of pathological prions from recombinant protein has also been reported (Barria et al., 2009; Kim et al., 2010; Makarava et al., 2011), activity appears to be dependent on the use of brain-derived template material or serial passage in cells or animals, suggesting that perhaps additional cofactors are required. Thus, we report the first evidence here that synthetic α-Syn PFFs alone, including both synthetic WT full-length human α-Syn and human α-Syn1-120Myc PFFs, can induce DLB/PD-like α-Syn pathology and transmit a lethal neurodegenerative α-synucleinopathy in vivo.
Our data also support the idea that pathological α-Syn can spread over considerable distances to many CNS regions, including cortical, midbrain, and brainstem neurons that are affected in DLB/PD (Braak et al., 2003; Dickson et al., 2009). The consistent observation that intracellular α-Syn inclusions in regions distant from the injection site is accompanied by the presence of α-Syn pathology within intermediary neuronal populations is also reminiscent of the hierarchal pattern of progression proposed for human DLB/PD (Braak et al., 2003; Del Tredici and Braak, 2008). Although propagation of LBs/LNs in human PD is postulated to start in the brainstem and ascend toward neocortical regions with disease progression (Braak et al., 2003; Del Tredici and Braak, 2008), the data presented here and recent studies (Volpicelli-Daley et al., 2011) indicate that the transmission of pathological α-Syn can occur bi-directionally within a network of interconnected populations.
The observation that inoculation into the cortex and striatum also resulted in robust α-Syn inclusions in brainstem and cerebellar nuclei, regions that do not share direct innervation with the injection sites, and that the path of transmission of pathological α-Syn does not appear to be restricted by either the presence or number of intermediary connections, suggests transsynaptic spreading as a possible mode of propagation for pathological α-Syn species. Although this mechanism has previously been proposed for transmission in both prion disease (Scott et al., 1992; Prinz et al., 2003) and CNS viral infection (Callaway, 2008) in neurons, additional studies examining the anatomical relationships between injection sites and affected areas will assist in elucidating the precise pathways involved in α-Syn spreading. Patients with PD exhibit elevated levels of multimeric α-Syn in the cerebrospinal fluid which may serve as transmissible seeds for subsequent inclusion formation (Tokuda et al., 2010). In contrast to our model, however, the primary source of α-Syn nucleating seeds in PD and DLB remains unknown (Braak et al., 2006). Despite the established presence of α-Syn pathology in olfactory and enteric neurons in PD (Wakabayashi et al., 1989; Daniel and Hawkes, 1992; Duda et al., 1999; Beach et al., 2010), there is currently no direct evidence that PD or DLB are either infectious or acquired from an external source via nasal or gastrointestinal routes. Rather, the release of pathological α-Syn species from dying neurons or by exocytosis represents a more probable source of transmissible α-Syn (Lee et al., 2008; Desplats et al., 2009; Emmanouilidou et al., 2010; Danzer et al., 2011; Hansen et al., 2011), uptake of which may trigger a reiterative pathogenic process like the one described here.
A frequently cited limitation of Tg models of α-synucleinopathies is the failure to recapitulate pathology in neurons of the substantia nigra and the motor impairments that accompany loss of dopamine function. Although we have demonstrated here that inoculation with either symptomatic M83 mouse brain lysates or α-Syn PFFs elicited α-Syn pathology in nigral neurons, our ability to detect motor deficits directly related to dopaminergic dysfunction and other possible neurological symptoms may actually be masked by the most striking feature of our transmission model—the rapidity with which injected animals develop pathology and disease. Nonetheless, our findings clearly suggest a direct relationship between the accumulation of pathological α-Syn and neurological disease. Indeed, the seemingly irreversible amplification and spread of pathological α-Syn observed here may explain the relentless neurological decline in patients with pathological α-synucleinopathies. Finally, these findings may extend to other protein-misfolding disorders, and thus have implications for developing disease-modifying therapies for DLB/PD and other neurodegenerative diseases linked to the accumulation misfolded protein aggregates.
MATERIALS AND METHODS
Animals.
M83 mice overexpressing human A53T α-Syn under the control of the mouse prion protein promoter (Giasson et al., 2002) were maintained on a C57BL/C3H background and α-Syn−/− mice (Abeliovich et al., 2000) were maintained on a C57BL/6 background. All housing, breeding, and procedures were performed according to the National Institutes of Health Guide for the Care and Use of Experimental Animals and approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Injection material.
Brainstem and spinal cord of aged symptomatic M83 mice were dissected from brains previously stored at −80°C. Tissue was sonicated in sterile PBS (100 mg per 1 ml of buffer) with a handheld probe (QSonica). Homogenates were cleared by centrifugation for 5 min (3,000 g, 4°C) and the resultant supernatant (lysate) was recovered and stored at −80°C until injection. Purification of recombinant α-Syn proteins and in vitro fibril assembly was performed as previously described (Murray et al., 2003; Luk et al., 2009) using human α-Syn1-120Myc or WT full-length human α-Syn (5 mg/ml). PFFs were collected after 5 d of incubation at 37°C. PFF preparations were diluted into sterile PBS and sonicated briefly before intracerebral injection.
Stereotaxic injections.
Male M83 mice (2–4 mo of age) were anesthetized with an intraperitoneal injection of ketamine hydrochloride (100 mg/kg) and xylazine (10 mg/kg), and stereotaxically injected with either brain lysate (5 µg total protein per brain) or recombinant α-Syn fibrils (5 µg per brain, unless otherwise indicated). Control M83 animals received either sterile PBS or brain lysate derived from 1-mo-old asymptomatic M83 mice. A single needle insertion (coordinates: +0.2 mm relative to bregma, 2.0 mm from midline) into the right forebrain was used to target the inoculum to both the somatosensory cortex and dorsal neostriatum located at a depth of 0.8 and 2.6 mm below the dura, respectively (Fig. S1 E). Material was injected via a Hamilton syringe at a rate of 0.1 µl per min (2.5 µl total per site) with the needle in place for ≥10 min at each target. Animals were inoculated at both sites unless otherwise indicated. After recovery from surgery, animals were monitored regularly, and sacrificed either upon the onset of paralysis or at various predetermined time points by overdose with ketamine/xylazine and then transcardial perfusion with PBS. For histological studies the brain and spinal cord were removed and underwent overnight postfixation in either neutral buffered formalin (Thermo Fisher Scientific) or 70% ethanol (in 150 mM NaCl), before being processed and embedded in paraffin. For biochemical studies, tissues were immediately frozen after removal and stored at −80°C until used.
Antibody generation.
Rabbits (PRF&L) were immunized with a synthetic peptide corresponding to residues 115–125 of the murine α-Syn (mSyn) conjugated via an additional N-terminal cysteine residue to keyhole limpet hemocyanin (KLH-CDMPVDPGSEAY). The resulting antisera were purified using an NHS-agarose column conjugated with recombinant full-length human α-Syn. Flowthrough fractions were further affinity-purified using agarose conjugated to recombinant mSyn. Polyclonal antibodies specific to α-Syn phosphorylated at serine 129 (pSer129) was generated by injecting rabbits with the KLH-conjugated peptide CAYEMPSEEGYQ (phosphorylated residue underlined). Phosphospecific antibodies were enriched by sequentially incubating antisera with NHS-agarose conjugated to recombinant α-Syn and the phosphopeptide.
Immunohistochemistry and mapping of α-Syn pathology.
Immunohistochemistry was performed on 6-µm-thick serial sections as previously described (Duda et al., 2000). Primary antibodies used and working dilutions are detailed in Table S1. For histological and cell mapping studies, coronal sections were stained using 3′-diaminobenzidine (Vector Laboratories) as a chromogen. Immunoreactive inclusions/cells and neurites were mapped at multiple rostrocaudal levels corresponding to ∼1.3, 0.26, −1.75, −3.0, −4.3, and −6.0 mm relative to Bregma. For double-labeling studies, immunoreactivity was revealed using the appropriate fluorescent secondary antibodies conjugated to Alexa Fluor 488 or 594 (Invitrogen). Images were captured using a DP71 digital camera connected to a BX51 microscope (Olympus). Collages were assembled using Photoshop CS2 software (Adobe).
Biochemical analysis.
Brain regions of interest were dissected, weighed, and sequentially extracted using high salt (HS) buffer (50 mM Tris, pH 7.5, 750 mM NaCl, and 5 mM EDTA), HS buffer containing 1% Triton-X100, RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS), 1% SDS buffer (50 mM Tris, pH 7.5, and 1% SDS), and 66% formic acid. Protease and phosphatase inhibitors (Roche) were added to buffers before use. For each extraction step, samples were sonicated and sedimented at 100,000 g for 30 min. 3 ml of buffer was used per gram of tissue in each extraction step. Protein concentrations were determined using the BCA assay (Thermo Fisher Scientific), and samples (20 µg total protein) were separated on SDS-polyacrylamide gels (4–20% gradient) and transferred onto nitrocellulose membranes for probing with various primary antibodies (Table S1). Target antigens were detected using an Odyssey FC scanner (LiCor) after incubation with the appropriate infrared secondary antibodies.
Online supplemental material.
Fig. S1 summarizes the α-Syn pathology observed in major CNS areas of M83 mice after inoculation with pathological α-Syn or control inoculum. Table S1 is a list of antibodies used in this study. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20112457/DC1.
Acknowledgments
We thank Drs. Kurt Brunden, Linda Kwong, Eddie Lee and Laura Volpicelli-Daley for helpful discussions and Sharon Xie for statistical analysis.
This work was supported by National Institutes of Health NS053488, the Picower Foundation, the Benaroya Foundation, the Jeff and Anne Keefer Fund and the Stein-Bellet Family Fund. K.C. Luk is supported in part by the University of Pennsylvania Institute for Translational Medicine and Therapeutics.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- α-Syn
- α-synuclein
- CNS
- central nervous system
- DLB
- dementia with LBs
- dpi
- d postinjection
- LB
- Lewy body
- LN
- Lewy neurite
- PD
- Parkinson’s disease
- PFF
- preformed fibril
- pSyn
- hyperphosphorylated α-Syn
References
- Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W.H., Castillo P.E., Shinsky N., Verdugo J.M., Armanini M., Ryan A., et al. 2000. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 25:239–252 10.1016/S0896-6273(00)80886-7 [DOI] [PubMed] [Google Scholar]
- Aguzzi A., Rajendran L. 2009. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 64:783–790 10.1016/j.neuron.2009.12.016 [DOI] [PubMed] [Google Scholar]
- Baba M., Nakajo S., Tu P.H., Tomita T., Nakaya K., Lee V.M.Y., Trojanowski J.Q., Iwatsubo T. 1998. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 152:879–884 [PMC free article] [PubMed] [Google Scholar]
- Barria M.A., Mukherjee A., Gonzalez-Romero D., Morales R., Soto C. 2009. De novo generation of infectious prions in vitro produces a new disease phenotype. PLoS Pathog. 5:e1000421 10.1371/journal.ppat.1000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach T.G., Adler C.H., Sue L.I., Vedders L., Lue L., White Iii C.L., Akiyama H., Caviness J.N., Shill H.A., Sabbagh M.N., et al. ; Arizona Parkinson’s Disease Consortium 2010. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 119:689–702 10.1007/s00401-010-0664-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. 1973. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 20:415–455 10.1016/0022-510X(73)90175-5 [DOI] [PubMed] [Google Scholar]
- Braak H., Del Tredici K., Rüb U., de Vos R.A.I., Jansen Steur E.N., Braak E. 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 24:197–211 10.1016/S0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- Braak H., de Vos R.A.I., Bohl J., Del Tredici K. 2006. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 396:67–72 10.1016/j.neulet.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Brundin P., Melki R., Kopito R. 2010. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 11:301–307 10.1038/nrm2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E.M. 2008. Transneuronal circuit tracing with neurotropic viruses. Curr. Opin. Neurobiol. 18:617–623 10.1016/j.conb.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F., Bolmont T., Crowther R.A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A.K., Beibel M., Staufenbiel M., et al. 2009. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11:909–913 10.1038/ncb1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D.F., George J.M. 1998. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 21:249-254 10.1016/S0166-2236(97)01213-7 [DOI] [PubMed] [Google Scholar]
- Daniel S.E., Hawkes C.H. 1992. Preliminary diagnosis of Parkinson’s disease by olfactory bulb pathology. Lancet. 340:186 10.1016/0140-6736(92)93275-R [DOI] [PubMed] [Google Scholar]
- Danzer K.M., Krebs S.K., Wolff M., Birk G., Hengerer B. 2009. Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J. Neurochem. 111:192–203 10.1111/j.1471-4159.2009.06324.x [DOI] [PubMed] [Google Scholar]
- Danzer K.M., Ruf W.P., Putcha P., Joyner D., Hashimoto T., Glabe C., Hyman B.T., McLean P.J. 2011. Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 25:326–336 10.1096/fj.10-164624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K., Braak H. 2008. A not entirely benign procedure: progression of Parkinson’s disease. Acta Neuropathol. 115:379–384 10.1007/s00401-008-0355-5 [DOI] [PubMed] [Google Scholar]
- Desplats P., Lee H.J., Bae E.J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S.J. 2009. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA. 106:13010–13015 10.1073/pnas.0903691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D.W., Braak H., Duda J.E., Duyckaerts C., Gasser T., Halliday G.M., Hardy J., Leverenz J.B., Del Tredici K., Wszolek Z.K., Litvan I. 2009. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 8:1150–1157 10.1016/S1474-4422(09)70238-8 [DOI] [PubMed] [Google Scholar]
- Duda J., Shah U., Arnold S.E., Lee V.M., Trojanowski J.Q. 1999. The expression of α-, β-, and γ-synucleins in olfactory mucosa from patients with and without neurodegenerative diseases. Exp. Neurol. 160:515–522 10.1006/exnr.1999.7228 [DOI] [PubMed] [Google Scholar]
- Duda J.E., Giasson B.I., Gur T.L., Montine T.J., Robertson D., Biaggioni I., Hurtig H.I., Stern M.B., Gollomp S.M., Grossman M., et al. 2000. Immunohistochemical and biochemical studies demonstrate a distinct profile of alpha-synuclein permutations in multiple system atrophy. J. Neuropathol. Exp. Neurol. 59:830–841 [DOI] [PubMed] [Google Scholar]
- Eisele Y.S., Obermüller U., Heilbronner G., Baumann F., Kaeser S.A., Wolburg H., Walker L.C., Staufenbiel M., Heikenwalder M., Jucker M. 2010. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 330:980–982 10.1126/science.1194516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E., Melachroinou K., Roumeliotis T., Garbis S.D., Ntzouni M., Margaritis L.H., Stefanis L., Vekrellis K. 2010. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30:6838–6851 10.1523/JNEUROSCI.5699-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. 2003. Description of Parkinson’s disease as a clinical syndrome. Ann.N.Y. Acad.Sci. 991:1–14 10.1111/j.1749-6632.2003.tb07458.x [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M.S., Shen J., Takio K., Iwatsubo T. 2002. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4:160–164 10.1038/ncb841 [DOI] [PubMed] [Google Scholar]
- Giasson B.I., Duda J.E., Quinn S.M., Zhang B., Trojanowski J.Q., Lee V.M.Y. 2002. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 34:521–533 10.1016/S0896-6273(02)00682-7 [DOI] [PubMed] [Google Scholar]
- Hansen C., Angot E., Bergström A.L., Steiner J.A., Pieri L., Paul G., Outeiro T.F., Melki R., Kallunki P., Fog K., et al. 2011. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 121:715–725 10.1172/JCI43366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.I., Cali I., Surewicz K., Kong Q., Raymond G.J., Atarashi R., Race B., Qing L., Gambetti P., Caughey B., Surewicz W.K. 2010. Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J. Biol. Chem. 285:14083–14087 10.1074/jbc.C110.113464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken J., Ingelsson M., Shin Y., Irizarry M.C., Hedley-Whyte E.T., Frosch M., Growdon J., McLean P., Hyman B.T. 2006. Clinical and biochemical correlates of insoluble alpha-synuclein in dementia with Lewy bodies. Acta Neuropathol. 111:101–108 10.1007/s00401-005-0027-7 [DOI] [PubMed] [Google Scholar]
- Kordower J.H., Chu Y., Hauser R.A., Freeman T.B., Olanow C.W. 2008. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14:504–506 10.1038/nm1747 [DOI] [PubMed] [Google Scholar]
- Lee H.J., Suk J.E., Bae E.J., Lee J.H., Paik S.R., Lee S.J. 2008. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int. J. Biochem. Cell Biol. 40:1835–1849 10.1016/j.biocel.2008.01.017 [DOI] [PubMed] [Google Scholar]
- Li J.Y., Englund E., Holton J.L., Soulet D., Hagell P., Lees A.J., Lashley T., Quinn N.P., Rehncrona S., Björklund A., et al. 2008. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 14:501–503 10.1038/nm1746 [DOI] [PubMed] [Google Scholar]
- Luk K.C., Song C., O’Brien P., Stieber A., Branch J.R., Brunden K.R., Trojanowski J.Q., Lee V.M.Y. 2009. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA. 106:20051–20056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald V., Halliday G.M. 2002. Selective loss of pyramidal neurons in the pre-supplementary motor cortex in Parkinson’s disease. Mov. Disord. 17:1166–1173 10.1002/mds.10258 [DOI] [PubMed] [Google Scholar]
- Makarava N., Kovacs G.G., Savtchenko R., Alexeeva I., Budka H., Rohwer R.G., Baskakov I.V. 2011. Genesis of mammalian prions: from non-infectious amyloid fibrils to a transmissible prion disease. PLoS Pathog. 7:e1002419 10.1371/journal.ppat.1002419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.J., Pan Y., Price A.C., Sterling W., Copeland N.G., Jenkins N.A., Price D.L., Lee M.K. 2006. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 26:41–50 10.1523/JNEUROSCI.4308-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M., Coomaraswamy J., Bolmont T., Kaeser S., Schaefer C., Kilger E., Neuenschwander A., Abramowski D., Frey P., Jaton A.L., et al. 2006. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 313:1781–1784 10.1126/science.1131864 [DOI] [PubMed] [Google Scholar]
- Murray I.V.J., Giasson B.I., Quinn S.M., Koppaka V., Axelsen P.H., Ischiropoulos H., Trojanowski J.Q., Lee V.M.Y. 2003. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 42:8530–8540 10.1021/bi027363r [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R., Geeraedts L.M.G., Veening J.G. 1982. The medial forebrain bundle of the rat. I. General introduction. J. Comp. Neurol. 206:49–81 10.1002/cne.902060106 [DOI] [PubMed] [Google Scholar]
- Polymenidou M., Cleveland D.W. 2011. The seeds of neurodegeneration: prion-like spreading in ALS. Cell. 147:498–508 10.1016/j.cell.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M., Heikenwalder M., Junt T., Schwarz P., Glatzel M., Heppner F.L., Fu Y.X., Lipp M., Aguzzi A. 2003. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature. 425:957–962 10.1038/nature02072 [DOI] [PubMed] [Google Scholar]
- Sampathu D.M., Giasson B.I., Pawlyk A.C., Trojanowski J.Q., Lee V.M.Y. 2003. Ubiquitination of alpha-synuclein is not required for formation of pathological inclusions in alpha-synucleinopathies. Am. J. Pathol. 163:91–100 10.1016/S0002-9440(10)63633-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.R., Davies D., Fraser H. 1992. Scrapie in the central nervous system: neuroanatomical spread of infection and Sinc control of pathogenesis. J. Gen. Virol. 73:1637–1644 10.1099/0022-1317-73-7-1637 [DOI] [PubMed] [Google Scholar]
- Spillantini M.G., Crowther R.A., Jakes R., Cairns N.J., Lantos P.L., Goedert M. 1998a. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 251:205–208 10.1016/S0304-3940(98)00504-7 [DOI] [PubMed] [Google Scholar]
- Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., Goedert M. 1998b. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA. 95:6469–6473 10.1073/pnas.95.11.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda T., Qureshi M.M., Ardah M.T., Varghese S., Shehab S.A., Kasai T., Ishigami N., Tamaoka A., Nakagawa M., El-Agnaf O.M. 2010. Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 75:1766–1772 10.1212/WNL.0b013e3181fd613b [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley L.A., Luk K.C., Patel T.P., Tanik S.A., Riddle D.M., Stieber A., Meaney D.F., Trojanowski J.Q., Lee V.M.Y. 2011. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 72:57–71 10.1016/j.neuron.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Takahashi H., Takeda S., Ohama E., Ikuta F. 1989. Lewy bodies in the enteric nervous system in Parkinson’s disease. Arch. Histol. Cytol. 52(Suppl):191–194 10.1679/aohc.52.Suppl_191 [DOI] [PubMed] [Google Scholar]
- Waxman E.A., Giasson B.I. 2008. Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J. Neuropathol. Exp. Neurol. 67:402–416 10.1097/NEN.0b013e3186fc995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman E.A., Giasson B.I. 2010. A novel, high-efficiency cellular model of fibrillar alpha-synuclein inclusions and the examination of mutations that inhibit amyloid formation. J. Neurochem. 113:374–388 10.1111/j.1471-4159.2010.06592.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.J., Wypych J., Steavenson S., Louis J.C., Citron M., Biere A.L. 1999. alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J. Biol. Chem. 274:19509–19512 10.1074/jbc.274.28.19509 [DOI] [PubMed] [Google Scholar]